There is a major gap in knowledge about the epidemiology of epilepsy in Mediterranean countries. The EPIBERIA group was formed with the aim of promoting the conducting of epidemiological studies in this area in order to improve this situation. This paper deals with the validation of a brief questionnaire for screening of patients with epilepsy in general population.
MethodsWe selected an English language questionnaire previously validated by the Ottman group. It was translated, modified to suit the characteristics of the Spanish population, and administered to a sample of 200 patients (93 epileptics and 107 non-epileptic patient controls) sampled consecutively from 5 Epilepsy Units scattered throughout Spain. Both groups were homogeneous in demographic variables, and the control group was representative of the general population.
ResultsWe obtained a sensitivity of 100% and a specificity of 74.77% for the less rigorous correction criteria of the questionnaire, with a sensitivity of 94.62% and a specificity of 99.07% for the most stringent ones. The positive predictive values (PPVs) ranged from 7.48% for the first case to 69.49% in the second, assuming a prevalence for epilepsy of 2%.
ConclusionsThe questionnaire EPIBERIA is a valid Spanish tool for epilepsy screening of epilepsy in the general population in Spain.
Existe una importante laguna de conocimiento sobre la epidemiología de la Epilepsia en los países de la cuenca mediterránea. El grupo EPIBERIA nace con el objetivo de promocionar la realización de estudios epidemiológicos en este ámbito capaces de paliar esta situación. El presente trabajo aborda la validación de un cuestionario breve de cribado de pacientes con epilepsia en población general.
MétodosSe seleccionó un cuestionario de origen anglosajón validado en inglés por el grupo de Ottman. Fue traducido, modificado para adaptarlo a las características de la población española y administrado a una muestra de 200 pacientes (93 epilépticos y 107 controles no epilépticos) extraídos de manera consecutiva de 5 Unidades de Epilepsia dispersas por España. Ambos grupos fueron homogéneos en variables demográficas y el grupo de control, representativo de la población general. Se realizó una estimación de la Sensibilidad (S), Especificidad (E), valores predictivos positivos (VPP) y valores predictivos negativos (VPN) para cuatro diferentes criterios de corrección del cuestionario.
ResultadosSe obtuvo una sensibilidad del 100% y una especificidad del 74,77% para el criterio menos riguroso y una sensibilidad del 94,62 y una especificidad del 99,07% para el criterio más estricto de corrección del cuestionario. Los VPP variaron entre el 7,48% en el primer supuesto al 69,49% en el segundo asumiendo una prevalencia pretest para la epilepsia del 2%.
ConclusionesEl cuestionario EPIBERIA es un instrumento válido como cuestionario de cribado de epilepsia en la población general en castellano en España.
There is a major gap in our knowledge of epilepsy epidemiology in Europe according to the first systematic review on that topic published by Forsgren et al.1 in 2005. This review stated that while epilepsy epidemiology was relatively well-studied in northern Europe (particularly in Scandinavian countries), this was2–12 not the case in central Europe and especially around the Mediterranean basin. The only high-quality study from the Mediterranean available at the date of the review was the one by Loiseau et al. carried out in France in 1990.13
The lack of literature from the Iberian Peninsula was even more obvious given its single study published by Luengo et al. in 2001.14 Furthermore, that study was characterised by external validity limitations resulting from its having been restricted to a very specific area (the industrial outskirts of Madrid) and being based on health centre records.
The 2007 study by Durá-Travé et al. is the only Spanish incidence study to have been published since Forsgen's review appeared.15 This 4-year-long study focused exclusively on paediatric patients in the region of Navarre.
In 2009, Banerjee et al.16 published a new systematic review of all epidemiological studies on epilepsy conducted since 1965, mainly addressing the methodological analysis and quality of the different studies. They found 48 prevalence studies, among which 29 used population-based surveys. The remaining studies were based on health centre records. Of those 29 population-based studies, only 2 were European (both Italian),17,18 displaying once again the scarcity of epidemiological data from Europe.
In a recent review of the subject, García-Martín19 revealed that since Banerjee's systematic review was published, additional studies have been completed in European countries, including Croatia,20 Russia,21 Ireland,22 and Spain, where another prevalence study covering the province of Huesca was published in 2009.23 The Huesca study was restricted to adolescents and based on health centre records. Due to its restricted geographical area, it is probably not representative of the general population in Spain.
In any case, current epidemiological data on epilepsy in Mediterranean countries are quite scarce and limited. In light of this problem, researchers usually extrapolate results from areas with very different social, healthcare, and geographical conditions, such as the Nordic countries or the United States.
The EPIBERIA project was created as a joint initiative between the Andalusian Epilepsy Society (SAdE) and the epilepsy study group within the Spanish Society of Neurology (GEESEN). The purpose of this initiative is to promote epidemiological studies in our setting in order to remedy the lack of data. The first objective set by this joint initiative was to conduct a population-based prevalence study in different geographical regions so as to increase the study's external validity and gather results that could be extrapolated to all of Spain.
The EPIBERIA study of epilepsy prevalence was designed as a two-phase study. The initial phase involves a screening process in which subjects who may suffer from epilepsy are pre-selected by means of a questionnaire directed at the general population. In the second phase, epilepsy experts analyse the pre-selected at-risk population in order to identify true cases of epilepsy. Similar strategies have already been used in several epidemiological studies on a large variety of neurological diseases, including epilepsy.24–32
This article presents the validation of a brief Spanish-language screening questionnaire that can be used during the first phase of the prevalence study, considering that no screening tools of this type had ever been validated for the population of Spain.
Materials and methodsAfter considering different options, we selected an English-language questionnaire which had been previously validated and used in epidemiological studies led by Ottman et al.33 This questionnaire was translated into Spanish and adapted to the characteristics of our target population. Appendix 2 shows the final version of the EPIBERIA questionnaire.
Our study sample included a total of 200 subjects recruited in 5 neurology departments which were heterogeneously distributed across different regions of Spain (Hospital Clínico de San Carlos in Madrid, Hospital General Universitario in Valencia, Hospital Universitario San Cecilio in Granada, Hospital Clínico Lozano Blesa in Zaragoza, and Complejo Hospitalario Torrecárdenas in Almería).
Researchers used a consecutive random sampling method and followed the steps listed below in order to homogenise the sample.
- 1.
Each centre had to include 40 patients in order to reach a total sample size of 200.
- 2.
These 40 patients were distributed in 2 groups of similar sizes. The first group included patients with a confirmed diagnosis of epilepsy. The second group was a control group in which one-third of the total subjects were healthy volunteers.
- 3.
In order to respect the typical age distribution in epileptic patients, age distribution in both groups had to follow the approximate proportions given below:
- -
50% aged 45 and younger;
- -
35% aged 46 to 59;
- -
15% aged 60 or older.
- -
- 4.
Subjects with cognitive decline were excluded.
Researchers simultaneously collected demographic data for the total sample, including age, sex, and educational level. The sample was divided into 4 categories: little or no schooling, primary education, secondary education, and university education.
In the control group, researchers collected morbidity data in order to place subjects in the following categories: headache, degenerative neurological disease, cerebrovascular disease, other types of neurological disease, non-neurological disease, and healthy volunteers.
In epileptic patient group, researchers collected variables related to the syndromic diagnosis, epilepsy aetiology, seizure type, number of antiepileptic drugs, epilepsy activity, and whether or not the epilepsy was drug-resistant (DRE).
Questionnaires were read aloud to subjects by nursing or administrative staff at the clinic. A neurologist supervised the administration of all questionnaires to ensure that the proper procedure was followed.
Researchers simultaneously entered results into a central database with web access that was created by the Spanish Society of Neurology's research office. The database was set to admit all records up to the limit of 40 patients per hospital.
Statistical analysisAfter the data collection phase, researchers ran a statistical analysis of the demographic variables collected for the total sample and any variables specific to either of the subgroups as outlined in the preceding section.
Following that, they calculated sensitivity (TPR), specificity (SPC), positive predictive values (PPVs), and negative predictive values (NPVs) for 4 different schemes for correcting the questionnaire.
The 4 correction schemes were as follows:
- -
Correction scheme 1: any questionnaire on which question 2 was answered with “yes” or “maybe”, regardless of the remaining responses, was considered positive. Where question 2 was answered as “no”, but any item on question 3 was answered as “yes” or “maybe”, the questionnaire was also considered positive.
- -
Correction scheme 2: Any questionnaire on which question 2 was answered as “yes” or “maybe”, regardless of the remaining responses, was considered positive. Where question 2 was answered as “no” but one of items A, E, or F on question 3 was answered as “yes” or “maybe”, the questionnaire was also considered positive.
- -
Correction scheme 3: any questionnaire on which question 2 was answered as “yes” or “maybe”, regardless of the remaining responses, was considered positive. Where question 2 was answered as “no” but at least 2 of items B, C, D, or G on question 3 were answered as “yes” or “maybe”, the questionnaire was also considered positive.
- -
Correction scheme 4: Any questionnaire in which question 2 was answered as “yes” or “maybe”, regardless of the remaining responses, was considered positive. Where question 2 was answered as “no” but 1 of items A, E, or F and at least 2 of the items B, C, or G on question 3 were answered as “yes” or “maybe”, the questionnaire was also considered positive.
Researchers calculated the PPVs for 3 different epilepsy prevalence scenarios using each of the correction schemes, according to expected values based on prior studies and the scenarios used in the validation study for subjects in the United States.33 The specific prevalence values were 1%, 2%, and 3%.
Calculations were performed using statistical software Epidat 3.2.
ResultsTable 1 shows general demographic data from the sample broken down by group. With regard to age and sex, there were no significant differences between the epileptic group and the non-epileptic group. There was a statistical difference between the 2 groups with regard to education. The non-epileptic group showed a higher educational level, and especially more university graduates, than the epileptic group.
General demographical data of the sample broken down by group. Chi-square test (categorical variables) or t-test (continuous independent variables).
| Epileptic subjects (%) | Non-epileptic subjects (%) | P | |
| Males (%) | 41/93 (44.08%) | 52/107 (48.59%) | NS |
| Healthy volunteers: 20/38 (52.63%) | |||
| Illness other than epilepsy: 32/69 (46.37%) | |||
| Mean age in years±SD | 46.2±16.12 | 45.87±16.52 | NS |
| Educational level | .003 | ||
| Primary education or functionally illiterate | 48/93 (51.6%) | 47/107 (44.59%) | |
| Secondary education | 35/93 (37.63%) | 28/107 (26.16%) | |
| University education | 10/93 (10.75%) | 32/107 (29.9%) | |
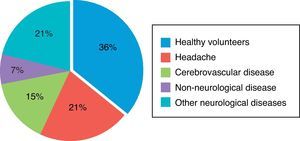
Fig. 1 shows morbidity data from the non-epileptic group. The largest subgroup was made up of healthy volunteers, which accounted for 36% of the sample (38/107). It was followed by subjects with headache and those with cerebrovascular disease, at 21% and 15% of the total respectively.
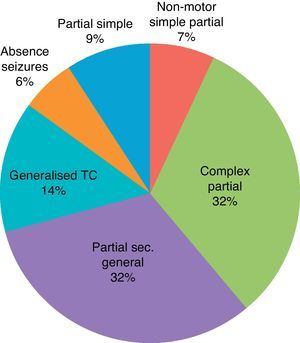
Table 2 and Figs. 2 and 3 show data regarding the general characteristics of epilepsy in the group of epileptic patients.
General characteristics of epilepsy in the epileptic subject group.
| Epileptic syndrome | |
| Generalised | 21 (22.58%) |
| Focal | 71 (76.34%) |
| Undetermined | 1 (1.07%) |
| Aetiology of epilepsy | |
| Idiopathic (genetic) | 21 (22.6%) |
| Symptomatic (structural) | 30 (32.25%) |
| Cryptogenic | 42 (45.16%) |
| Predominant type of seizure | |
| Simple partial motor seizures (type I.A.1) | 13 (13.97%) |
| Non-motor simple partial seizures (type I.A 2–4) | 11 (11.82%) |
| Complex partial seizure seizures (type I.B) | 48 (51.61%) |
| Partial secondarily generalised seizures (type I.C) | 47 (50.53%) |
| Absence seizures (type II.A) | 20 (21.5%) |
| Myoclonic seizures (type II.B) | 9 (9.67%) |
| Generalised tonic–clonic seizures (type II.D) | 9 (9.67%) |
| No. of antiepileptic drugs (AEDs) at time of inclusion | |
| No treatment | 1 (1.07%) |
| Monotherapy | 41 (44.08%) |
| Bitherapy | 35 (37.63%) |
| Polytherapy (>2 AEDs) | 16 (17.2%) |
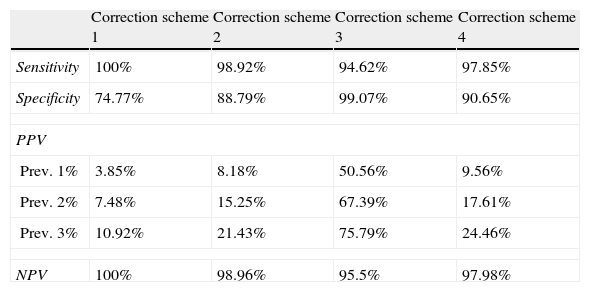
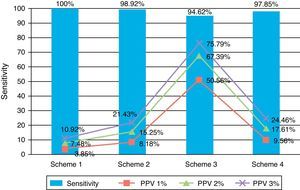
Table 3 and Fig. 4 show TPR and SPC values plus the PPV and NPV for each of the screening questionnaire's correction schemes and in the 3 prevalence scenarios extrapolated from prior studies.
Values for sensitivity, specificity, PPV, and NPV obtained for each scheme used to correct the questionnaire.
| Correction scheme 1 | Correction scheme 2 | Correction scheme 3 | Correction scheme 4 | |
| Sensitivity | 100% | 98.92% | 94.62% | 97.85% |
| Specificity | 74.77% | 88.79% | 99.07% | 90.65% |
| PPV | ||||
| Prev. 1% | 3.85% | 8.18% | 50.56% | 9.56% |
| Prev. 2% | 7.48% | 15.25% | 67.39% | 17.61% |
| Prev. 3% | 10.92% | 21.43% | 75.79% | 24.46% |
| NPV | 100% | 98.96% | 95.5% | 97.98% |
Of the total sample, 39.78% of the patients were resistant to AEDs.
DiscussionAs mentioned in the introduction, we are unaware of the existence of any Spanish-language epilepsy screening tests validated for the population of Spain.
Any existing questionnaires used for this purpose have been taken from English-language literature. While some have been translated into Spanish and used for epidemiological studies in Latin America, they were not validated prior to use. A single exception may be the population-based study carried out by Marco Tulio Medina et al.34 in Honduras in 2005, which used a Spanish-language questionnaire with 4 questions. This questionnaire is a version of the one previously employed by Aziz et al.35 for epidemiological studies in third-world countries with non-Spanish-speaking populations. The authors of the Honduran article describe a preliminary pilot study of 20 epileptic families.
In 1992, Placencia et al.28 validated a questionnaire derived from one that had previously been published by the World Health Organization (WHO). This questionnaire initially included 20 questions; results from its normative study were mediocre. Nevertheless, a sub-analysis of these results showed that a cluster of 9 questions selected from the 20 initial questions reached a TPR of 79.3%, a SPC of 92.9%, and a PPV of 18.3% (of the 9 questions, 3 positive responses were required in order for the result of the screening to be positive). Interestingly, this test was used in several epidemiological population-based studies in Latin America, but there is no evidence that its Spanish-language version was ever validated.36,37
The questionnaire by Aziz et al.35 was validated in 1994. The strong points of the questionnaire were that it was simpler than others while offering similar results.
More recently, the English-language version of the questionnaire we would later adapt for our study was validated for the population of the United States by Ottman et al.33 in 2010.
Some of these questionnaires have been shown to be useful not only in the clinical diagnosis of epilepsy, but also for diagnosing seizure type. This is true of the questionnaires by Ottman et al. in 199038 and by Reutens at al. in 1992.39 Some researchers recently proposed using computer-assisted methods for the telephone interview. These methods have also been shown to be useful in diagnosing epilepsy and the seizure type.40
In general, all these questionnaires, including Ottman's English-language version, show TPR values above 90% and SPC values of about 50%, although such results may be modified by changing the test's correction scheme.34
The questionnaire being validated in the study displays characteristics which set it apart from other questionnaires, which is why we chose it for the EPIBERIA study. Its main distinguishing feature is that it includes questions that address subjects’ symptoms. In theory, this makes it more applicable to the general population, outside of the healthcare setting, which is a necessary consideration when conducting a population-based study. Additionally, these types of questionnaires are very likely to maximise TPR values, as is required of a questionnaire used for screening purposes.
We would like to make a few comments on the translated version. Authors of the English-language version used the term ‘seizure disorders’, but translating the term literally into Spanish would produce a confusing result. We therefore opted for the term ‘epilepsia’, despite its having some negative connotations in our area.
With regard to item G of question number 3, the English-language questionnaire refers to ‘spells’, a non-specific term that may be translated literally as ‘episodios’. We opted to modify the item by providing an explanation of the symptoms which may be attributed to experiential or sensory auras in order to communicate more effectively with our Spanish population (see Appendix 2).
Age and sex distributions of the samples are homogeneous between epileptic and non-epileptic groups.
The epileptic group contained a lower percentage of subjects with university education than the non-epileptic group (10.75% vs 29.9%). Although this difference might appear to be a bias, it correctly reflects the situation in the general population: it is a well-known fact that the percentage of epileptic patients who attend university is significantly lower than that of the general population.41
As shown in Fig. 1, the group of non-epileptic subjects was heterogeneous. Healthy volunteers accounted for more than one-third of this group; the other two-thirds were patients with neurological diseases that are prevalent in the general population, such as headaches (21%) or cerebrovascular disease (15%). Patients with a variety of other neurological diseases accounted for an additional 21% of the sample. We achieved our goal of constituting a control group representing the non-epileptic general population.
Table 2 and Fig. 2 show all syndromic and aetiological subgroups within the group of epileptic patients, and all types of seizures that may be expected in an adult epileptic population. Moreover, percentages of the different subgroups are similar to percentages reported in large epidemiological studies of reference. Most patients were treated with monotherapy, but the percentage of patients treated with 2 or more drugs was significant (Table 2).
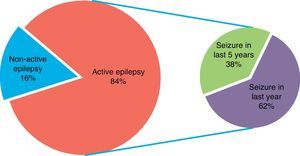
Concerning epilepsy activity, 84% of the sample had active epilepsy, understood as seizures in the previous 5 years. Moreover, more than half of the sample had experienced seizures in the preceding year.
Last of all, nearly 40% of the patients met criteria for DRE according to the ILAE's consensus definition of 2010.42
Table 3 shows TPR, SPC, PPV, and NPVs. Fig. 4 displays these values as a graph.
TPR values are the highest when correction scheme 1 is applied, which is to be expected, although this may result in SPC values being below 75%. This produces a PPV of 7.48%, assuming an epilepsy prevalence of 2%. This means that if we use correction scheme 1, we should anticipate that only 1 out of every 13 patients who enter the second phase of the prevalence study will actually suffer from epilepsy.
On the other end of the spectrum, if we use the strictest correction scheme (scheme 3), sensitivity decreases to 94.62%. According to this correction scheme, more than half of the patients advancing to the second phase would be true epileptics. However, the number of cases it would exclude due to false negatives would be unacceptable for purposes of a prevalence study such as ours.
Correction scheme 2 yields results that are situated between the 2 extremes, with a TPR of 98.92% and a PPV of 15%, assuming an epilepsy prevalence of 2%. Use of this correction scheme would limit entry into the second phase of the study, but some cases would risk being excluded due to false negatives.
In conclusion, results from the validation study confirm the EPIBERIA questionnaire as a validated Spanish-language screening test for use in epidemiological studies of epilepsy prevalence in Spain's general population. The fact that the study design includes 5 regions within Spain increases its external validity. We therefore propose that this questionnaire be used in future EPIBERIA projects.
FundingEPIBERIA project received private financial support from research funds for the SAdE and the Spanish Neurology Society's epilepsy study group, and funds donated by the EISAI and BIAL pharmaceutical companies for research purposes.
Conflicts of interestThe EPIBERIA project as a whole has received donations from the EISAI and BIAL pharmaceutical companies.
We would like to thank Dr Ruth Ottman for her valuable comments on the final version of this manuscript.
Ayala, J.L.; García-Morales, I.; Hernández-Ramos, F.J.; Mauri-Llerda, J.A.; Matías-Guiu, J.; Parejo-Carbonell, B.; Quiroga-Subirana, P.A.; Sánchez-Alvarez, J.C.; Sancho-Rieger, J.; Vázquez-Gutiérrez, F., and Serrano-Castro, P.J.
See Appendix 1.
Please cite this article as: Serrano-Castro PJ, et al. Validación en castellano de un cuestionario breve útil para cribado epidemiológico de epilepsia en España: Cuestionario EPIBERIA. Neurología. 2013;28:24–32.