Epilepsy, which is present in 0.5–1% of the paediatric population, is one of the most frequent childhood neurological disorders. Approximately 20% to 30% of these cases will be drug-resistant. The objective of this study is to describe the impact of vagal nerve stimulation (VNS) on seizures and quality of life in a sample of 30 patients.
MethodsDescriptive, retrospective study of all patients with a VNS device implanted between 2008 and 2013 in a single paediatric hospital, based on patients’ medical records. Quality of life was assessed using the Spanish scale for quality of life in children with epilepsy, completed by means of a telephone interview.
ResultsWe describe a population of 19 boys (64%) and 11 girls (36%) with a mean age at seizure onset of 21 months (1–144 months). The mean age of VNS implantation was 11.89 years. Follow-up periods ranged from 6 to 36 months. Mean reduction in seizures at 6 months was 38%, with a reduction of 43% at 12 months, 42% at 24 months, and 54% at 36 months. At least half of all patients were classified as responders. According to the quality of life scale, 54% of the families rated the effect of VNS as either very good or good while 39% rated it as fair.
ConclusionsVNS is a safe palliative treatment that is generally well tolerated. It is partially effective for controlling drug-resistant epilepsy and exerts a positive effect on quality of life.
La epilepsia es uno de los trastornos neurológicos más frecuentes de la infancia, presentándose en un 0,5–1%. Aproximadamente un 20–30% de los pacientes son farmacorresistentes. El objetivo de este trabajo es describir en 30 pacientes el impacto sobre las crisis y la calidad de vida del estimulador del nervio vago (ENV).
MétodosSe trata de un estudio descriptivo, retrospectivo, mediante revisión de las historias clínicas de todos los pacientes a quienes se les colocó el ENV entre el 2008 y 2013 en nuestro centro. La calidad de vida fue valorada mediante la escala de calidad de vida en el niño con epilepsia (CAVE), obtenida por medio de una entrevista telefónica.
ResultadosSe incluyeron 19 niños (64%) y 11 niñas (36%) con una mediana de comienzo de las crisis de 21 meses (1–144 meses). La edad promedio de colocación del ENV fue de 11,89 años. El tiempo de seguimiento fue de 6–36 meses. A los 6 meses la reducción de las crisis en promedio fue del 38%, a los 12 meses del 43%, a los 24 meses del 42% y a los 36 meses del 54%. De todos los pacientes evaluados al menos un 50% se catalogaron como respondedores. Según la CAVE un 54% de las familias encontró el efecto del ENV como bueno o muy bueno y un 39% como regular.
ConclusionesEl ENV es un tratamiento paliativo, generalmente bien tolerado, parcialmente efectivo para el control de la epilepsia refractaria en pediatría y con repercusiones positivas sobre la calidad de vida.
Epilepsy is one of the most frequent neurological diseases in childhood, presenting in 0.5% to 1% of children. In 20% to 30% of patients, antiepileptic drugs (AED) fail to control seizures.1,2 Epilepsy is considered drug-resistant when the patient does not respond to 2 first-line AEDs in monotherapy or to combined therapy with 2 first-line AEDs appropriately chosen for the type of seizure and syndrome and dosed at the maximum tolerated dose.3 Drug-resistant epilepsy has a significant and negative impact on development, learning, behaviour, and quality of life in patients and their families and therefore poses a major challenge for doctors.1,2 Some treatment alternatives for patients with resistant epilepsy include the ketogenic diet, vagus nerve stimulation (VNS), and functional epilepsy surgery.
The first experimental studies of VNS were carried out in the 1980s and results indicated good tolerability and a significant reduction in seizure frequency.4–6 The antiepileptic mechanism underlying VNS is not fully understood; however, it is believed that the intermittent electric stimulation of the left vagus nerve desynchronises the thalamic-cortical circuits involved in seizure propagation.5,7 The vagus nerve terminates in the solitary nucleus, which projects to other nuclei of the brain stem; activation of these nuclei may control epileptic activity.8
VNS was approved by the US Food and Drug Administration in 1997 as a neuromodulator adjuvant therapy for patients aged over 12 years with drug-resistant partial epilepsy.9,10 VNS is regarded as a safe and effective palliative treatment for patients with severe drug-resistant epilepsy who are not eligible for functional epilepsy surgery.3,7
The first study conducted in children (60 patients) was published by the Paediatric VNS Study Group in 1999 and revealed a reduction in seizure frequency.8 Since then, several studies have shown similar effectiveness and safety rates in both children and adults with different types of seizures and epileptic syndromes of different aetiologies. Effectiveness improves when VNS is used for a long period of time. The mean reduction in seizure frequency in paediatric patients treated with VNS ranges from 30% to 62% and the percentage of respondents varies from 26% to 77%.8,11
The purpose of our study is to describe the impact of VNS on seizure frequency and quality of life in a series of 30 patients. As far as we know, this is the first study evaluating the results of VNS in a paediatric hospital in Spain.
Patients and methodsWe conducted a descriptive retrospective study in a single centre; all medical histories were reviewed and we applied the CAVE questionnaire to the families by telephone in order to assess quality of life in children with epilepsy.
We included data from all patients treated with VNS at Hospital Sant Joan de Déu between April 2008 and May 2013. The minimum follow-up time was 6 months. Patients were considered eligible for VNS if they had drug-resistant epilepsy (as defined in the introduction) and were not candidates for functional epilepsy surgery. Informed consent forms were signed by the families of all patients. The exclusion criterion was paediatric patients with progressive diseases. All patients underwent a comprehensive evaluation before having the VNS device implanted, including a complete neurological examination, a complete neuropsychological study, continuous video-EEG monitoring (2 to 5 days), and a brain MRI scan. All patients were assessed in the epilepsy unit by our group of specialists working together.
We gathered the following variables from patients’ medical histories: sex, date of birth, anomalies detected by the neurological examination, age at epilepsy onset, type of seizure, aetiology of epilepsy, and seizure frequency. Seizure frequency was calculated as the total number of seizures (of any type) the patient experienced in a month. Likewise, we gathered information about the AEDs the patients used (only for those receiving therapeutic doses for at least 2 months) and the results from the EEG and MRI studies. Results from the neuropsychological study were classified into the following categories by IQ scores: average (>84), borderline (71-84), mild intellectual disability (50-70), moderate intellectual disability (35-49), severe intellectual disability (20-34), and profound intellectual disability (<20).
VNS devices were implanted by the neurosurgery department at our hospital (García-Fructuoso). An incision was performed at the left cervical level at approximately C5-C6. Interfascial dissection was performed to expose the neurovascular bundle of the neck; the vagus nerve was located between the carotid artery and the jugular vein and dissected along 4 to 5cm approximately. A bipolar electrode was placed around the vagus nerve and connected to the pulse generator, which was implanted subcutaneously in the left part of the thorax. We checked that the device worked correctly before closing the incisions. It was turned on 2 to 3 weeks after surgery in an outpatient consulting room. The initial parameters were output current 0.25mA, pulse 0.5ms, frequency 30Hz, and a 30s/5min on/off cycle. Output current was progressively increased by 0.5mA every 7 days until reaching a maximum output current of 2 to 2.5mA, depending on the patient's tolerance and response.
The same paediatric neurologist (Sanmartí) was responsible for short-term follow-up and adjusting parameters of the VNS device. We recorded the date of device implantation; adverse reactions; monthly seizure frequency in follow-up appointments at 6, 12, 24, and 36 months after surgery; and overall favourable effects. Patients were classified as responders if they presented a reduction in seizure frequency ≥50%. We changed the type of stimulation (for example, short-cycle) and AEDs administered when seizure control was not optimal.
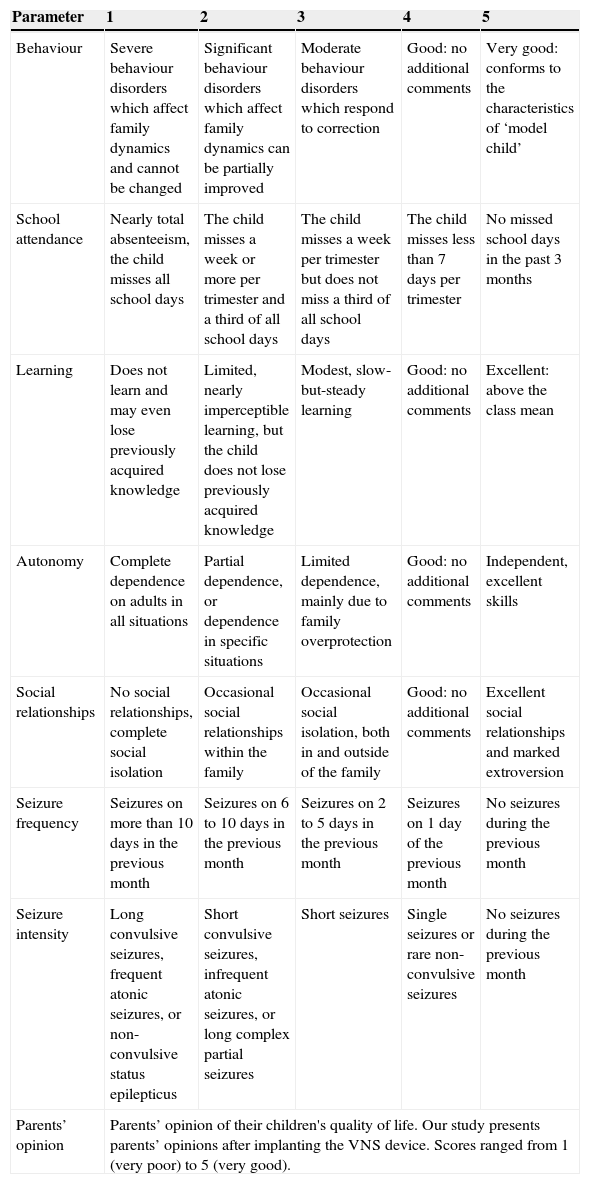
We assessed each patient's quality of life by administering the CAVE questionnaire to their families. Telephone interviews were conducted by 2 interviewers (Ulate-Campos and Cean-Cabrera). The families were asked about the following items covered by the CAVE questionnaire: behaviour, school attendance, learning, autonomy, social relationships, seizure frequency, seizure intensity, and parents’ general opinion on VNS. Responses to each item were classified as very good (5), good (4), average (3), poor (2), and very poor (1), according to the scale described in the CAVE questionnaire. Parents’ opinions on their children's quality of life after surgery were classified subjectively (Table 1).
Summary of the CAVE questionnaire to assess quality of life in children with epilepsy.
| Parameter | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Behaviour | Severe behaviour disorders which affect family dynamics and cannot be changed | Significant behaviour disorders which affect family dynamics can be partially improved | Moderate behaviour disorders which respond to correction | Good: no additional comments | Very good: conforms to the characteristics of ‘model child’ |
| School attendance | Nearly total absenteeism, the child misses all school days | The child misses a week or more per trimester and a third of all school days | The child misses a week per trimester but does not miss a third of all school days | The child misses less than 7 days per trimester | No missed school days in the past 3 months |
| Learning | Does not learn and may even lose previously acquired knowledge | Limited, nearly imperceptible learning, but the child does not lose previously acquired knowledge | Modest, slow-but-steady learning | Good: no additional comments | Excellent: above the class mean |
| Autonomy | Complete dependence on adults in all situations | Partial dependence, or dependence in specific situations | Limited dependence, mainly due to family overprotection | Good: no additional comments | Independent, excellent skills |
| Social relationships | No social relationships, complete social isolation | Occasional social relationships within the family | Occasional social isolation, both in and outside of the family | Good: no additional comments | Excellent social relationships and marked extroversion |
| Seizure frequency | Seizures on more than 10 days in the previous month | Seizures on 6 to 10 days in the previous month | Seizures on 2 to 5 days in the previous month | Seizures on 1 day of the previous month | No seizures during the previous month |
| Seizure intensity | Long convulsive seizures, frequent atonic seizures, or non-convulsive status epilepticus | Short convulsive seizures, infrequent atonic seizures, or long complex partial seizures | Short seizures | Single seizures or rare non-convulsive seizures | No seizures during the previous month |
| Parents’ opinion | Parents’ opinion of their children's quality of life. Our study presents parents’ opinions after implanting the VNS device. Scores ranged from 1 (very poor) to 5 (very good). | ||||
The statistical analysis was performed using SPSS statistical software and Excel. A multivariate linear regression model was used to analyse reduction in seizure frequency at 6, 12, 24, and 36 months after surgery given the following independent variables: sex, focus (focal or multifocal), seizure type (symptomatic or cryptogenic), age at implantation of VNS device (younger or older than 12 years), and disease progression time before VNS surgery (less or more than 6 years). We assumed a 95% confidence interval (CI). P-values <.05 were considered statistically significant.
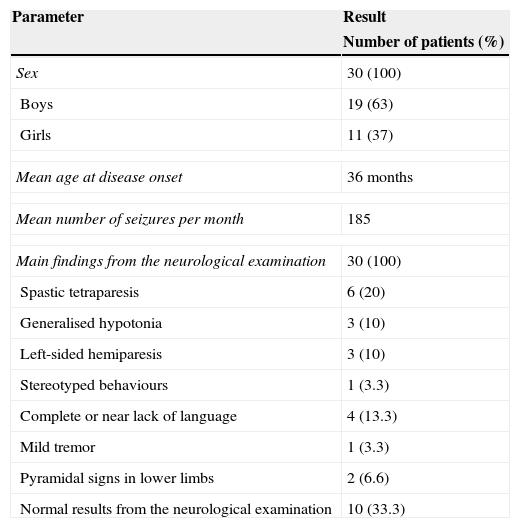
ResultsA total of 30 patients were treated with VNS between April 2008 and May 2013: 19 boys (63%) and 11 girls (37%). The main pathological findings from the neurological examination performed before surgery are summarised in Table 2. Results were normal in 10 patients (33%).
Patients’ epidemiological and clinical characteristics.
| Parameter | Result |
|---|---|
| Number of patients (%) | |
| Sex | 30 (100) |
| Boys | 19 (63) |
| Girls | 11 (37) |
| Mean age at disease onset | 36 months |
| Mean number of seizures per month | 185 |
| Main findings from the neurological examination | 30 (100) |
| Spastic tetraparesis | 6 (20) |
| Generalised hypotonia | 3 (10) |
| Left-sided hemiparesis | 3 (10) |
| Stereotyped behaviours | 1 (3.3) |
| Complete or near lack of language | 4 (13.3) |
| Mild tremor | 1 (3.3) |
| Pyramidal signs in lower limbs | 2 (6.6) |
| Normal results from the neurological examination | 10 (33.3) |
Mean age at first seizure was 36 months (median, 21 months; range, 1-144 months). Twenty-five patients (83%) had multifocal epilepsy, one patient (3.3%) had temporal epilepsy, and 4 (13.7%) presented extratemporal epilepsy. Extratemporal epilepsy affected the following areas: right parietal (1 patient), left frontal (1), right centro-parietal (1), and left posterior quadrant (1).
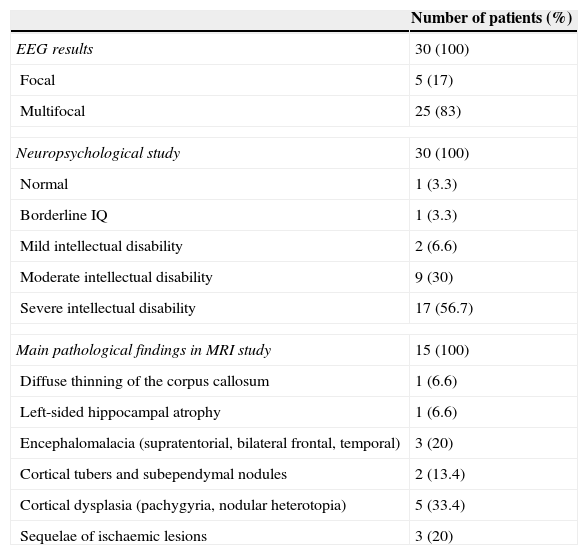
The mean number of seizures before VNS treatment was 185 per month (range, 3-1140 seizures per month), and the mean number of AEDs used before VNS treatment was 8 (range, 5-12 AEDs). According to the neuropsychological study, intellectual disability was severe in 17 patients, moderate in 9, and mild in 2; in contrast, one patient had borderline intellectual functioning and another had normal IQ scores (Table 3).
Results from complementary tests (n=30).
| Number of patients (%) | |
|---|---|
| EEG results | 30 (100) |
| Focal | 5 (17) |
| Multifocal | 25 (83) |
| Neuropsychological study | 30 (100) |
| Normal | 1 (3.3) |
| Borderline IQ | 1 (3.3) |
| Mild intellectual disability | 2 (6.6) |
| Moderate intellectual disability | 9 (30) |
| Severe intellectual disability | 17 (56.7) |
| Main pathological findings in MRI study | 15 (100) |
| Diffuse thinning of the corpus callosum | 1 (6.6) |
| Left-sided hippocampal atrophy | 1 (6.6) |
| Encephalomalacia (supratentorial, bilateral frontal, temporal) | 3 (20) |
| Cortical tubers and subependymal nodules | 2 (13.4) |
| Cortical dysplasia (pachygyria, nodular heterotopia) | 5 (33.4) |
| Sequelae of ischaemic lesions | 3 (20) |
Brain MRI scan findings were pathological in 15 patients (50%); these findings are shown in Table 3. Epilepsy was classified as symptomatic in 15 patients and cryptogenic in the remaining 15. Among patients with symptomatic epilepsy, 2 presented neurocutaneous syndromes (tuberous sclerosis complex), 4 had infectious diseases (herpes virus encephalitis, 2; pneumococcal meningoencephalitis, 1; unidentified, 1). Five had malformations of cortical development (one with LIS1 mutation), 3 presented sequelae of ischaemic lesions, and one exhibited ring chromosome 20.
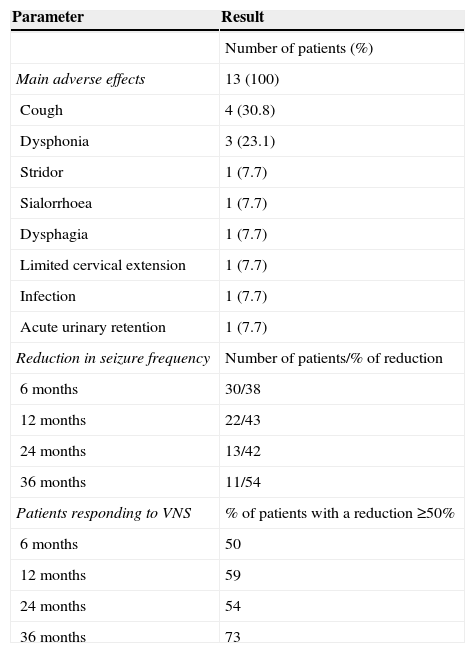
Mean age at implantation of VNS device was 11.89 years (range, 3.91-24.33 years). Follow-up times ranged from 6 to 36 months; no patients died during that period. Thirteen patients (43.3%) experienced adverse effects, including stridor, dysphagia, dysphonia, cough, sialorrhoea, urinary retention, and limited cervical extension. However, all adverse effects were minimal (Table 4). Only one patient experienced a superficial infection of the surgical wound. The infection manifested as redness in the surgical wound and resolved after the patient was treated with antibiotics for 7 days. Adverse effects were transient in most patients, and in no cases did the device have to be removed. One patient had to undergo surgery for a second time due to device malfunction, even though the device had been tested and found to work properly during the first surgical procedure. A patient classified as responder presented increasingly severe urinary retentions a few months later and required urinary catheterisation. This was considered a secondary effect of vagal nerve hyperstimulation; the parameters therefore had to be adjusted until the patient tolerated the treatment well and symptoms disappeared. At present, the patient still has the device in place and presents a reduction in seizure frequency of 90%.
Results of VNS implantation.
| Parameter | Result |
|---|---|
| Number of patients (%) | |
| Main adverse effects | 13 (100) |
| Cough | 4 (30.8) |
| Dysphonia | 3 (23.1) |
| Stridor | 1 (7.7) |
| Sialorrhoea | 1 (7.7) |
| Dysphagia | 1 (7.7) |
| Limited cervical extension | 1 (7.7) |
| Infection | 1 (7.7) |
| Acute urinary retention | 1 (7.7) |
| Reduction in seizure frequency | Number of patients/% of reduction |
| 6 months | 30/38 |
| 12 months | 22/43 |
| 24 months | 13/42 |
| 36 months | 11/54 |
| Patients responding to VNS | % of patients with a reduction ≥50% |
| 6 months | 50 |
| 12 months | 59 |
| 24 months | 54 |
| 36 months | 73 |
Mean reduction in seizure frequency at 6 months was 38%; 50% of patients were responders. At 12 months, mean reduction in seizure frequency in 22 patients was 43% and 59% of patients were responders. At 24 months, seizure frequency decreased by 42% in 13 patients, and responders accounted for 53%. By 36 months, 11 patients presented a mean seizure reduction of 54% and the responder rate was 72%. None of our patients achieved a reduction of 100% in seizure frequency. In the last follow-up consultation after implantation of the VNS device, 14 patients (47%) presented the same seizure pattern they had before surgery. While 20 patients used the same number of AEDs after surgery, 5 were administered one fewer AED, another patient was administered 2 fewer AEDs, and 4 used an additional AED.
The multivariate linear regression analysis of seizure frequency reduction at 6 months showed no statistically significant effects for any of the following independent variables: sex (CI: −78 to 37; P=.48), focus (CI: −26 to 97; P=.25), age at implantation of VNS device (CI: −43 to 64; P=.70), aetiology of epilepsy (CI: −72 to 34; P=.47), and disease progression time before VNS surgery (CI: −70 to 38; P=.54). The number of patients attending follow-up appointments at 12, 24, and 36 months decreased, which resulted in an increase in standard errors and made it difficult to obtain statistically significant results. The same analysis was also performed for these periods, but is not presented in this study.
Mean scores on the CAVE questionnaire in 28 patients were as follows: behaviour, 3 (moderate behaviour disorders although children conform to behavioural norms); school attendance, 4 (fewer than 7 school days missed per trimester); learning, 3 (modest but steady learning, slow knowledge acquisition); autonomy, 2 (partial dependence or dependence in specific situations); social relationships, 3 (occasionally isolated, both within and outside the family environment); seizure frequency, 2 (6 to 10 seizure days during the past month); and seizure intensity, 2 (short convulsive seizures, infrequent atonic seizures, long complex partial seizures, either with or without secondary generalisation). According to the parents, scores for all parameters were better after surgery. However, we were unable to perform a more accurate comparison since most parents could not answer specific questions about their children's state before surgery (some patients have been treated with VNS for more than 60 months). Parents also rated the effects of VNS treatment as very good (25%), good (28.6%), average (39.3%), poor (3.6%), and very poor (3.6%). Parents frequently reported improvements in their children's capacity for attention, and some reported that their children were more active, communicative, alert, autonomous, and cheerful. Although some parents reported similar seizure frequency rates to those before surgery was performed, seizures were less intense or did not result in falls. As a result, children were more independent and could leave their homes.
DiscussionThis study is the first evaluation of responses to VNS and the opinion of patients’ families to be conducted in a series of Spanish patients from a paediatric hospital.
The epidemiology characteristics of our population before VNS treatment are comparable to those described in other studies. Mean age at disease onset was 3 years. The studies conducted by Klinkenberg et al. and Elliot et al. report mean ages of 2 years and 4 months, and 2 years and 8 months, respectively, which enable better comparison of the results.1,7 The population receiving VNS treatment is drug-resistant, which means that most of these patients have been treated with multiple AEDs. Patients in our study had been taking a mean of 8 AEDs before surgery, which is in line with other studies reporting 7 to 8 AEDs.7 Our population presents a high percentage of moderate to severe intellectual disability (87%). Although the neuropsychological study was not carried out at the same point in disease progression for all patients, our findings are consistent with those described by Chen et al., who found that all patients with drug-resistant epilepsy in their study had intellectual disability or borderline intellectual functioning.9 These findings support the hypothesis that seizures have a negative impact on cognitive function, and that cognitive impairment exacerbates with disease progression time and greater difficulty achieving seizure control. It is therefore essential to find new, effective treatments for these patients.1,12
We found that 13 out of the 22 patients in follow-up for 12 months (59%) achieved a reduction in seizure frequency of 50%; similarly, Elliot et al. report seizure reductions of 50% in 64.8% of their patients.1 Parker et al. describe a series of 16 paediatric patients with a mean age of 11 years who experienced a mean reduction of 17% in seizure frequency at 12 months of treatment.13
Furthermore, control of epilepsy in our patients improved over time, from 38% at 6 months to 54% at 36 months. It has been suggested that the number of patients responding to treatment increases in the long term, with a reduction in seizure frequency of as much as 42% at 18 months.8 Follow-up is therefore necessary to make sure seizure frequency actually decreases. The number of AEDs changed in 33% (10) of our patients, and 6 of them (60%) required fewer AEDs.
Although VNS was approved for focal epilepsy, it has now been proved an effective treatment for several other epileptic syndromes. Nearly all patients in our population (83%) presented multifocal epilepsy and achieved satisfactory results. No statistically significant differences in seizure frequency reduction at 6 months were found between patients with focal epilepsy and those with multifocal epilepsy. Thompson et al. demonstrated that VNS was beneficial for both generalised epilepsy and partial epilepsy in paediatric patients.10
Reductions in seizure frequency 6 months after implanting the VNS device were similar in patients with symptomatic epilepsy and in those with cryptogenic epilepsy, as observed by Chen et al.9
Some suggest that patients younger than 12 years may respond better to VNS. However, we found no statistically significant differences in seizure frequency reduction 6 months after surgery between patients who were younger than or older than 12 at time of treatment. No other studies have found a statistically significant connection between implantation of the VNS device at ages younger than 12 years and better control of the disease. This suggests that the age at implantation of the device is not a key factor for VNS to be effective.1,4,14 As our results show, implantation of the device a shorter time after first seizure has not been correlated with greater reductions in seizure frequency; however, it is significantly correlated with quality of life.14,15
Most studies report a positive impact of VNS on quality of life, especially in the areas of alertness, global interaction, and night-time sleep.4,9 These findings were frequently reported by our patients’ families. It remains unclear whether these effects are directly related to stimulation itself or to seizure frequency reduction. In our study, 53.6% of the families regarded the effects of VNS on their children's quality of life as either good or very good. This technique is a treatment alternative that may improve overall quality of life for both patients and their families.
The technique has been shown to reduce total healthcare costs in paediatric patients, mainly in terms of hospital stays and visits to the emergency department.8 Although this aspect was not evaluated in our study, most families did mention making fewer emergency department visits and fewer hospital stays after surgery.
Although 43.3% of patients experienced adverse effects, all were transient, mild, and related to stimulation; the 3 most frequent ones were cough, dysphonia, and stridor. However, since our patients presented considerable cognitive impairment, they may have not been able to describe some of the adverse effects VNS may cause. The surgical infection rate for this procedure range from 3% to 5%,4,11 although this adverse effect was described only in one patient in our series.
The limitations of our study include its retrospective design, the small sample size, and the drop-outs during the follow-up period that decreased our numbers at 12, 24, and 36 months after surgery. Furthermore, our population was heterogeneous in terms of age at implantation of the device. Our results may have been affected by changes in AEDs around the time of surgery (10 patients). However, these changes were necessary since our priority was achieving better seizure control.
The present study confirms that VNS is a safe treatment with few and transient adverse effects and which exerts a positive impact on seizure frequency, seizure duration, and quality of life.
Conflicts of interestThe present study has been presented as an oral communication at the Annual Meeting of the Spanish Society of Paediatric Neurology.
Please cite this article as: Ulate-Campos A, Cean-Cabrera L, Petanas-Argemi J, García-Fructuoso G, Aparicio J, López-Sala A, et al. Resultados de la colocación del estimulador del nervio vago en epilepsia y calidad de vida en un hospital pediátrico. Neurología. 2015;30:465–471.