Speech and language disturbances are very frequent in progressive supranuclear palsy (PSP). Therefore, they are part of the diagnostic criteria set forth by the International Parkinson and Movement Disorder Society (MDS) for the disease and are considered a core clinical feature. However, more studies are needed to characterize the linguistic profile of PSP, thus being able to assist in the differential diagnosis. Additionally, studies assessing linguistic differences among PSP phenotypes are needed. The objective of this study is to analyze the language alterations presented by patients with PSP, as well as its different phenotypes, and differentiate them from those presented in patients with Parkinson's disease (PD).
MethodsAn extensive cognitive and linguistic assessment was administered to 13 PSP patients, 19 PD patients and 19 healthy controls (HC) with similar sociodemographic features. Language assessment included evaluation of: syntactic processing, object naming, and phonetic and semantic fluencies. We included a subgroup of 6 PSP patients, 19 PD patients and 19 HC for further analysis of language. This analysis included, in addition to the general evaluation, the assessment of alternating fluency, comprehension, naming, automatic speech, repetition, object recognition, verbal and written instructions, writing to dictation, and oral expression.
ResultsWe found greater impairment on phonetic, semantic, and alternating fluencies, following verbal instructions, repetition, syntactic processing and writing (without phonetic paragraphia) in the PSP group compared to patients with PD and HC. Distinguishing linguistic features of PSP with a less marked reduction than the previously mentioned features were automatic speech, fluency of speech, and naming. Language analysis did not distinguish between PSP phenotypes.
ConclusionsLanguage disturbances distinguish PSP from PD and HC but were not able to discriminate PSP phenotypes.
Las alteraciones del habla y el lenguaje son muy frecuentes en la parálisis supranuclear progresiva (PSP). Por ello, forman parte de los criterios diagnósticos postulados por la Sociedad Internacional de Parkinson y Trastornos del Movimiento (International Parkinson and Movement Disorder Society, MDS) para la enfermedad, y son consideradas una característica clínica central. No obstante, se necesitan más estudios que caractericen el perfil lingüístico de la PSP, pudiendo así ayudar en el diagnóstico diferencial. Además, se necesitan estudios que evalúen las diferencias lingüísticas entre los fenotipos de PSP. El objetivo de este estudio es analizar las alteraciones del lenguaje que presentan los pacientes con PSP, así como sus diferentes fenotipos, y diferenciarlas de las que se presentan en la enfermedad de Parkinson (EP).
MétodosSe realizó una evaluación cognitiva y lingüística extensa a 13 pacientes con PSP, 19 pacientes con EP y 19 controles sanos (CS) con características sociodemográficas similares. La evaluación del lenguaje incluyó la evaluación del: procesamiento sintáctico, la denominación de objetos y la fluencia fonética y semántica. Se incluyó también a un subgrupo de 6 pacientes con PSP, 19 pacientes con EP y 19 CS, para un análisis adicional del lenguaje. En este análisis se incluyó, además de la evaluación general, la evaluación de la fluencia alternante, la comprensión, la denominación, el habla automática, la repetición, el reconocimiento de objetos, seguimiento de instrucciones verbales y escritas, la escritura al dictado y la expresión oral.
ResultadosSe observó un mayor deterioro en las medidas de fluencia fonética, semántica y alternante, el seguimiento de instrucciones verbales, la repetición, el procesamiento sintáctico y la escritura (sin paragrafía fonética) en el grupo de PSP en comparación con los pacientes con EP y los CS. Características lingüísticas distintivas de la PSP con una reducción menos marcada que las características anteriores fueron el habla automática, la fluidez del habla y una menor denominación. El análisis del lenguaje no permitió distinguir entre los fenotipos de PSP.
ConclusionesLas alteraciones del lenguaje discriminan la PSP y la EP y la PSP de los CS, pero no fueron capaces de discriminar los fenotipos de PSP.
Progressive supranuclear palsy (PSP) is a neurodegenerative disease characterized by motor dysfunction, behavioral symptoms, and cognitive decline.1,2 Motor dysfunction in PSP includes postural instability, falls, rigidity, akinesia, and supranuclear gaze palsy.3 PSP is identified as a primary tauopathy,4 and distinct subtypes have been established, with the classic PSP Richardson's syndrome (PSP-RS) being the most common.5 Other subtypes have been described based on varying initial or predominant symptoms during the evolution of the disease.6 These subtypes include PSP with predominant Parkinsonism (PSP-P), progressive gait freezing (PSP-PGF), corticobasal syndrome (PSP-CBS), and predominant frontal presentation (PSP-F).3 Some patients develop prominent language deficits in the so-called PSP-speech-language (PSP-SL) phenotype,7 including progressive apraxia of speech (AOS) and nonfluent/agrammatic primary progressive aphasia (nfaPPA).8,9
Clinical diagnosis of PSP can be difficult due to shared motor and non-motor symptoms10 such as cognitive impairment and behavioral alterations1,2,11 which are also common in other neurodegenerative diseases, including Parkinson's disease (PD). As the early stages of PSP and PD may present with overlapping clinical manifestations PSP may be misdiagnosed as PD.12 Early cognitive changes in PSP have been primarily described as a frontal dysexecutive syndrome,13,14 manifesting as difficulty with planning and organization6 and a decrease in cognitive flexibility, sustained attention, and processing speed.6,15 Other affected domains include language, memory, visuospatial capacities, and social cognition.13,15,16 The International Parkinson and Movement Disorder Society (MDS)-endorsed PSP study group established criteria to characterize a variety of clinical presentations related to PSP clinical syndrome (PSPs). They ascertained speech and language as having the highest degree of diagnostic certitude in the cognitive domain (C1) followed by frontal cognitive/behavioral presentation (C2) and corticobasal syndrome (C3), thus establishing speech and language as a fundamental core criterion in the diagnosis of PSP.10 Accordingly, tests assessing various aspects of language may be useful in distinguishing PD from atypical parkinsonism conditions.
Linguistic impairment in PSP has been characterized by a reduction in spelling accuracy,17 naming,18 sentence comprehension, sentence repetition, and lexico-semantic and syntactic processing.19 One of the most frequent aspects of the language changes seen in patients with PSP in which pronounced deficits have been consistently found is verbal fluency.6,20 A decline in propositional speech is also a feature of PSP. In some cases where the speech impairment is more serious this may be described as dynamic aphasia.21 This is characterized by a pronounced decline in propositional speech while other primary language functions, such as naming, comprehension, and repetition remain intact.20–23 Characteristic writing impairments in PSP include letter omission,24,25 micrographia,26 and dysgraphia regarding the use of phonemic additions, substitutions, and spelling errors.24,25
When compared to patients with progressive nonfluent aphasia (PNFA) without PSP, patients with the PSP-SL phenotype present with a more serious reduction in spontaneous speech rate (words per minute) but with fewer speech production errors.7 PNFA patients without PSP present significantly worse in spelling than those with the PSP-SL phenotype.7 Patients with this phenotype have been described as presenting with decreased auditory verbal comprehension (following sequential commands), grammar comprehension, semantic and phonetic fluency, repetition,27 word and sentence comprehension, and naming7 when compared to controls. Greater impairment in picture description tasks in the PSP-SL phenotype has been described as a reduction in speech rate, phonetic errors, and a greater number of syntactic errors,7,27 pointing toward underlying phonetic and syntactic dysfunction.
Several characterizations of language alterations have been identified in PD. These include a mild deficiency in speech, naming, fluency, and grammar.28,29 Additionally, impaired phonetic, semantic,30 and action fluencies,31 and difficulty in comprehension of sentences with complex syntactic structure32 have been described in PD. Findings have shown that PD patients use simplified syntactic structure33 and produce fewer grammatically correct sentences in spontaneous speech.33 Object and action semantics32 and object and action naming34 have also been found to be impaired in PD, although it has been reported that action naming is more affected than object naming.34
The present study aimed to further characterize linguistic distinctions between PD and PSP by means of a comprehensive language assessment. Our objective was to establish how insight into linguistic deficiencies can contribute to distinctions between PSP and PD and determine the most effective language assessments that distinguish the two diseases. Additionally, we explored the capacity of language assessments to discern between PSP phenotypes.
Materials and methodsParticipantsA total of 51 participants were recruited from the movement disorders unit at Hospital de la Santa Creu i Sant Pau in Barcelona, Spain. Of the 51 participants, 19 had a clinical diagnosis of PD, 13 had been clinically diagnosed with PSP, and 19 were healthy controls (HC). The PSP group was made up of three distinct phenotypes, PSP-RS (n=6), PSP-PGF (n=5), and PSP-SL (n=2). PSP diagnosis as well as PSP phenotype were established by criteria set forth by the MDS for the diagnosis of PSP (MDS-PSP).10 All participants were native bilingual Spanish-Catalan speakers. Of the 13 PSP participants, a subgroup of 6 participants partook in an additional linguistic assessment. The PSP phonotypes included in this group were PSP-RS (n=3), PSP-PGF (n=2), and PSP-SL (n=1). We administered the Token Test, an alternating verbal fluency task, and the Mississippi Aphasia Screening Test (MAST) to this group for a more comprehensive assessment of linguistic functions. We added this subgroup to strengthen the assessment through comprehension of spoken language, alternating fluency, and the MAST which is comprised of several linguistic subscales. The subgroup was comprised of fewer participants due to difficulty of some patients to come to the hospital for a second visit. For clarity purposes, this subgroup will be referred to as the PSP-MAST (Mississippi Aphasia Screening Test) subgroup. Criteria for exclusion in the study were auditory difficulties or a history of developmental, psychiatric, or neurological disorders other than PD or PSP. All controls had scores within the normal range on the Frontal Assessment Battery (FAB) with a cutoff score of 12, and on the Montreal Cognitive Assessment (MoCA) with a cutoff score of 26. All measures were performed with the approval of the local institutional review board. Participants and caregivers gave written informed consent. The investigation was carried out in accordance with the Declaration of Helsinki.
Neuropsychological and language assessmentTo assess global cognitive function we administered the Montreal Cognitive Assessment (MoCA)35 and the Frontal Assessment Battery (FAB).36 The FAB is a short scale regarded for its sensitivity in detecting frontal related deficiencies in PD36,37 and PSP,36,38 and the MoCA has shown to be a sensitive instrument to detect cognitive impairment in PD39 and PSP.40 To assess verbal memory, we administered the Buschke Free and Cued Selective Reminding Test (FCSRT).41 We evaluated language using several neuropsychological assessments. To evaluate oral comprehension and syntactic processing, subsections of the Boston Diagnostic Aphasia Examination (BDAE) were administered.42 The specific subsections were touching A with B, reversible possessives, and embedded sentences. Naming and lexical retrieval was evaluated using the Boston Naming Test (BNT).43 Verbal fluency was measured by the number of unique words produced in 60s. Phonetic fluency was assessed by producing words beginning with the letter p, a version of the FAS verbal fluency test44 with comparable values oriented to the Spanish population.45 Semantic fluency was measured by the number of unique animal names produced.42
The additional language assessment for the PSP-MAST subgroup was comprised of the shortened version of the Token Test,46 the Mississippi Aphasia Screening Test (MAST),47 and an alternating fluency task. The Token Test measures receptive language capabilities through comprehension of spoken language. The Mississippi Aphasia Screening Test addresses a broad range of linguistic functions and is comprised of 9 subscales which measure expressive and receptive language capacities including naming, automatic speech, repetition, verbal fluency (free speech), writing to dictation, verbal instructions, written instructions, object recognition, and yes/no response to a stimulus question. Alternating fluency was assessed using the alternating fluency task from the Parkinson's Disease Cognitive Rating Scale (PD-CRS),48 in which participants were required to produce a word beginning with the letter “s” followed by an article of clothing (which could begin with any letter), alternating between the two for a duration of 60s.
Statistical analysesStatistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) v28.0. Descriptive statistics were used to classify sociodemographic properties of groups. Sociodemographic and clinical variables are stated as means±standard deviations. Differences between groups were analyzed with analyses of variance (ANOVA). Scheffe's test was used for post-hoc analysis. Categorical variables were analyzed with χ2. ANCOVA with age as covariate was used for analyses comparing the PSP, PD, and HC on cognitive and linguistic assessments, and Bonferroni's test was performed to analyze differences between groups. Values of p<0.05 were considered statistically significant.
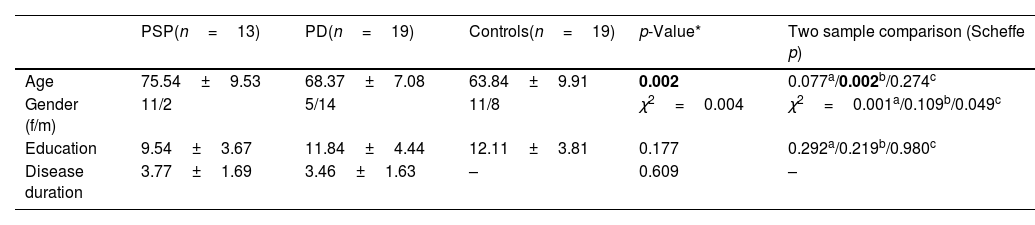
ResultsDemographicsRegarding the 13 PSP participants, mean age was 75.5±9 years and disease duration was 3.7±1.6 years. The 19 PD patients had a mean age of 68.3±7.1 years, and mean disease duration of 3.4±1.6 years. HC had a mean age of 63.8±9.9 years. PSP patients were significantly older than the HC group (p=0.002) but no significant differences were found for educational level (p=0.219) or gender (p=0.109). The PSP, PD, and HC groups were matched for education (p=0.177) and the PSP and PD groups were matched for disease duration (p=0.609) (Table 1).
Clinical and sociodemographic characteristics of all groups.
| PSP(n=13) | PD(n=19) | Controls(n=19) | p-Value* | Two sample comparison (Scheffe p) | |
|---|---|---|---|---|---|
| Age | 75.54±9.53 | 68.37±7.08 | 63.84±9.91 | 0.002 | 0.077a/0.002b/0.274c |
| Gender (f/m) | 11/2 | 5/14 | 11/8 | χ2=0.004 | χ2=0.001a/0.109b/0.049c |
| Education | 9.54±3.67 | 11.84±4.44 | 12.11±3.81 | 0.177 | 0.292a/0.219b/0.980c |
| Disease duration | 3.77±1.69 | 3.46±1.63 | – | 0.609 | – |
| PSP-MAST(n=6) | PD(n=19) | Controls(n=19) | p-Value* | Two sample comparison (Scheffe p) | |
|---|---|---|---|---|---|
| Age | 71.50±9.29 | 68.37±7.08 | 63.84±9.91 | 0.096 | 0.727d/0.159e/0.259f |
| Gender (f/m) | 5/1 | 5/14 | 11/8 | χ2=0.026 | χ2=0.013d/0.258e/0.049f |
| Education | 9.67±4.93 | 11.84±4.44 | 12.11±3.81 | 0.463 | 0.554d/0.477e/0.982f |
| Disease duration | 3.69±1.73 | 3.50±1.54 | – | 0.800 | – |
Bold p values denote significant group differences.
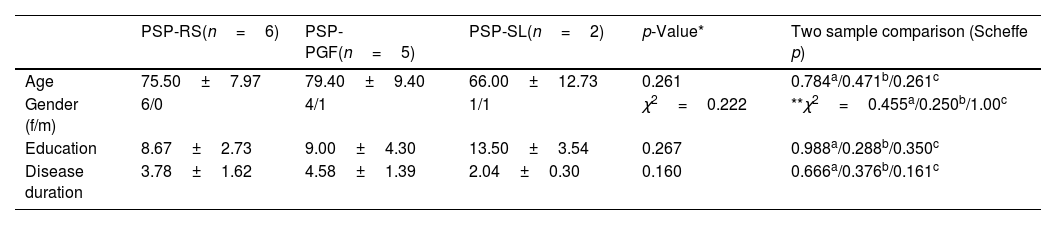
The PSP-MAST subgroup (n=6) had a mean age of 71.5±9.3 years and mean disease duration of 3.6±1.7 years. This subgroup was made up of PSP Richardson's syndrome (PSP-RS), PSP with progressive gait freezing (PSP-PGF), and PSP with predominant speech or language disorder (PSP-SL) phenotypes. The PSP-MAST subgroup, PD group, and HC group were comparable in age (p=0.096), education (p=0.463), and disease duration (p=0.800) (Table 1). Regarding PSP phenotypes, there were no significant differences in age (p=0.261), gender (p=0.222), education level (p=0.267), or disease duration (p=0.160) between the PSP-RS group, PSP-PGF group, and PSP-SL group. We did not find significant differences in age (p=0.249), education level (p=0.457), or disease duration (p=0.714) between PSP-RS, PSP-PGF, or PSP-SL phenotypes in the PSP-MAST subgroup (Table 2).
Clinical and sociodemographic characteristics of PSP phenotypes.
| PSP-RS(n=6) | PSP-PGF(n=5) | PSP-SL(n=2) | p-Value* | Two sample comparison (Scheffe p) | |
|---|---|---|---|---|---|
| Age | 75.50±7.97 | 79.40±9.40 | 66.00±12.73 | 0.261 | 0.784a/0.471b/0.261c |
| Gender (f/m) | 6/0 | 4/1 | 1/1 | χ2=0.222 | **χ2=0.455a/0.250b/1.00c |
| Education | 8.67±2.73 | 9.00±4.30 | 13.50±3.54 | 0.267 | 0.988a/0.288b/0.350c |
| Disease duration | 3.78±1.62 | 4.58±1.39 | 2.04±0.30 | 0.160 | 0.666a/0.376b/0.161c |
| Clinical and sociodemographic characteristics of PSP phenotypes MAST subgroup | ||||
|---|---|---|---|---|
| PSP-RS(n=3) | PSP-PGF(n=2) | PSP-SL(n=1) | p-Value* | |
| Age | 73.33±2.08 | 76.00±12.73 | 57.00±– | p=0.249 |
| Gender (f/m) | 3/0 | 2/0 | 0/1 | χ2=0.05 |
| Education | 8.00±0.00 | 9.00±8.49 | 16.00±– | p=0.457 |
| Disease duration | 3.72±1.67 | 4.38±2.53 | 2.25±– | p=0.714 |
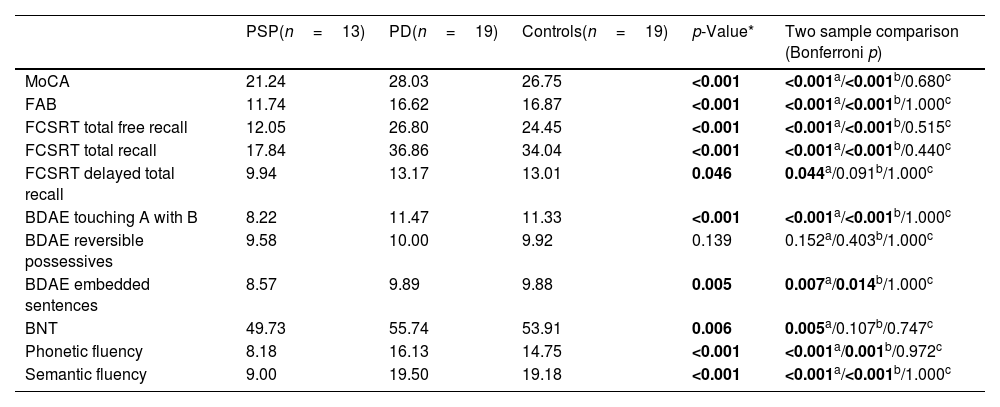
PSP patients performed significantly worse than PD and HC groups on the FAB (p<0.001), MoCA (p<0.001), FCSRT total free recall (p<0.001) and FCSRT total recall (p<0.001). PSP patients showed significantly worse scores than PD patients on syntactic processing measured using the BDAE touching A with B (p<0.001) and BDAE embedded sentences (p=0.007) tasks. PSP patients also performed worse on naming (p=0.005), and phonetic (p<0.001) and semantic (p<0.001) fluencies. In comparison to HC, the PSP group performed significantly worse on assessments of syntactic processing BDAE touching A with B (p<0.001) and BDAE embedded sentences (p=0.014), and phonetic (p=0.001) and semantic (p<0.001) fluencies. The only assessment that did not show any statistically significant difference between PSP with either PD or HC groups was the BDAE reversible possessives test (p=0.139). There was no significant difference between HC and PSP on the BDAE reversible possessives (p=0.403) or naming (p=0.107) assessments. Differences in results between PD and HC groups were not significant in any task (Table 3). Because the PD and PSP groups differed significantly on the MoCA, we conducted a complimentary analysis with a subgroup of the PD (n=5) and PSP (n=8) patients that performed similarly on the MoCA (p=0.064). The two groups were also homogeneous in age (p=1.000), education (p=0.400), and disease duration (p=1.000). The results were the same across all tests except only one subscale, the embedded sentences task, which did not reach significance (p=0.173). No significant outcomes were found between PSP phenotypes (Supplementary Table 1).
Cognitive and linguistic performance between PSP, PD, and controls.
| PSP(n=13) | PD(n=19) | Controls(n=19) | p-Value* | Two sample comparison (Bonferroni p) | |
|---|---|---|---|---|---|
| MoCA | 21.24 | 28.03 | 26.75 | <0.001 | <0.001a/<0.001b/0.680c |
| FAB | 11.74 | 16.62 | 16.87 | <0.001 | <0.001a/<0.001b/1.000c |
| FCSRT total free recall | 12.05 | 26.80 | 24.45 | <0.001 | <0.001a/<0.001b/0.515c |
| FCSRT total recall | 17.84 | 36.86 | 34.04 | <0.001 | <0.001a/<0.001b/0.440c |
| FCSRT delayed total recall | 9.94 | 13.17 | 13.01 | 0.046 | 0.044a/0.091b/1.000c |
| BDAE touching A with B | 8.22 | 11.47 | 11.33 | <0.001 | <0.001a/<0.001b/1.000c |
| BDAE reversible possessives | 9.58 | 10.00 | 9.92 | 0.139 | 0.152a/0.403b/1.000c |
| BDAE embedded sentences | 8.57 | 9.89 | 9.88 | 0.005 | 0.007a/0.014b/1.000c |
| BNT | 49.73 | 55.74 | 53.91 | 0.006 | 0.005a/0.107b/0.747c |
| Phonetic fluency | 8.18 | 16.13 | 14.75 | <0.001 | <0.001a/0.001b/0.972c |
| Semantic fluency | 9.00 | 19.50 | 19.18 | <0.001 | <0.001a/<0.001b/1.000c |
MoCA: Montreal Cognitive Assessment; FAB: Frontal Assessment Battery; FCSRT: Free and Cued Selective Reminding Test; BDAE: Boston Diagnostic Aphasia Examination; BNT: Boston Naming Test.
Bold p values denote significant group differences.
Similar to the PSP group, the PSP-MAST subgroup performed significantly worse than PD and HC groups on the FAB (p<0.001) and MoCA (p<0.001). PSP-MAST patients showed significantly greater cognitive impairment than PD patients on assessments of comprehension (p<0.001), repetition (p<0.001), following verbal instructions (p=0.007), writing without phonetic paragraphia (p=0.002), and alternating fluency (p=0.004). No significant difference was found between the PSP-MAST and PD groups on measures of naming (p=0.055), automatic speech (p=0.047), or oral expression (p=0.055). In comparison to HC, the PSP-MAST group performed significantly worse on assessments of automatic speech (p=0.047), repetition (p<0.001), following verbal instructions (p=0.004), writing without phonetic paragraphia (p=0.003), and alternating fluency (p<0.001). No significant difference was found between the PSP-MAST and HC groups on measures of naming (p=0.055) or oral expression (p=0.055). Results between PD and HC were not significant (Table 4). Because the PD and PSP-MAST groups differed significantly on the MoCA, we conducted a complimentary analysis with a subgroup of the PD (n=5) and PSP-MAST (n=4) patients that performed similarly on the MoCA (p=0.531). The two groups were also homogeneous in age (p=0.926), education (p=0.758), and disease duration (p=0.811). The results were the same across all tests except two subscales, the MAST following verbal instructions (p=0.120) and the MAST writing without phonetic paragraphia (p=0.372) subscales, which did not reach significance. Outcomes were not significant between PSP phenotypes on the FAB, MoCA, Token Test, MAST, or alternating fluency task (Supplementary Table 2).
Cognitive and linguistic performance between PSP-MAST subgroup, PD, and controls.
| PSP-MAST(n=6) | PD(n=19) | Controls(n=19) | p-Value* | Two sample comparison (Scheffe p) | |
|---|---|---|---|---|---|
| MoCA | 23.17±5.42 | 28.05±1.39 | 27.42±1.31 | <0.001 | <0.001a/0.001b/0.696c |
| FAB | 11.67±3.08 | 16.63±1.12 | 17.26±0.99 | <0.001 | <0.001a/<0.001b/0.419c |
| Token | 31.50±3.21 | 34.95±1.13 | 35.00±1.05 | <0.001 | <0.001a/<0.001b/0.994c |
| MAST naming | 9.67±0.816 | 10.00±0.00 | 10.00±0.00 | 0.038 | 0.055a/0.055b/1.000c |
| MAST automatic speech | 8.33±2.73 | 9.74±0.65 | 9.79±0.92 | 0.036 | 0.058a/0.047b/0.991c |
| MAST repetition | 8.33±1.51 | 9.89±0.46 | 9.84±0.50 | 0.019 | <0.001a/<0.001b/0.973c |
| MAST yes/no responses | 20.00±0.00 | 20.00±0.00 | 20.00±0.00 | – | – |
| MAST object recognition | 10.00±0.00 | 10.00±0.00 | 10.00±0.00 | – | – |
| MAST following verbal instructions | 9.00±1.67 | 9.95±0.23 | 10.00±0.00 | 0.003 | 0.007a/0.004b/0.965c |
| MAST following written instructions | 10.00±0.00 | 10.00±0.00 | 10.00±0.00 | – | – |
| MAST writing to dictation | 8.50±1.98 | 9.89±0.46 | 9.84±0.38 | 0.001 | 0.002a/0.003b/0.979c |
| MAST oral expression/fluency | 9.17±2.04 | 10.00±0.00 | 10.00±0.00 | 0.038 | 0.055a/0.055b/1.000c |
| MAST total | 93.00±6.99 | 99.47±0.84 | 99.42±1.12 | <0.001 | <0.001a/<0.001b/0.998c |
| Alternating fluency | 5.83±3.82 | 13.95±5.62 | 15.21±4.35 | <0.001 | 0.004a/<0.001b/0.731c |
MoCA: Montreal Cognitive Assessment; FAB: Frontal Assessment Battery; MAST: Mississippi Aphasia Screening Test.
Bold p values denote significant group differences.
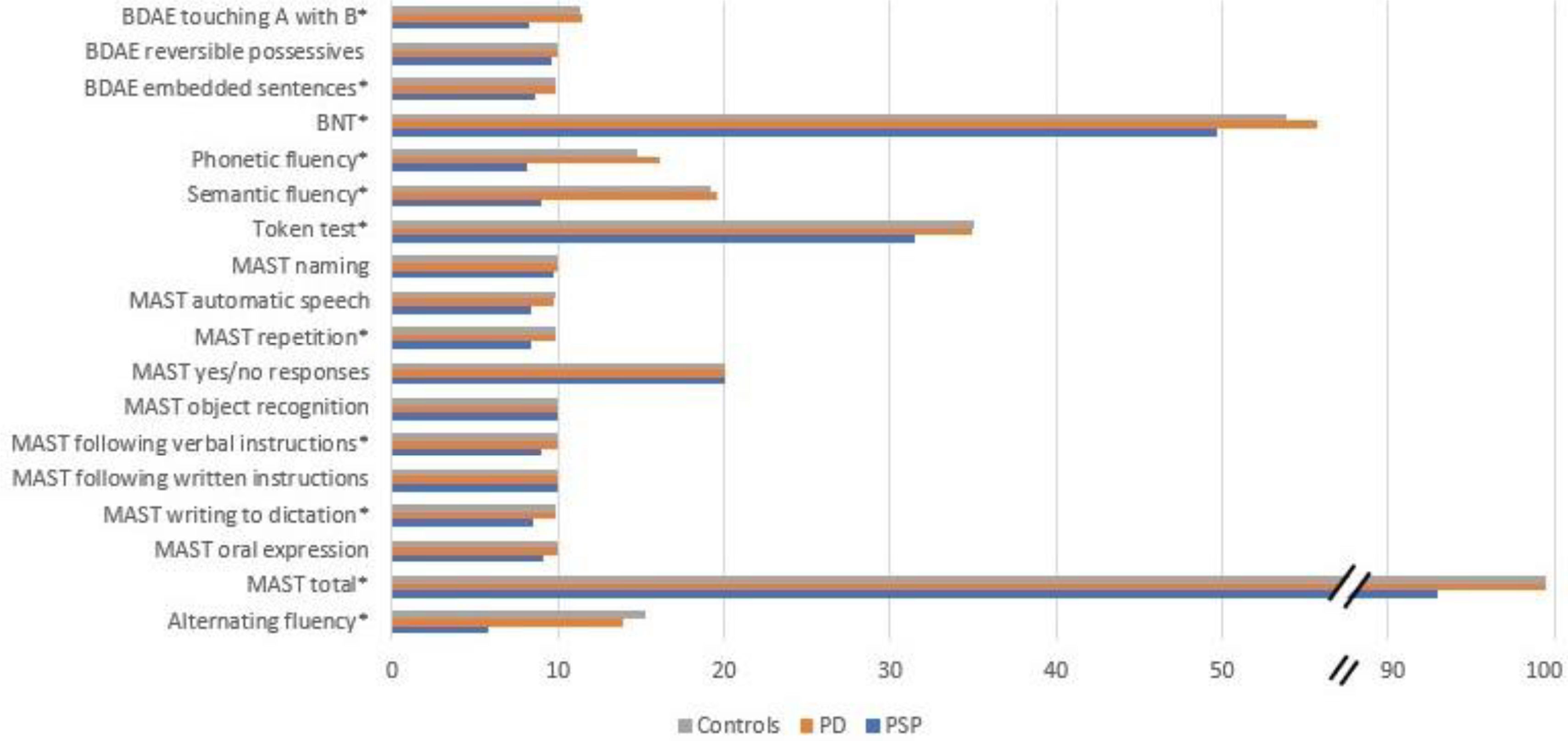
The present study evaluated linguistic differences in patients with PD and PSP and differences between PSP phenotypes. We found that the majority of tests in our battery revealed significant differences between PD and PSP, with a worse performance in the PSP group (Fig. 1). The linguistic tests that significantly discriminated between PD and PSP were the BDAE touching A with B and BDAE embedded sentences assessments, the BNT, phonetic, semantic, and alternating fluencies, the Token Test, and the repetition, following verbal instructions, and writing to dictation subscales of the MAST. The MAST total score also revealed a significant difference between PD and PSP. Automatic speech, oral expression, and the BDAE reversible possessives assessment did not discriminate between our sample of PD and PSP patients. Furthermore, the PD group scored similarly to the control group across all assessments.
Summary of linguistic performance between groups. BDAE: Boston Diagnostic Aphasia Examination; BNT: Boston Naming Test; MAST: Mississippi Aphasia Screening Test. *Significant at p<.05 between PD and PSP. Note: MAST naming and MAST oral expression reached significance among groups, with a significance of p=.055 between PD and PSP. Performances are illustrated with raw scores.
Mild impairment of sentence comprehension has been demonstrated in PSP.19,49 Lexico-semantic dysfunction has been found in PSP through a reduction in naming, word comprehension, semantic association, speech rate, and number of words in spoken language.19 Lexico-semantic and executive functioning may affect sentence comprehension rather than syntactic dysfunction alone.19 This may explain our finding of a mild impairment of receptive language capabilities through comprehension of spoken language. Although we found no differences between groups concerning object recognition, yes/no responses, and following written instructions subtests of the MAST, the following verbal instructions subtest of the MAST revealed a significantly lower score in the PSP group than the PD and HC groups, as did the Token Test. Furthermore, similar to previous studies,14,18,19,50 we found a mild reduction in confrontational naming in the PSP group. Two measures of syntactic comprehension, the BDAE touching A with B task and the BDAE embedded sentences task, were significantly impaired in the PSP group. It is important to note however, that vertical eye movements are required to scan pictures used in the syntactic comprehension tests, which may put PSP patients at a slight disadvantage when compared to PD patients and controls. Other studies have found that visual scanning or visual attention may affect performance on tests that employ word or picture-matching tasks.23,49
The pronounced alteration of verbal fluency in PSP patients has been well documented and has been maintained to be caused by executive deficiency.51 While results have shown that there is generally a stronger deficit in phonetic fluency52,53 than semantic fluency,50,52 our results yielded similar verbal fluency production between phonetic and semantic fluency, a result which has also been found.15
Imaging studies have revealed that phonetic fluency generally relies on frontal regions while semantic fluency also activates temporal areas.54 In general and in the early stages of the disease, PSP patients have frontal and midbrain atrophy while temporal and parietal areas remain fairly intact.55 Because PSP patients in our study performed so similarly in phonetic and semantic fluency, this may be an indication that executive dysfunction is an underlying cause of overall deficiency in verbal fluency in PSP, and not phonetic fluency alone. Another possible explanation for the similar results in phonetic and semantic fluency in the PSP group is that PSP patients have diminished processing speed and a reduction in initiation, which may influence verbal fluency output.56 However, diminished processing speed may not be the only underlying factor, as PSP patients quickly run out of words, despite the amount of time available to them.56 This effect may be due to sustained activation57 and is consistent with the PSP participants in our study. This outcome is different from that observed in PD patients, who are able to continue producing words throughout the allotted one-minute time frame. It has been reported that producing seven words or less during the phonetic fluency task differentiates PSP patients from those with other movement disorders.58 Alternating fluency was also significantly impaired in the PSP group, and they performed slightly worse on alternating than phonetic and semantic fluency which may be due to the added demands of set-shifting.
Agrammatism59 and irregularities in language production tasks such as fewer words and sentences, fewer morphemes, perseveration, and reduced use of novel words and sentences19,21,23,25,60 have been described in PSP. These elements reflect our finding of reduced expressive language capabilities assessed by the MAST. We also found a significant reduction in repetition in the PSP-MAST group. PSP patients have been found to perform better on word and repetition tasks relative to a PNFA patients,7 and this may indicate that a slight decrease in repetition is an indicator of PSP, while a steeper decrease in repetition would be expected in patients with PNFA.
Another feature typically affected within the expressive language capabilities in PSP patients is dysgraphia. Our study measured dysgraphia in relation to correct codification without phonetic paragraphia. The use of unintended phonemes, phonemic and/or letter omissions and substitutions, and phonological orthographic errors appear to be a feature of PSP. This may be a distinguishing factor between PSP and PD as the PSP groups performed more poorly.
There are marked distinctions between PD and PSP regarding language. One clear difference is the performance on verbal fluency tasks. Our results show that PSP patients are significantly more impaired than PD patients on this assessment. We found significant impairment in writing (characterized by orthographic/phonetic errors), naming, sentence comprehension, sentence repetition, and lexico-semantic and syntactic processing when compared to PD and HC. There was also a significant reduction in automatic speech and oral expression. These tests may prove useful in discriminating the diseases on a clinical level. Distinguishing linguistic features include reduced word production in PSP and a higher amount of orthographic and semantic errors. Another important distinguishing factor is that language deficiency can be seen early in the disease progression of PSP53 while language decline and general cognitive decline usually occurs later in PD.17 While language assessment revealed discriminatory capacity between PD and PSP, there were no differences between the PSP-RS, PSP-PGF, or PSP-SL groups.
The linguistic impairment is consistent with marked frontal deficits observed in PSP patients.3 Frontal impairment characterized by a mild deficiency in expressive language was also found in our sample in oral expression, automatic speech, and repetition. This result and the frontal-executive demand required for verbal fluency further support the presence of frontal cognitive deficiency as well as a pronounced impairment in the frontal-subcortical network.61
Memory impairment was also observed in the PSP group. Attention and working memory dysfunction are features of dysexecutive syndrome linked to reduced frontal-subcortical gray matter in PSP.62 Regarding verbal learning, our results support the notion that impaired attention, working memory and dysfunctional strategic search of stored information63 could cause memory deficits. This may be linked to frontally mediated faulty organizational and temporal capacities regarding encoding and retrieval.63 However, in contrast to previous findings,63 PSP patients in our study performed significantly worse on delayed recall suggesting disruption in encoding and retrieval of stored information. It is important to note that the PD patients had significantly higher scores on the MoCA, and future studies should include PD and PSP patients with similar MoCA scores.
Distinctions such as naming, word comprehension, and number of information units have been reported with PSP-RS performing worse than non-RS.19 Differences have also been found between the PSP-RS and PSP with predominant Parkinsonism (PSP-P) phenotypes regarding phonetic and semantic fluencies, with more impairment in the PSP-RS groups than in the PSP-P group.15 Furthermore, a separate study reported altered semantic fluency in both PSP-RS and PSP with predominant corticobasal syndrome (PSP-CBS) when compared to PSP-PGF, with worse performance in the PSP-CBS group followed by the PSP-RS group.64 Therefore, while our results showed no significant distinction between PSP phenotypes on any language test or linguistic measure, a finding similar to previous studies,65 future studies may aim to evaluate these linguistic assessments between subtypes in a larger cohort of PSP phenotypes.
An interesting finding was the lack of significant differences between the PSP-SL phenotype compared to other PSP phenotypes. While this may be due to the small number of PSP-SL patients included in this study, the PSP-SL phenotype may only present mild linguistic distinctions from other PSP phenotypes. One recent study comparing PSP phenotypes which included a larger cohort of PSP-SL participants found that among naming, phonetic, and semantic fluencies, only semantic verbal fluency was impaired in the PSP-SL group compared to other PSP phenotypes.15 A future study assessing language performance between PSP phenotypes with a larger group of PSP-SL participants would be useful to determine the linguistic distinctions of this specific phenotype. In our sample, although it did not reach statistical significance, the PSP-SL group was younger than the PSP-RS and PSP-PGF groups and they also had a slightly shorter disease evolution time, which may have had an effect on performance outcomes.
Qualitatively, in our sample, PSP-RS appear to have a slight impairment at the lexical semantic level with a greater reduction in naming and semantic fluency. This was similar for the PSP-PGF patients, although this group had slightly higher semantic fluency scores. The PSP-RS group also had a large reduction in alternating fluency when compared to the PSP-PGF and PSP-SL group, suggesting that this reduction may be due to worse executive control affecting lexical-semantic retrieval. Worse performance in sentence comprehension, syntactic processing, and semantic and alternating fluencies in the PSP-RS and PSP-PGF groups may reflect a lexico-semantic and executive component underlying these reductions. The presence of a reduction in automatic speech in the PSP-RS and PSP-PGF groups may be attributed to a motor component19 or memory impairment in the case of automatic speech.
Our study has several limitations, one of which is the low number of PSP participants in the PSP-MAST subgroup. Secondly, the PSP patients included in this study were not pathologically confirmed diagnoses. Diagnoses were made in accordance with validated clinical criteria. Lastly, there was a significant age difference between the PSP and HC groups. However, after controlling for the age difference the main findings of the study were still apparent. Importantly, apart from this difference all groups were homogenous in age, education level, and disease duration.
The main contribution of the current study is a comprehensive and detailed linguistic characterization of PSP, particularly regarding discriminatory capacity between PSP and PD in distinct linguistic domains. Overall, the most discriminating linguistic features between PD and PSP were phonetic, semantic, and alternating fluencies, following verbal instructions, repetition, syntactic processing, and writing without phonetic paragraphia. Additional distinguishing linguistic features with a slighter reduction were automatic speech, fluency of speech, and naming. Criteria provided by the MDS-PSP study group establish speech and language as the highest degree of diagnostic certainty in the cognitive domain and, accordingly, our findings may be useful on a clinical level.
Conflict of interestNo potential conflict of interest was reported by the authors.
The authors wish to thank the patients, their families, and healthy volunteers for their participation in this study.