The study aims to quantify the types of antiepileptic drugs (AED) prescribed in neurology consultations.
MethodsThis descriptive, observational study included a sample of 559 patients older than 14 years, diagnosed with epilepsy, and receiving pharmacological treatment. Data were collected at outpatient consultations by 47 Spanish neurologists in May 2016. Epilepsy was defined based on the International League Against Epilepsy classification. According to the year of marketing, AEDs were categorised as classic (before 1990) or new (after 1990). We performed a descriptive analysis of qualitative and quantitative variables.
ResultsFemale patients accounted for 54.6% of the sample. Mean age was 42.7 years; mean age of onset was 22.4. Regarding epilepsy type, 75.7% of patients experienced partial seizures, 51.5% were symptomatic, 32.4% had refractory epilepsy, 35.6% had been seizure-free for the previous year, and 59.2% had associated comorbidities.
A total of 1103 AED prescriptions were made; 64.6% of prescriptions were for new AEDs; 85.4% of patients received new AEDs. Patients received a mean of 2 AEDs (range, 1–5). A total of 59.6% of patients received polytherapy.
The most frequently prescribed AEDs were levetiracetam (42.6%), valproic acid (25.4%), lamotrigine (19.5%), carbamazepine (17.9%), and lacosamide (17.5%). No AED was employed exclusively as monotherapy. The most frequently prescribed AEDs for generalised and partial seizures were valproic acid (48.2%) and levetiracetam (43.2%), respectively. Valproic acid was less frequently prescribed to female patients. Patients with refractory epilepsy or with associated comorbidities were more frequently prescribed a combination of new and classic AEDs (48.7% and 45.6%, respectively) than only one type of AED.
ConclusionsThe majority of patients received new AEDs. The combination of classic and new AEDs was more frequently prescribed to patients with refractory epilepsy or with associated comorbidities.
Cuantificar el tipo de fármacos antiepilépticos (FAEs), empleados en epilepsia, en consultas de neurología.
Material y métodoEstudio descriptivo observacional sobre una muestra de 559 pacientes mayores de 14 años con epilepsia y en tratamiento farmacológico, recogidos en consultas ambulatorias por 47 neurólogos en España en mayo del año 2016.
Para las clasificaciones clínicas de la epilepsia se utilizaron las de la Liga Internacional contra la Epilepsia.
Los FAEs se clasificaron según el año de comercialización: clásicos (anteriores al 1990) y nuevos (los posteriores).
Se realiza análisis descriptivo de las variables cualitativas y cuantitativas.
ResultadosDemográficos: 54,6% mujeres; edad media 42,7 años; edad media de inicio de la epilepsia 22,4 años.
ClínicosPredominan las crisis parciales: 75,7%; sintomáticas: 51,5% y con epilepsia farmacoresistente: 32,4%. Pacientes libres de crisis en el último año 35,6%. Comorbilidad asociada 59,2 %. Tratamiento: número de FAEs empleados 1103; 64,6% FAEs nuevos. 85,4% de los pacientes tratados con FAEs nuevos. La media y rango de FAEs empleados: 2 (1–5). 59,6% recibían politerapia.
FAEs más utilizados: levetiracetam (LEV) 42,6%, valproico (VPA) 25,4%, lamotrigina (LTG) 19,5%, carbamacepina (CBZ) 17,9% y lacosamida (LCM) 17,5%. Ningún FAE empleado exclusivamente en monoterapia. El más utilizado en crisis generalizadas fue VPA 48,2% y en parciales LEV 43,2%. VPA fue menos utilizado en mujeres. Los pacientes sin control de sus crisis (48,7%) o co-morbilidad asociada (45,6%) recibían la combinación de ambos tipos de FAEs, en mayor porcentaje, que de forma aislada.
ConclusionesLa mayoría de pacientes toman FAEs nuevos. La asociación de ambos tipos de FAEs se emplea en mayor medida en los pacientes sin control de sus crisis o co-morbilidad asociada.
Epilepsy is a severe neurological disease associated with social stigma, multiple comorbidities, and a considerable economic burden.
According to the World Health Organization’s 2015 Global Burden of Disease Study, epilepsy is the neurological disease associated with the second highest number of years of life lost or years lived with disability.1
In Europe, epilepsy affects around 6 million people, with 400 000 new cases diagnosed each year. Half of patients report feeling stigmatised; the disease reduces their life expectancy by 2 to 10 years and the associated mortality rate is 2–3 times higher in these patients than in the general population. Around 60% of patients show psychiatric, neurological, or intellectual disorders. The total cost of epilepsy in Europe amounts to €20 billion per year.2,3
In the Spanish population older than 18 years, the age- and sex-adjusted lifetime prevalence of epilepsy is 14.87 per 1000 population, whereas the active prevalence is 5.79 cases per 1000 population.4
Population studies have revealed an increase in antiepileptic drug (AED) use, which varies between countries. However, not all AEDs are used exclusively to control epileptic seizures, as they have other indications (psychiatric disorders, chronic pain, migraine, eating disorders, drug abuse). According to the year they entered the market, AEDs are classified into classic (before 1990) or new AEDs (after 1990). The former group includes carbamazepine (CBZ), clobazam (CLB), clonazepam (CZP), ethosuximide (ESM), phenobarbital (PB), phenytoin (PHT), primidone (PRM), and valproic acid (VPA), whereas the group of new AEDs includes eslicarbazepine (ESL), felbamate (FBM), gabapentin (GBP), lacosamide (LCM), levetiracetam (LEV), lamotrigine (LTG), oxcarbazepine (OXC), perampanel (PMP), pregabalin (PGB), rufinamide (RFM), retigabine (RTG), tiagabine (TGB), topiramate (TPM), vigabatrin (VGB), and zonisamide (ZNS).
A study conducted in 7 European countries between 2001 and 2009 and analysing AED use for all indications reports an overall increase in AED use, which is mainly due to increased prescription of new AEDs. The prevalence of AED prescriptions in 2001 in the participating countries ranged from 88 to 144 prescriptions per 100 000 population, showing a 6%–15% increase by the end of the study period.5
Long-term AED treatment contributes to the high direct cost (medical, pharmacological) of epilepsy treatment. Over the past 25 years, numerous treatment options for epilepsy have been introduced. New AEDs are more expensive than the classic drugs; using the former as the first line of treatment may therefore have a great economic impact on long-term treatment.6 Seventeen new AEDs have been marketed over the past 20 years, although the use of these AEDs has had no impact on the number of seizure-free patients.7,8
The available evidence on cost-effectiveness from clinical trials does not support preferential use of the new AEDs, either in monotherapy or in combination therapy, over classic AEDs, or the use of a new AED over other AEDs of the same group. These studies suggest that the new AEDs are cost-effective in monotherapy when classic AEDs are ineffective, poorly tolerated, or contraindicated.9
Our study is the first to analyse AED prescription by demographic and clinical characteristics in patients with epilepsy older than 14 years throughout Spain.
Material and methodsStudy populationOur sample included 559 patients with epilepsy older than 14 years who were receiving AEDs and attended at neurology departments. Patients gave informed consent to participate and data were provided by 47 neurologists from across Spain in April and May 2016.
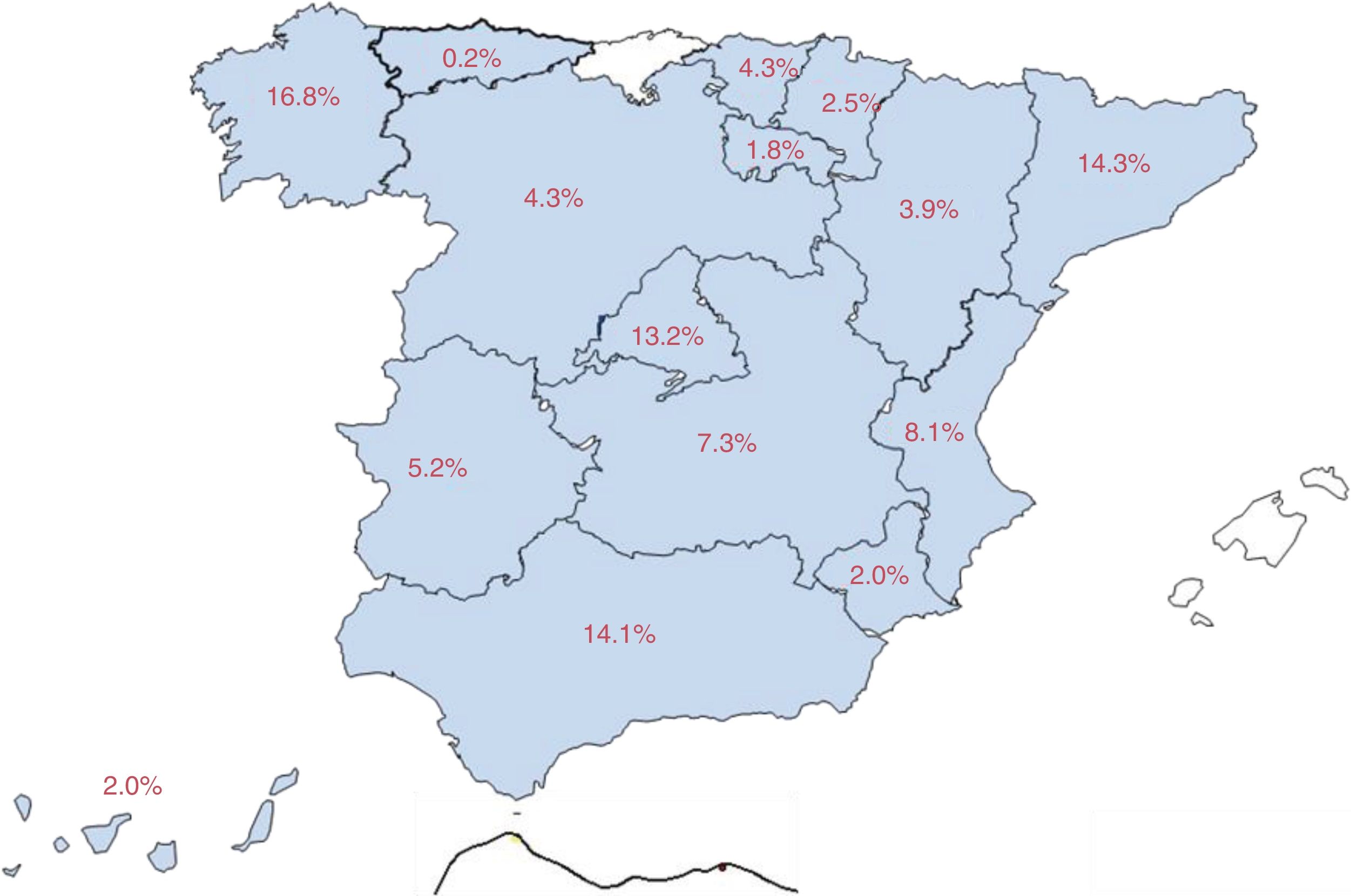
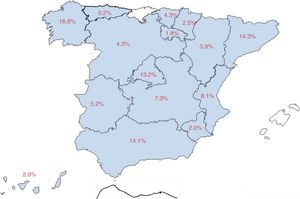
Eighty-three percent of patients were attended at epilepsy units (58.7% from reference units, 15.9% from refractory epilepsy units, and 8.4% from medical/surgical units), and the remaining 17% at neurology departments. Fig. 1 shows the distribution of our sample by autonomous community.
MethodsAll patients were randomly included in a database previously validated in 2015; we recorded demographic and clinical characteristics, treatments received at the time of inclusion, and previous treatments (if known).
The patients included met the 2005–2014 ILAE diagnostic criteria for epilepsy10,11; seizures and aetiological diagnoses were classified according to the 1989 ILAE classification.12
According to the classification established by Shorvon in 2011,13 symptomatic epilepsy was classified into predominantly genetic or due to developmental causes, and predominantly acquired.
Drug-resistant epilepsy was diagnosed according to the 2010 ILAE criteria.14
Statistical analysisWe performed a descriptive analysis of both qualitative and quantitative variables. Qualitative variables were expressed as absolute frequencies and percentages, and quantitative variables as means, standard deviations (SD), medians, ranges, confidence intervals (CI) or 25th and 75th percentiles (p25–p75), and number of cases.
We used the chi-square test to compare qualitative variables between groups. Quantitative variables were compared using the Mann–Whitney U test or the Kruskal–Wallis test.
Significance was established at P<.05. All analyses were conducted using SPSS statistical software, version 19.0.
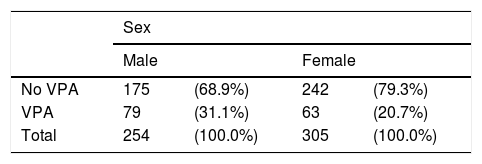
ResultsDemographic characteristicsOf the 559 patients included in the study, 254 (45.4%) were men and 305 (54.6%) were women.
Mean age in our sample was 42.7 years (SD: 16.8); the median age was 41.0 years (range, 13–91; 95% CI, 41.3–44.1)
Clinical characteristicsThe mean age at epilepsy onset was 22.4 years (SD: 19.1); the median was 17.0 years (range, 0–90; p25-p75: 8–32). Patients with drug-resistant epilepsy were younger than the remaining patients (P<.001), with a mean age of 15.8 years (SD: 13.7) and a median age of 13.0 years (range, 0–56).
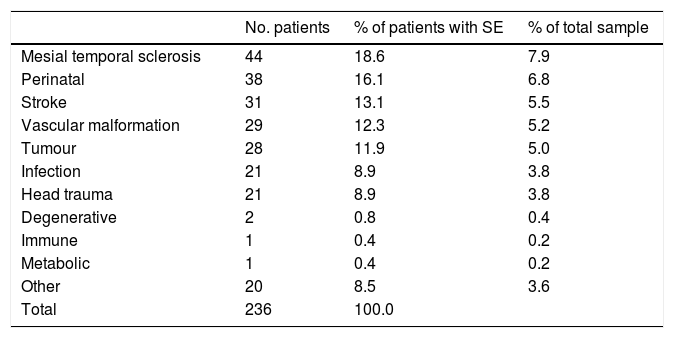
Over half of the patients had symptomatic epilepsy (n=288; 51.5%); 171 patients (30.6%) had cryptogenic epilepsy and the remaining 100 (17.9%) had idiopathic epilepsy. Symptomatic epilepsy was predominantly acquired in 82.5% of the sample and predominantly genetic or due to developmental causes in 17.5%. Table 1 summarises the causes of acquired symptomatic epilepsy in our sample.
Aetiology of symptomatic epilepsy.
| No. patients | % of patients with SE | % of total sample | |
|---|---|---|---|
| Mesial temporal sclerosis | 44 | 18.6 | 7.9 |
| Perinatal | 38 | 16.1 | 6.8 |
| Stroke | 31 | 13.1 | 5.5 |
| Vascular malformation | 29 | 12.3 | 5.2 |
| Tumour | 28 | 11.9 | 5.0 |
| Infection | 21 | 8.9 | 3.8 |
| Head trauma | 21 | 8.9 | 3.8 |
| Degenerative | 2 | 0.8 | 0.4 |
| Immune | 1 | 0.4 | 0.2 |
| Metabolic | 1 | 0.4 | 0.2 |
| Other | 20 | 8.5 | 3.6 |
| Total | 236 | 100.0 |
SE: symptomatic epilepsy.
By seizure type, 70.5% of patients had partial seizures, 26% had generalised seizures, and 3.5% had unknown onset seizures.
Patients were classified into 5 clinical groups:
- 1.
In remission: presenting no seizures over the past 2 years (24% of patients)
- 2.
Active, non–drug-resistant epilepsy requiring treatment changes, which are considered effective by the researcher (30.2% of patients)15
- 3.
Drug-resistant epilepsy according to the ILAE criteria (32.4% of patients)14
- 4.
Newly diagnosed epilepsy: less than 6 months since diagnosis or treatment onset (2.5% of patients)
- 5.
Treated with surgery or non-resective techniques (10.9% of patients)
With the exception of patients with newly diagnosed epilepsy, 35.6% of patients had remained seizure-free over the last year of follow-up.
Comorbidities were observed in 59.2% of the sample; these were medical in 30.6% of patients, psychiatric in 29.7%, and neurological in 28.4%. Around 4.1% of patients had more than one comorbidity. Patients with drug-resistant epilepsy were more likely to present comorbidities that the remaining patients (70.2% vs 54%; P<.001).
Pharmacological treatmentAll patients in our sample were receiving AEDs, regardless of whether they met diagnostic criteria for active epilepsy according to the 1993 ILAE definition.16
Approximately 72% of patients had previously received AEDs other than those reported at the inclusion consultation; 51% had taken at least 2 different AEDs. The reasons for discontinuation were lack of effectiveness in 45.1% of patients, intolerance in 16.5%, both reasons in 32.4%, and unknown in 6%.
Patients had been taking AEDs for a mean of 17.9 years (SD: 15.0) and a median of 14 years (range, 0–73). Patients with drug-resistant epilepsy had been receiving AEDs for significantly longer than the remaining patients (P<.001): a mean of 24.4 years (SD: 15.0) and a median of 23 (range, 2–67).
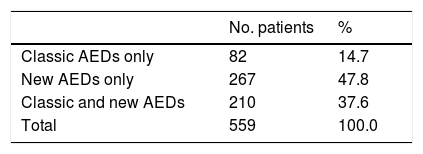
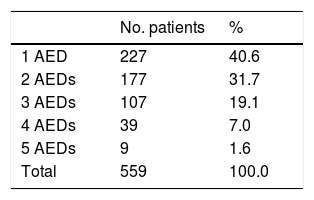
Overall, our sample received 1103 AEDs: 64.6% were new AEDs and 35.4% were classic AEDs. The distribution of classic and new AEDs in our sample is shown in Table 2. Of all patients, 85.4% were taking new AEDs; 40.6% of patients were receiving monotherapy and 59.4% combination therapy (2–5 AEDs) (Table 3).
The most frequently used new AEDs in our sample were LEV (42.6%), VPA (25.4%), LTG (19.5%), CBZ (17.9%), LCM (17.5%), and ESL (12.7%).
By seizure type, the most frequently used AEDs were LEV (43.2%), LCM (22.1%), CBZ (21.4%), VPA (19.2%), LTG (17.3%), and ESL (15.4%) for partial seizures, and VPA (48.2%), LEV (41.1%), LTG (23.4%), ZNS (9.9%), and ESL (6.4%) for generalised seizures.
By sex, men were taking the following AEDs: LEV (46.1%), VPA (31.1%), CBZ (19.7%), LCM (18.1%), LTG (18.1%), and ESL (12.2%); women received LEV (39.7%), VPA (20.7%), LTG (20.7%), LCM (17%), CBZ (16.4%), and ESL (13.1%).
Significantly more men than women received VPA: 31.1% vs 20.7% (P=.005). Forty-three of the 63 women treated with VPA (68.3%) were aged 15 to 45 years. No significant differences between sexes were observed for the remaining AEDs (Table 4).
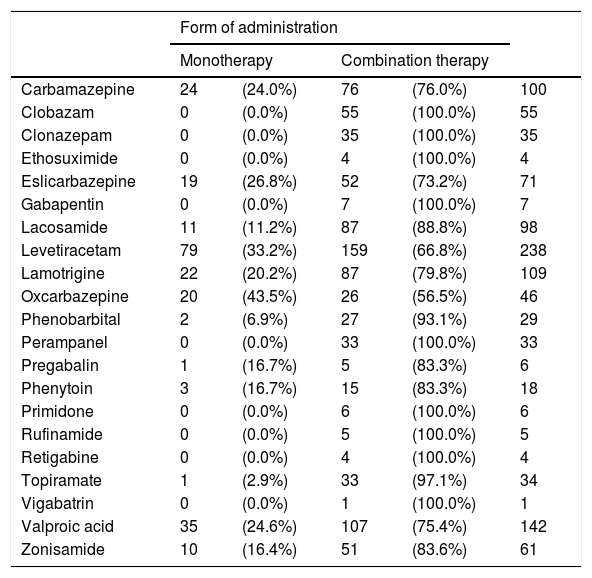
None of the AEDs used were administered in monotherapy exclusively, although some of them were administered in combination therapy exclusively (CLB, CZP, ESM, GPT, PMP, PRM, RFM, TGB, and VGB). Table 5 lists the AEDs currently prescribed and the form of administration in our sample.
Types of AEDs currently prescribed and form of administration in our sample.
| Form of administration | |||||
|---|---|---|---|---|---|
| Monotherapy | Combination therapy | ||||
| Carbamazepine | 24 | (24.0%) | 76 | (76.0%) | 100 |
| Clobazam | 0 | (0.0%) | 55 | (100.0%) | 55 |
| Clonazepam | 0 | (0.0%) | 35 | (100.0%) | 35 |
| Ethosuximide | 0 | (0.0%) | 4 | (100.0%) | 4 |
| Eslicarbazepine | 19 | (26.8%) | 52 | (73.2%) | 71 |
| Gabapentin | 0 | (0.0%) | 7 | (100.0%) | 7 |
| Lacosamide | 11 | (11.2%) | 87 | (88.8%) | 98 |
| Levetiracetam | 79 | (33.2%) | 159 | (66.8%) | 238 |
| Lamotrigine | 22 | (20.2%) | 87 | (79.8%) | 109 |
| Oxcarbazepine | 20 | (43.5%) | 26 | (56.5%) | 46 |
| Phenobarbital | 2 | (6.9%) | 27 | (93.1%) | 29 |
| Perampanel | 0 | (0.0%) | 33 | (100.0%) | 33 |
| Pregabalin | 1 | (16.7%) | 5 | (83.3%) | 6 |
| Phenytoin | 3 | (16.7%) | 15 | (83.3%) | 18 |
| Primidone | 0 | (0.0%) | 6 | (100.0%) | 6 |
| Rufinamide | 0 | (0.0%) | 5 | (100.0%) | 5 |
| Retigabine | 0 | (0.0%) | 4 | (100.0%) | 4 |
| Topiramate | 1 | (2.9%) | 33 | (97.1%) | 34 |
| Vigabatrin | 0 | (0.0%) | 1 | (100.0%) | 1 |
| Valproic acid | 35 | (24.6%) | 107 | (75.4%) | 142 |
| Zonisamide | 10 | (16.4%) | 51 | (83.6%) | 61 |
Bitherapy was prescribed for 31.7% of patients; the most frequent drug combinations were LEV+VPA (9.6%), LEV+ESL (9%), and LEV+LCM (9%).
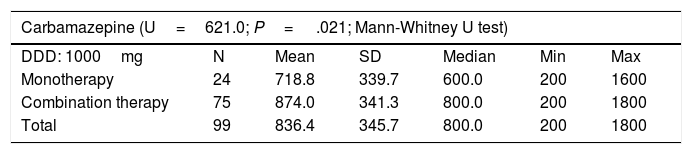
Patients treated with CBZ, ESL, LCM, LEV, OXC, VPA, or ZNS in combination therapy received higher doses than those treated with the same drugs in monotherapy. Table 6 shows the mean daily dose of each of these AEDs in monotherapy and combination therapy, as well as the defined daily dose established for monotherapy by the World Health Organization.17
Daily dose of carbamazepine, eslicarbazepine, lacosamide, levetiracetam, oxcarbazepine, valproic acid, and zonisamide in monotherapy and in combination therapy.
| Carbamazepine (U=621.0; P= .021; Mann-Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 1000mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 24 | 718.8 | 339.7 | 600.0 | 200 | 1600 |
| Combination therapy | 75 | 874.0 | 341.3 | 800.0 | 200 | 1800 |
| Total | 99 | 836.4 | 345.7 | 800.0 | 200 | 1800 |
| Eslicarbazepine (U=333.5; P=.023; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 800 mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 19 | 842.1 | 226.9 | 800.0 | 400 | 1200 |
| Combination therapy | 52 | 1042.3 | 346.0 | 1000.0 | 400 | 2000 |
| Total | 71 | 988.7 | 329.3 | 800.0 | 400 | 2000 |
| Lacosamide (U=169.5; P=.013; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 300 mg | N | Mean | SD | Median | Min. | Max. |
| Monotherapy | 8 | 225.0 | 88.6 | 200.0 | 100 | 400 |
| Combination therapy | 87 | 336.5 | 127.0 | 350.0 | 100 | 700 |
| Total | 95 | 327.1 | 127.7 | 300.0 | 100 | 700 |
| Levetiracetam (U=3792.5; P=.001; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 1500 mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 73 | 1490.4 | 742.9 | 1000.0 | 250 | 3000 |
| Combination therapy | 158 | 2024.1 | 896.9 | 2000.0 | 250 | 4000 |
| Total | 231 | 1855.4 | 885.3 | 2000.0 | 250 | 4000 |
| Oxcarbazepine (U=155.0; P=.015; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 1000 mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 20 | 1006.5 | 407.7 | 990.0 | 300 | 1800 |
| Combination therapy | 26 | 1336.5 | 474.9 | 1200.0 | 600 | 2400 |
| Total | 46 | 1193.0 | 472.0 | 1200.0 | 300 | 2400 |
| Valproic acid (U=992.5; P=.001; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 1500 mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 32 | 993.8 | 380.9 | 1000.0 | 500 | 2100 |
| Combination therapy | 107 | 1355.6 | 503.8 | 1500.0 | 300 | 2500 |
| Total | 139 | 1272.3 | 500.9 | 1200.0 | 300 | 2500 |
| Zonisamide (U=43.0; P=.001; Mann–Whitney U test) | ||||||
|---|---|---|---|---|---|---|
| DDD: 200 mg | N | Mean | SD | Median | Min | Max |
| Monotherapy | 10 | 160.0 | 69.9 | 150.0 | 100 | 300 |
| Combination therapy | 51 | 358.8 | 118.2 | 400.0 | 100 | 600 |
| Total | 61 | 326.2 | 133.7 | 300.0 | 100 | 600 |
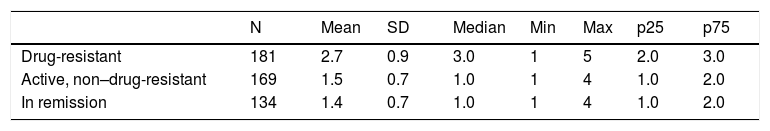
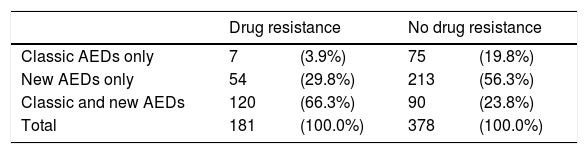
Patients with drug-resistant epilepsy received more AEDs than those with non–drug-resistant epilepsy or in remission: a mean of 2.7 AEDs (SD: 0.9) and a median of 3 (range, 1–5) (Table 7). These patients are more frequently treated with a combination of new and classic AEDs (66.3%), whereas patients with non–drug-resistant epilepsy are treated with either classic AEDs only (20%) or new AEDs only (56%) (Table 8).
Resistance to antiepileptic drugs, broken down by type of antiepileptic drug.
| Drug resistance | No drug resistance | |||
|---|---|---|---|---|
| Classic AEDs only | 7 | (3.9%) | 75 | (19.8%) |
| New AEDs only | 54 | (29.8%) | 213 | (56.3%) |
| Classic and new AEDs | 120 | (66.3%) | 90 | (23.8%) |
| Total | 181 | (100.0%) | 378 | (100.0%) |
P=.001 (chi-square test).
AED: antiepileptic drug.
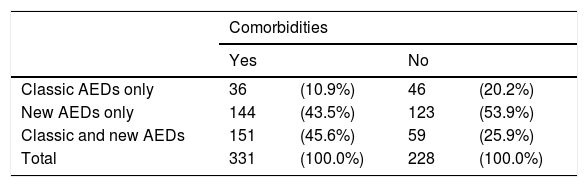
Patients with associated comorbidities were more likely to receive a combination of both types of AEDs than those without (45.6% vs 25.9%) (Table 9).
Types of antiepileptic drugs administered to patients with and without comorbidities.
| Comorbidities | ||||
|---|---|---|---|---|
| Yes | No | |||
| Classic AEDs only | 36 | (10.9%) | 46 | (20.2%) |
| New AEDs only | 144 | (43.5%) | 123 | (53.9%) |
| Classic and new AEDs | 151 | (45.6%) | 59 | (25.9%) |
| Total | 331 | (100.0%) | 228 | (100.0%) |
P=.001 (chi-square test).
AED: antiepileptic drug.
Patients with newly diagnosed epilepsy were initially treated with the following AEDs: LEV (50%), VPA (21.4%), LCM (21.4%), and CBZ (7.1%). Sixty-four percent of patients received new AEDs only, 22% received classic AEDs only, and 14% received a combination of both types.
DiscussionThe clinical classification of epileptic seizures depends on the reliability of the semiological data gathered, the availability of diagnostic tests (particularly neuroimaging), and the classification used. According to population studies, 20% to 66% of patients display partial seizures, and symptomatic epilepsy is observed in 14% to 39% of patients.18 A study conducted by Cockerell et al.19 in the United Kingdom between 1984 and 1997, including a large sample of patients with newly diagnosed epilepsy gathered from primary care records, reports partial seizures in 52% of the sample; aetiology was unknown in 59% of patients. In a population study conducted in Spain in 2013 and including patients with active epilepsy, Serrano Castro et al.4 reported partial seizures in 59% of the sample; half of the cases were symptomatic.
In hospital-based studies, these figures are higher due to greater professional expertise and more readily available technology. In our sample, partial seizures accounted for 75% of all seizures and 51.5% of patients had symptomatic epilepsy. This is in line with results from other national and international studies. In the study by Luengo et al.,20 conducted in 2000 in Madrid, 63% of patients had partial seizures and 45% had symptomatic epilepsy. In a study conducted by García Martín et al.21 in Málaga in 2010, 75.5% of patients had partial seizures and 47.6% had symptomatic epilepsy. Similar results are reported by other international studies: a 2014 study conducted in Korea, including data from clinical records, found that 78% of seizures were partial seizures and that 65% of patients had symptomatic epilepsy.22
The most frequent causes of symptomatic epilepsy in most epidemiological studies are vascular malformations, trauma, and congenital disorders.4,20,22,23 In our sample, the most frequent cause of symptomatic epilepsy was mesial temporal sclerosis associated with hippocampal atrophy, in 18.6% of patients; the incidence of the remaining causes is similar to that reported in the literature. This may be due to the fact that most of our patients were recruited from epilepsy units.
According to a literature review conducted in 2007, 20% to 40% of patients have drug-resistant epilepsy.24 Incidence varies depending on how drug-resistant epilepsy is defined, the type of study (population or clinical), and time since diagnosis (newly diagnosed vs chronic epilepsy); and prognosis depends mainly on aetiology or the type of epileptic syndrome.25
In a clinical series gathered from the epilepsy unit at the Western Infirmary Hospital in Glasgow, most patients showed sustained progression of epilepsy (59% showed good seizure control and 25% had drug-resistant epilepsy), and 16% of patients alternated periods of remission and recurrences.26 Four consecutive analyses of a series of patients recruited between 1982 and 2012 from the same unit revealed rates of remission (of at least one year) of 64% in the first (1982–1997) and the last periods analysed (1982–2012).27 In our study, 32.4% of patients had drug-resistant epilepsy; a similar percentage was reported in the Glasgow study, although the remission period in that study was set at ≥ 2 years.
AED use has increased overall in recent years due to these drugs’ indication for other diseases and to the emergence of new AEDs. Abasolo Osinaga et al.28 analysed AED use for all indications in the Basque Country (1992–2004) and observed an increase in the defined daily dose per 1000 inhabitants per day (DID), from 5.53 DID at the beginning of the study period to 9 DID at the end, as well as an increase in AED use; use of the classic AEDs CBZ and VPA remained stable, whereas use of PB and PHT decreased. In a similar study conducted in Norway, analysing AED use in patients with and without epilepsy from 2008 to 2012, 53% of AEDs prescribed in 2012 were prescribed to patients without epilepsy. The AEDs most frequently prescribed to patients with epilepsy were LTG, LEV, CBZ, and VPA. The number of women taking VPA decreased by 26% over the study period. New AEDs were the most frequently prescribed drugs; retention rates of new AEDs were higher than those of classic AEDs for all indications.29 In another study conducted in Norway, including 2004–2009 data, Landmark et al.30 observed that 49% of drugs prescribed to patients with epilepsy were new AEDs; use of these drugs showed marked differences according to age and sex. The AEDs most frequently prescribed to women were new AEDs. A study conducted in the United Kingdom between 1993 and 2008, analysing the drugs prescribed by primary care physicians, revealed an increase in the prescription of LTG (from 2% to 17%) and LEV (0% to 8.6%) over the study period; by the end of the study, the most frequent drug combination was VPA+LTG.31
In Germany, Strzelczyk et al.32 studied 2 groups of patients with active epilepsy who were evaluated in 2003 and in 2008 and studied changes in prescriptions over this 5-year period; 45% of patients used new AEDs in 2003, compared to 70% in 2008. The most frequently used AEDs were CBZ (40%), VPA (37%), LTG (32%), LEV (22%), and TPM (11%) in the 2003 group, and LTG (41%), LEV (36%), VPA (32%), CBZ (16%), and OXC (15%) in the 2008 group. In our study, new AEDs accounted for 65% of the drugs used, and were prescribed to 85% of patients. LEV was the most frequently used AED for partial seizures in both sexes, both in monotherapy and in combination therapy, whereas VPA was most frequently indicated for generalised seizures.
There is little evidence supporting use of the new AEDs in monotherapy or in combination with classic AEDs.
The SANAD study,33 a nonblinded randomised controlled trial including over 7000 outpatients from hospitals in the United Kingdom, compared the efficacy of CBZ against GBP, LTG, OXC, and TPM for partial onset seizures, and found that LTG is a cost-effective alternative to CBZ. In patients with generalised or unclassified epilepsy, VPA was found to be better tolerated than TPM and more efficacious than LTG.34 The study is regarded as class III by several researchers due to its open-label design, the titration schedule used, and the imprecise classification of seizures.35
The KOMET study, conducted by Trinka et al.36 in 2013, compared the efficacy of LEV against that of extended-release VPA and controlled-release CBZ, each in monotherapy, and found no differences between them. Efficacy, tolerance, and time to treatment withdrawal of all 3 drugs was similar in patients with newly diagnosed focal or generalised epilepsy.
Few studies have compared the efficacy of different new AEDs and their results are inconclusive. Some studies compare these drugs in combination therapy. LEV, LTG, and LCM seem to achieve better seizure control and higher retention rates than other new AEDs.37,38
The benefits of the new AEDs are most visible when adjunctive treatment with these drugs is compared against placebo. Beyenburg et al.39 conducted a systematic review and meta-analysis of the effectiveness of adjunctive treatment against placebo for drug-resistant epilepsy, reporting a weighted pooled risk difference of 6% for seizure control and a weighted pooled risk difference of 21% for 50% seizure reduction in favour of AEDs.39
In a systematic review, Wilby et al.9 conclude that the new AEDs may be a cost-effective option in monotherapy when classic AEDs are ineffective or contraindicated, or cause adverse reactions.9
New AEDs are initially used as adjunctive treatment; if they meet the standards of regulatory agencies, they are subsequently incorporated to treatment in monotherapy. At present, most of the new AEDs have been approved for use in monotherapy.
The main clinical practice guidelines for epilepsy recommend most of the new AEDs (GBP, LEV, LTG, OXC, TPM, ZNS) in monotherapy for partial seizures, all of them as adjunctive therapy, and LEV, LTG, TPM, and ZNS for generalised tonic-clonic seizures. Although the guidelines published have varying levels of evidence and strengths of recommendation for AEDs, whether classic or new, they generally coincide in that treatment must be adapted to each patient’s personal and clinical characteristics.40–46 LCM has recently been approved for use in monotherapy for partial seizures.
The main limitations of the classic AEDs PB, PRM, PHT, CBZ, and VPA are the fact that they are metabolised by the liver, which may cause interactions between the AED and other types of drugs or endogenous pathways of hepatic metabolism; their teratogenic potential; and their negative impact on cognitive function. Most new AEDs have a better tolerability profile and cause fewer interactions.47,48
The new AEDs are safer than classic AEDs. In a recent meta-analysis of randomised controlled trials on the short-term tolerability of the new AEDs in adults for all indications, LEV and GBP showed the best tolerability profiles.49 However, several new AEDs, including FBM and RTG, have been withdrawn from the market due to severe toxicity; likewise, the use of VGB in some refractory childhood epilepsies has been restricted due to toxicity issues.
The somatic comorbidity of epilepsy is associated with increased need for healthcare, poorer quality of life, and premature death.50 Around 50% of adults with active epilepsy have at least one associated comorbidity, which may be the cause or consequence of epilepsy, or a risk factor for developing the condition.51 In our study, 59% of patients had comorbidities, which were closely related to drug resistance.
Teratogenicity is an important factor for women of childbearing age. Tomson et al.52 conducted a systematic review of prospective epilepsy and pregnancy registries and conclude that the risk of congenital malformations is greater for children of women receiving classic AEDs, especially VPA, than for those of women taking new AEDs. The Spanish Agency for Medicines and Medical Devices and numerous experts discourage use of VPA for women of childbearing age and underscore the need for further studies into the new AEDs.53,54 In our series, 20.7% of women (68% were women of childbearing age) were taking VPA; while VPA use was less frequent in women than in men (31%), this remains a high percentage.
Patients with drug-resistant epilepsy, who frequently have more comorbidities, are more likely to receive combination therapy (classic+new AEDs), which may be explained by the use of a greater number of AEDs in these patients.
Pharmacological treatment represents 3% of the total cost (direct and indirect) of epilepsy in Europe.55 A literature review on the cost of new treatments for epilepsy concludes that the cost of AEDs (which is higher in the case of the new drugs) should not be the only factor to be considered when selecting the most appropriate AED, given that the main objective of epilepsy treatment is to achieve seizure control with no adverse drug reactions. Selecting AEDs based on their cost is unlikely to be cost-effective in the long term, especially when considering indirect costs, such as those associated with shortened working lives.56 Most authors therefore do not recommend changing brand-name AEDs for generic AEDs, or new AEDs for classic AEDs, based exclusively on AED cost, especially in cases of good seizure control.57
Experts continue to recommend selecting AEDs according to seizure type, epileptic syndrome, patient age and sex, concomitant medications, comorbidities, and AED efficacy, safety, and tolerability. The new AEDs are safer and better tolerated than classic AEDs, as reflected by greater indication of these drugs in our study.
LimitationsOur study population is not fully representative of the population of patients with epilepsy in Spain since it mainly includes patients attended at epilepsy units, who have difficulty controlling seizures, more frequently visit neurology outpatient consultations (especially at epilepsy units), and take more AEDs.
Ethical approvalThe study was classified by the Spanish Agency for Medicines and Medical Devices as “post-authorisation study with a design other than prospective follow-up” (EPA-OD; study no. EPI-EPI-2015-01) on 27 March 2015.
It was approved by the Research Ethics Committee of the region of Almería on 29 July 2015.
The data included were reviewed by the scientific committee of the study (JM Mercadé, FJ López, and P Serrano).
Conflicts of interestAll authors are members of the Spanish Society of Neurology and have no conflicts of interest to declare.
AcknowledgementsWe would like to thank Patricia Santagueda (BIODATOS) for the statistical analysis of our data.
| Dr Juan Mercadé Cerdá | Málaga |
| Dr José Ángel Mauri Llerda | Zaragoza |
| Dr Pedro Serrano Castro | Almería |
| Dr Francisco Javier López González | Santiago de Compostela |
| Dr Jerónimo Sancho Rieger | Valencia |
| Dr Jaime Parra Gómez | Madrid |
| Dr Juan Luis Becerra Cuñat | Badalona |
| Dr Jordi Ciurans Molist | Badalona |
| Dr Juan Carlos Sánchez Álvarez | Granada |
| Dr Joaquín Ojeda | Madrid |
| Dr Isabel Ybor Gorrin | Madrid |
| Dr Fernando Ayuga Loro | Toledo |
| Dr María Rosa Querol Pascual | Badajoz |
| Dr Aránzazu Alfaro Sáez | Orihuela |
| Dr Rosana Saiz Díaz | Madrid |
| Dr Manuel Toledo Argany | Barcelona |
| Dr Javier Abella Corral | Ferrol |
| Dr María Gomez Eguilaz | Logroño |
| Dr Marian Barcala Simó | Tarragona |
| Dr Carmen Arenas Cabrera | Seville |
| Dr Clara Isabel Cabeza Álvarez | Toledo |
| Dr Vicente Bertol Alegre | Zaragoza |
| Dr Javier Díaz de Terán | Madrid |
| Dr Júlia Miró Lladó | Hospitalet de Llobregat |
| Dr Mercè Falip Centelles | Hospitalet de Llobregat |
| Dr Laura Pulido Fontes | Pamplona |
| Dr Juan José Poza Aldea | San Sebastián |
| Dr Asier Gómez | Valencia |
| Dr Montserrat Asensio Asensio | Alicante |
| Dr Maribel Chamorro Muñoz | Málaga |
| Dr M. Dolores Castro Vilanova | Vigo |
| Dr Amaya Castela | Seville |
| Dr Inés Aranzabal Alustiza | Baracaldo |
| Dr Esteban Santamarina Pérez | Barcelona |
| Dr María José Aguilar Amat Prior | Madrid |
| Dr Patricia Esteve | Tortosa |
| Dr María Luisa Galiano Fraguas | Madrid |
| Dr Juan Miguel Galán Barranco | Seville |
| Dr Xiana Rodríguez Osorio | Santiago de Compostela |
| Dr Santiago Fernández Fernández | Barcelona |
| Dr Alfonso Falcón García | San Pedro de Alcántara |
| Dr David Sopelana Garay | Albacete |
| Dr José M. Flores Galdo | Tenerife |
| Dr DiegoTortosa Conesa | Murcia |
| Dr Luis Redondo Verge | Seville |
| Dr María Pardo Parrado | Ourense |
| Dr Dulce Campos Blanco | Valladolid |
Please cite this article as: Mercadé Cerdá JM, et al. Estudio observacional multicéntrico español sobre el empleo de fármacos antiepilépticos en consultas de neurología. Neurología. 2020;35:115–125.