The brain is a series of networks of functionally and anatomically connected, bilaterally represented structures; in epilepsy, activity of any part of the brain affects activity in the other parts. This is relevant for understanding the pathophysiology, diagnosis, and prognosis of the disease.
ObjectivesIn this study, we present a state-of-the-art review of the neurophysiological view of epilepsy as a disease affecting neural networks.
ResultsWe describe the basic and advanced principles of epilepsy as a disease affecting neural networks, based on the use of different clinical and mathematical techniques from a neurophysiological perspective, and signal the limitations of these findings in the clinical context.
ConclusionsEpilepsy is a disease affecting complex neural networks. Understanding these will enable better management and prognostic confidence.
La epilepsia es un conjunto de redes de estructuras cerebrales representadas bilateralmente, que están funcional y anatómicamente conectadas, en la epilepsia, la actividad de cualquier parte del cerebro afecta la actividad de las demás. Esto es relevante para el entendimiento de la fisiopatología, etiología, el diagnóstico y la prognosis de esta enfermedad.
ObjetivoRevisar el estado del arte en cuanto al entendimiento de la visión neurofisiológica de la epilepsia como una enfermedad de redes neuronales.
DesarrollSe describen los principios básicos y avanzados de la epilepsia como enfermedad de redes neuronales usando distintos métodos clínicos y matemáticos con una visión neurofisiológica, indicando las limitaciones de estos hallazgos en el contexto clínico.
ConclusionesLa epilepsia es una enfermedad de redes neuronales complejas cuyo entendimiento permitirá mejorar los tratamientos disponibles y la certeza pronostica.
Epilepsy is a chronic disorder of the central nervous system that affects individuals of all ages and geographical origins.1 According to the Global Burden of Disease Study 2016, epilepsy accounts for 0.5% of disability-adjusted life-years for all diseases and injuries and 5.0% of disability-adjusted life-years for neurological disorders. In 2016, epilepsy (idiopathic and secondary) was estimated to affect 45.9 million individuals (range, 39.9-54.6).2
Definition of epilepsy and epileptic seizureEpilepsy is a disorder of the brain characterised by an enduring predisposition to generate epileptic seizures, which has neurobiological, cognitive, psychological, and social consequences.3 A seizure is defined as a transient occurrence of signs or symptoms caused by excessive or synchronous neuronal activity in the brain.3
Epilepsy is a disease of the brain meeting either of the following criteria: 1) at least 2 unprovoked (or reflex) seizures occurring less than 24 hours apart, or 2) an unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) following 2 unprovoked seizures occurring over the next 10 years.4
The International League Against Epilepsy classification has recently been updated to reflect the current understanding of epilepsy and its pathophysiological mechanisms, according to the latest scientific evidence.5 Once seizure type is established, the following step is to diagnose the epilepsy type: focal, generalised, combined (focal and generalised), or unknown.5 This classification is based on a complex neural network of neurophysiological and neuroanatomical data from neurons and brain lobes and hemispheres.5
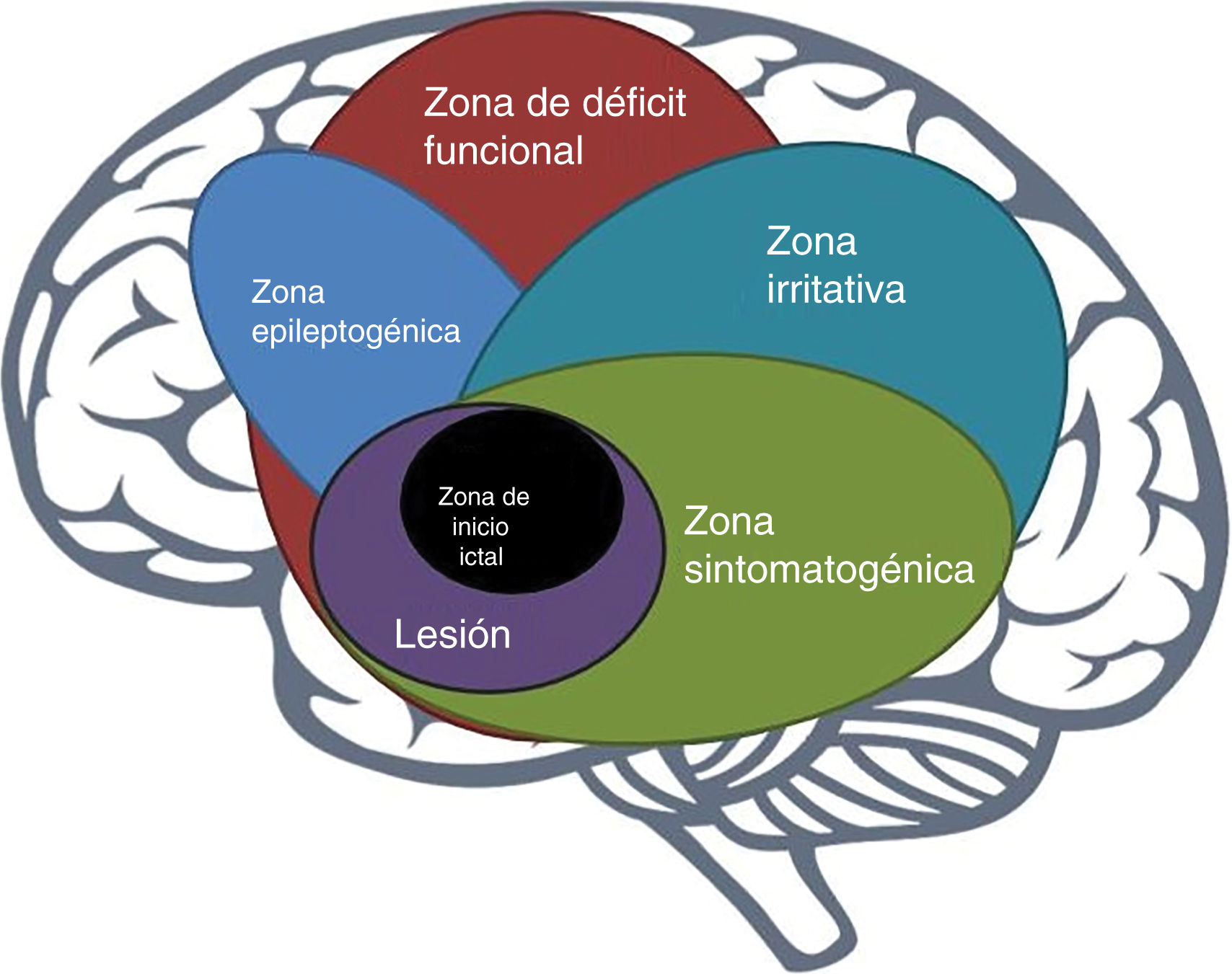
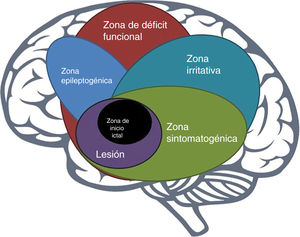
The epileptogenic zoneIn 1993, Lüders et al. defined the epileptogenic zone as “area of cortex that is necessary and sufficient for initiating seizures and whose removal (or disconnection) is necessary for complete abolition of seizures” (Fig. 1).6 This concept is relevant only in the context of the definition of 5 cortical zones proposed for preoperative assessment:
- 1.
Irritative zone: area of cortex that generates interictal epileptiform activity;
- 2.
Seizure-onset zone: area of the cortex where clinical seizures initiate;
- 3.
Symptomatogenic zone: area of cortex that, when activated, produces the initial ictal signs or symptoms;
- 4.
Epileptogenic lesion: macroscopic lesion which is causative of the epileptic seizures because the lesion itself is epileptogenic or by secondary hyperexcitability; and
- 5.
Functional deficit zone: area of cortex that is not functioning normally in the interictal period.7
However, direct preoperative measurement of the epileptogenic zone is not possible; rather, its delineation is a purely conceptual exercise incorporating data from a wide range of tests and components of a preoperative evaluation.7
Epilepsy as an abnormal neural networkIn 2002, Spencer defined epilepsy as a disorder of neural networks, which constitute a functionally and anatomically connected and bilaterally represented set of cortical and subcortical structures in which activity in any one part affects activity in the others.8 This approach is essential for some therapies, such as deep brain stimulation,9 vagus nerve stimulation,10 or responsive neurostimulation11; these treatments, targeting any region of the neural network, should in theory be as effective as those targeting the specific “focus” of epileptic activity.8 It is also a key factor in identifying the anatomical distribution of epileptogenic processes, including epilepsy surgery.12
In humans, the study of epilepsy as a network disorder is based on neuroimaging (MRI, single-photon emission CT, positron emission tomography)13 and neurophysiological studies (EEG, transcranial magnetic stimulation, magnetoencephalography [MEG]), which have varying spatial and contrast resolutions and present different advantages and limitations for the structural or functional analysis of brain regions.14 Some of these techniques are invasive (intraoperative electrocorticography, stereo EEG, depth electrodes) but present limited spatial resolution.15 All studies aim to evaluate ictal (epileptic seizures) or interictal states (interval between epileptic seizures) to identify the epileptogenic areas involved in each type of epilepsy.16
Methods for recording and analysis of epileptic networks using electroencephalography signalsSeveral methods have been developed to analyse epileptic networks using frequency or time-frequency analysis of EEG signals (recorded through invasive17 or non-invasive procedures18), epileptogenicity maps,19 or the epileptogenicity index.20 In the following sections, we describe some general principles and their applications in epileptic neural networks.
Connectivity analysisThis approach is based on mathematical estimations of the relationship between 2 signals from different parts of the brain.19,21,22 Connectivity analysis enables the analysis of neuroimaging and neurophysiological data obtained during the ictal and interictal periods.23 For example, dynamic coupling of functional MRI (fMRI) and interictal EEG has shown a strong, time-dependent association between fMRI local connectivity and interictal EEG in focal epilepsy.23 Coupling of both techniques usually identifies more consistent epileptic networks than either technique alone.23–25 Several linear (coherence, linear regression analysis) and nonlinear statistics (mutual information, nonlinear regression analysis, similarity indices based on state space trajectories reconstructed from observed signals) have been used.26,27 Some studies have shown that nonlinear methods (nonlinear correlation coefficient, transfer entropy, phase synchronisation, and mutual information) provide a more efficient measure of connectivity than linear methods (eg, cross-correlation coefficient) using interictal epileptiform activity recorded with non-invasive dense EEG.26,28 Correlations between time series may be estimated directly using time-domain features, frequency-domain features, or wavelets, in which time resolution may be adapted as a function of frequency.29,30
Causality analysisCausal relationships and the direction of information flow within a multivariate time series have been thoroughly studied under the concept of Granger causality.31 This approach has relevant applications in neuroscience in general, and in the study of epilepsy in particular.32 Granger causality is a statistical concept based on prediction, according to which if a signal X1 “Granger-causes” (or “G-causes”) a signal X2, then past values of X1 should contain information that helps predict X2 beyond the information contained in past values of X2 alone. The mathematical formula is based on linear regression modelling of stochastic processes.31 The definition of Granger causality has also been applied to nonlinear cases in the time and frequency domains, based on non-parametric statistics.32 Two of these techniques are the direct directed transfer function, which estimates the relationships between direct and indirect flows,33 and partial directed coherence, a Granger causality measure in the frequency domain.32 Granger causality may help to localise seizure networks based on interictal data, as demonstrated in a study including 25 patients with focal epilepsy.34
Graph theory–based analysisThe origins of graph theory date to 1735, when Leonhard Euler solved the classic Königsberg bridge problem, concerning whether it is possible to take a walk through the town of Königsberg (now Kaliningrad) that crossed each of the 7 bridges once and only once. Euler used the terms “node” for landmasses and “edge” for bridges, and proved that the problem has no solution. In graph theory, a graph G is defined as a set of vertices (nodes) and edges connecting them. In a graph G = (V, E), for example, V denotes a finite set of vertices and E denotes the set of edges connecting those vertices, with E being a subset of the Cartesian product V × V (pairs of different nodes). G is used to represent the conditional dependencies between nodes; more specifically, each edge or connection between nodes represents a conditional dependency. This approach enables structural and functional analyses of varying levels of complexity. Depending on its application in neurology, the network may be modelled by directed or undirected edges, although graphical models of brain networks frequently use undirected edges since most brain connections are reciprocal.35,36
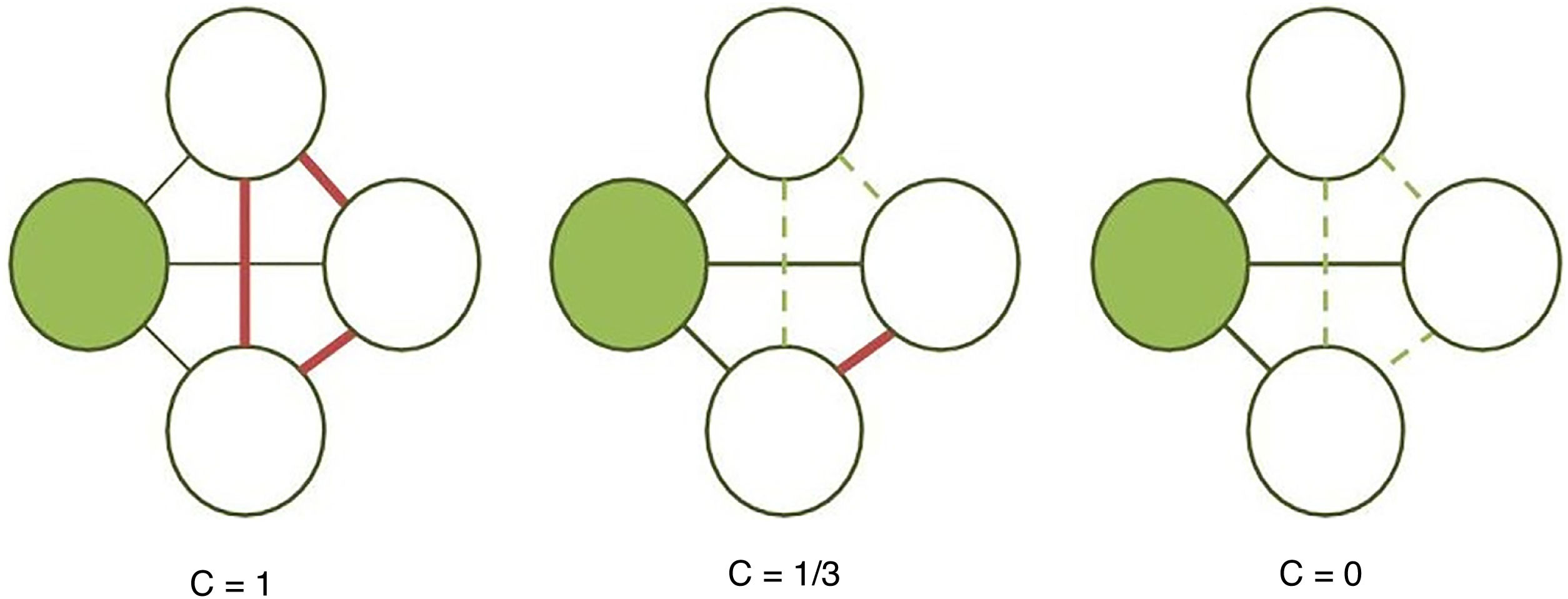
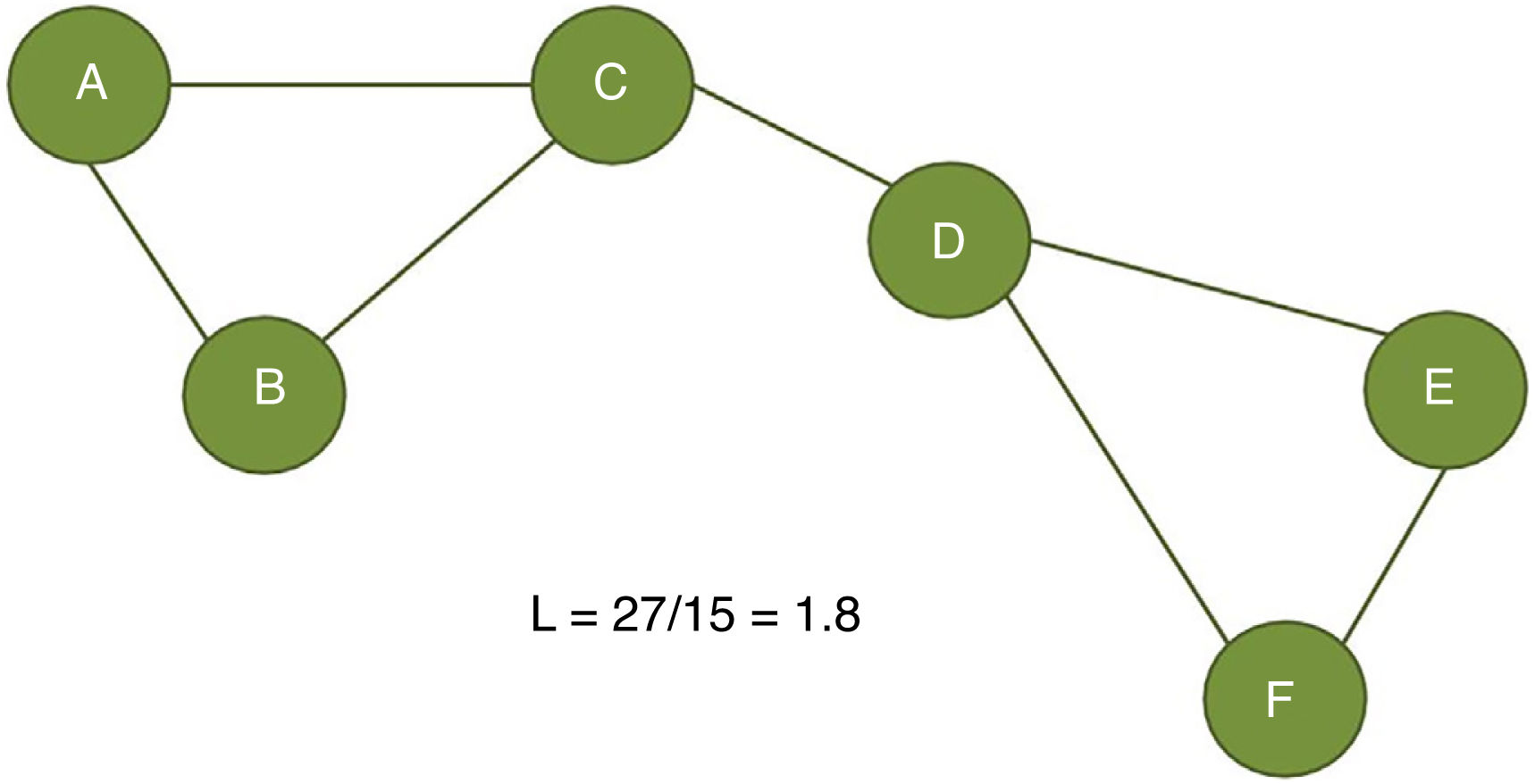
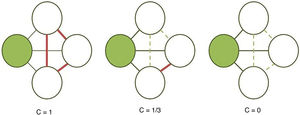
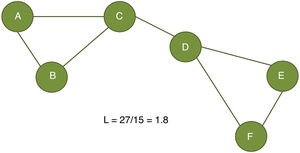
The clustering coefficient (Fig. 2) and characteristic path length (Fig. 3) are 2 of the most frequently used parameters in graph theory. The clustering coefficient measures the density of connections surrounding a given node; it is linked to local network efficiency and quantifies the robustness of a network against the loss of a given node.37 The overall clustering coefficient is the average clustering coefficient across all nodes in the network. Fig. 2 compares a node with a high clustering coefficient against a node with a low clustering coefficient. The characteristic path length quantifies the average value of the shortest paths between all pairs of nodes in the network. Networks with a short characteristic path length are considered globally efficient.38 The interaction between clustering and path length may be used to characterise the global topological properties of a network. Regular lattice networks present high clustering and path length values, while random networks show low clustering and path length. Small-world networks present high clustering coefficients and low path length and are therefore considered both locally and globally efficient.21,38 On an intermediary scale, we may assess communities of nodes, or modules; nodes within a module are more densely connected to one another than to nodes in other modules.39,40
Calculation of the clustering coefficient (C). The clustering coefficient of a given node (shaded circle) is the proportion of established connections (thick continuous lines) between its neighbours (white circles) over all possible connections (dashed lines). In the examples presented here, the clustering coefficient is 1 in the first network, as all possible connections are established; 1/3 in the second network, since only 1 of 3 possible connections is established; and 0 in the third, as the neighbouring nodes are not connected.
Calculation of the characteristic path length (L). The characteristic path length is defined as the average distance between all node pairs in a graph. In the example presented here, we measured the distance between the 15 possible pairs of nodes (total distance of 27) and divided it by the number of pairs (15).
Preliminary studies provide evidence that focal epilepsy is associated with alterations in brain network topology.38 The graph theory may be used in the interictal,41 ictal,42 and preictal periods.43 One of the main advantages of the graph theory is that it simplifies the complex interactions between neuroimaging and neurophysiological data; however, our ability to interpret the results is still limited.35,36,38
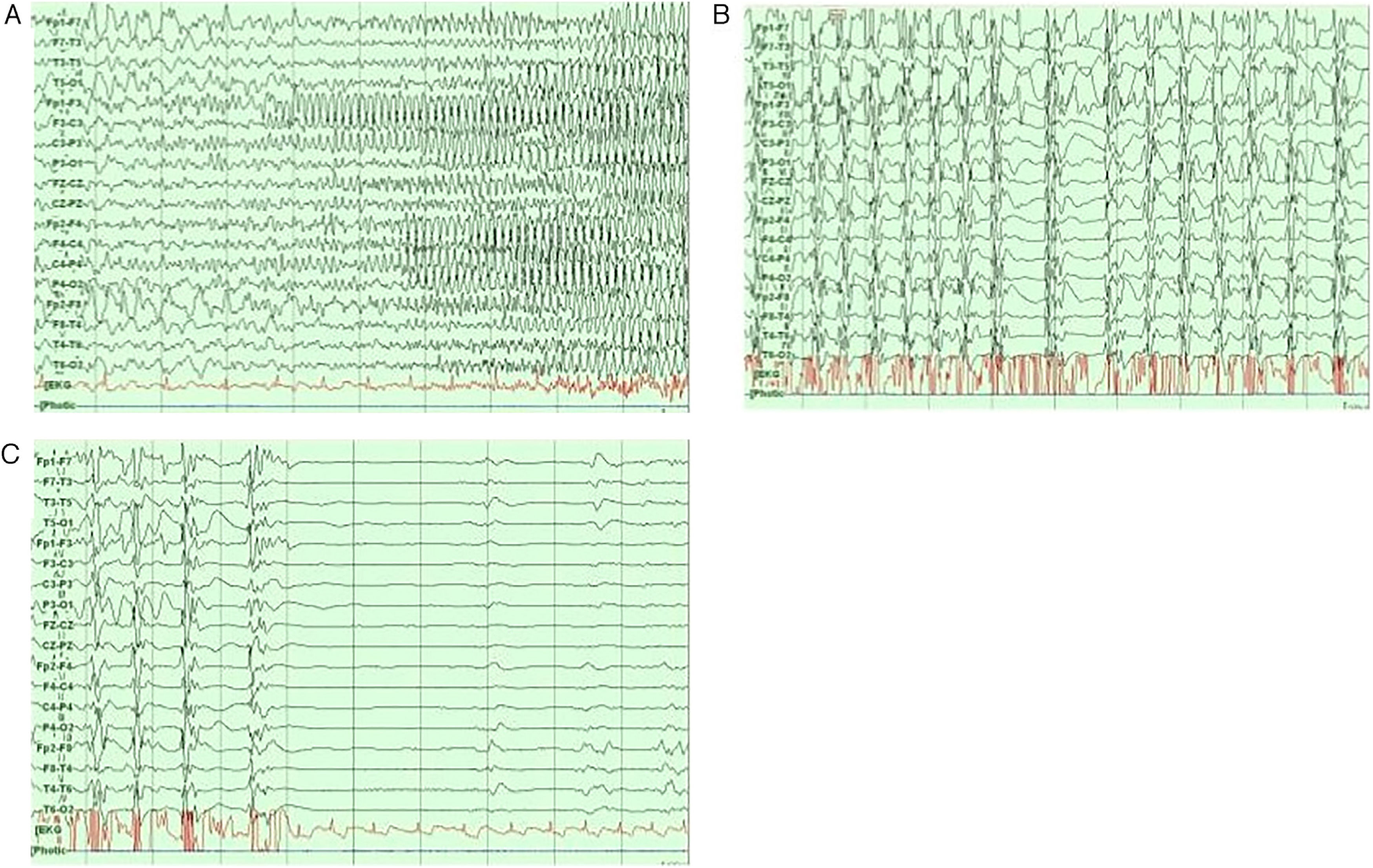
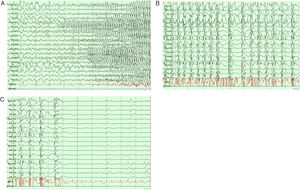
Neurophysiological networks in epileptic seizuresFocal and generalised epilepsy present different EEG patterns during the interictal and ictal periods (Fig. 4).44 Recent studies have used wide-band EEG to measure high-frequency oscillations (80-600 Hz).45,46 These are linked to a wide range of physiological and pathological events; fast ripples (250-600 Hz), in particular, seem to reflect pathological processes associated with epileptogenicity.46
The most common stereo-EEG pattern during ictal events is low-voltage fast discharges, which are frequently preceded by spikes or trains of slow-wave complexes.47 The frequency of low-voltage fast discharges range from beta ranges (15-30 Hz; eg, mesial temporal lobe epilepsy [TLE]) to higher frequencies (gamma range, 30-100 Hz; eg, neocortical seizures).47,48 At the beginning of a seizure, stereo-EEG may be used to calculate the epileptogenicity index, which combines an analysis of both spectral and temporal parameters and is linked to the propensity of brain area to generate fast discharges (12.4-97 Hz), with a view to predicting the initial seizure-onset zone.47,49 Neuroimaging data can be used to create parametric statistical maps of high-frequency oscillations at seizure onset; these are called epileptogenicity maps.21 For example, in a study using stereo-EEG, Aubert et al.50 used the original calculation of the epileptogenicity index in 36 patients with malformations of cortical development, finding that 30% presented a strictly focal epileptogenic zone organisation, whereas the rest presented more than one epileptogenic zone, with a bilateral organisation. Another study compared 15 patients with bilateral mesial TLE against 15 patients with unilateral mesial TLE, and found that the latter group more frequently presented maximal epileptogenicity in hippocampal structures, whereas patients with bilateral mesial TLE presented maximal values in subhippocampal areas (entorhinal cortex, temporal pole, parahippocampal cortex). Another study found that the epileptogenicity index is higher in patients with neocortical TLE and normal MRI findings than in those with hippocampal sclerosis.52 This is also true for such other focal epilepsies as frontal lobe,53 parietal lobe,54 and occipital lobe epilepsy.55
The postoperative prognosis of TLE is associated with the number of epileptogenic zones, while focal epilepsies present better prognosis.50,52 The epileptogenicity index may also be used with MEG signals. For example, Bouet et al.56 studied the spatial relationship between MEG spiking volume, seizure-onset zone location as determined with the epileptogenic index, and lesion volume delineated on brain MRI in 11 patients with focal cortical dysplasia. Stereo-EEG demonstrated a significant correlation between MEG spiking activity and the epileptogenicity index in 8 patients, 7 of whom presented good surgical outcomes. Recently, stereo-EEG and the epileptogenicity index have been used for patient reassessment after epilepsy surgery failure.57
Some computational models provide a framework for studying the influence of neural networks and local tissue properties, and exploring alternative resection strategies.58 However, these models must be validated in vivo in animal models of epilepsy or in the clinical setting.
Functional connectivity during epileptic seizuresAs mentioned earlier, an epileptic seizure is currently defined as excessive or synchronous neuronal activity in the brain.3 Brain structures present marked desynchronisation at seizure onset,59 but synchronisation increases during seizure propagation and termination.37,60 The available information on this topic is limited to TLE, as this is the most frequent indication for presurgical assessment.14,48 These findings have been observed in patients with other focal epilepsies affecting the frontal and occipital lobes,60 and may be more common than in studies using standard EEG.61
Animal and human studies have shown that the seizure-onset zone is functionally organised in small clusters of neurons, which present synchronous firing in tiny microdomains (< 1 mm diameter) during epileptogenesis and ictogenesis.59 However, the exact role of these microseizures in seizure genesis and propagation is yet to be understood. For example, rather than propagating outwards from a restricted cortical site, epileptic seizures may be generated from clusters of spatially distant microdomains.62
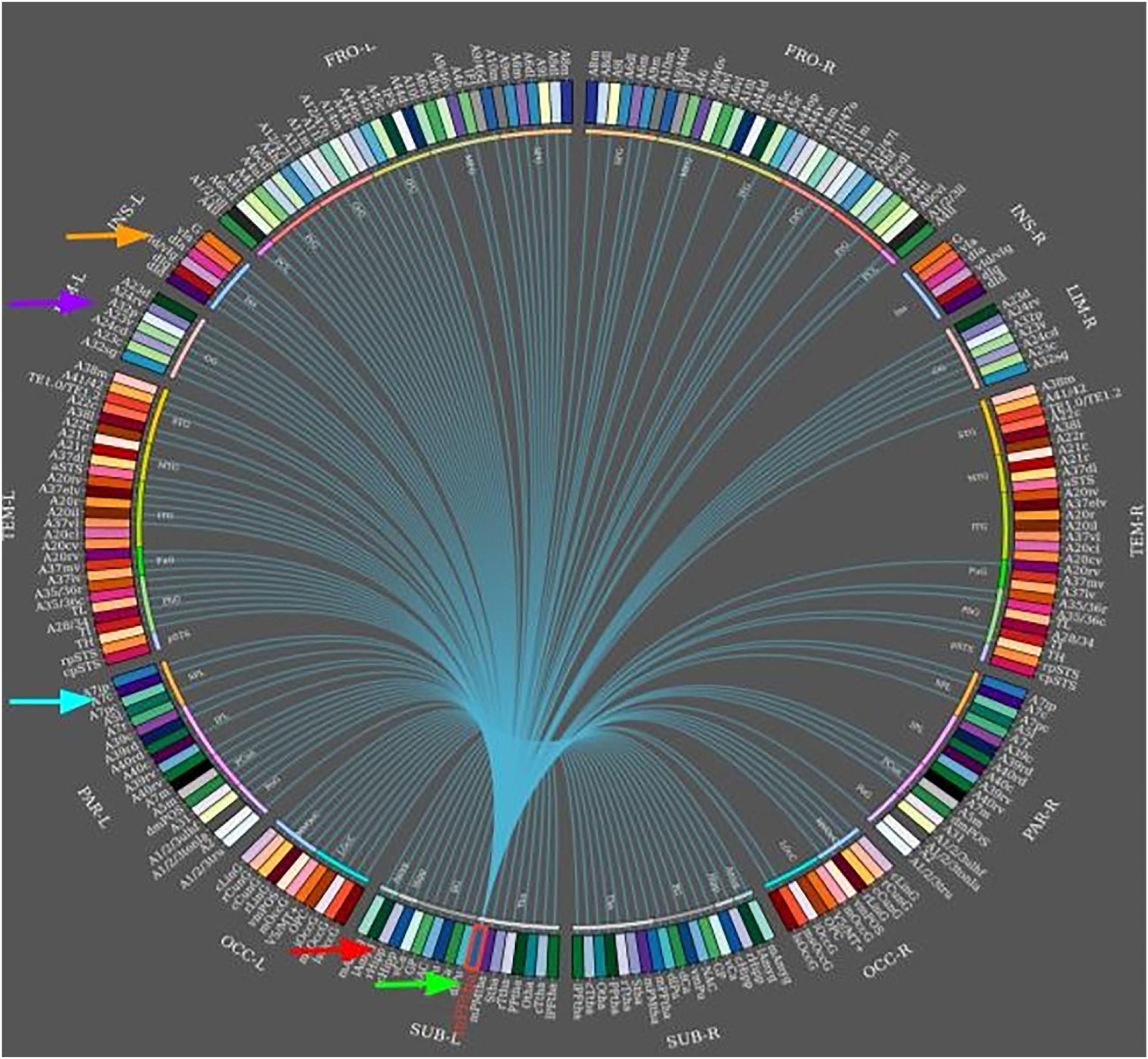
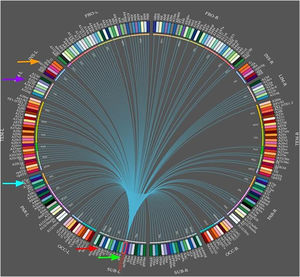
During temporal lobe seizures, a strong correlation in epileptic activity has been observed between the thalamus and temporal lobe structures, which may manifest as dystonic posturing. Neural networks can be used to predict surgical outcomes. In a case-control study by Morgan et al.,65 including 26 presurgical patients with mesial TLE and 44 healthy controls, 3T MRI was used for functional and structural connectivity mapping across an 8-region network of seizure propagation, including the hippocampus, insula, thalamus, precuneus and mid cingulate (Fig. 5). The authors confirmed that whole-network propagation connectivity patterns were consistent with the mesial TLE model predicting surgical failure.64,65 In any case, other studies of patients with mesial TLE have shown that the basal ganglia do not generate specific EEG epileptic activity; despite this, they do reflect changes in the distribution of the ictal epileptic activity.66
Interictal epileptiform discharges are biomarkers of epilepsy; although they usually indicate the irritative zone, on occasion they may also indicate the epileptogenic zone and other cortical zones defined in presurgical evaluation.7,67 Several studies into TLE68 and extratemporal lobe epilepsy69,70 have observed complex interictal distributions between the medial and lateral cortices or between cortical and subcortical areas.70 Brain regions with interictal epileptiform discharges frequently show increased interictal synchrony in structures located in the epileptogenic zone in patients with mesial TLE as compared with patients with neocortical epilepsies, and are linked to the duration of epilepsy.71 These patterns of functional connectivity in interictal epileptiform discharges may be useful in understanding the action mechanisms of such neuromodulatory therapies as vagus nerve stimulation72 or deep brain stimulation. In a small study including 6 patients with epilepsy, stereo EEG was performed during vagus nerve stimulation. Functional connectivity, analysed with deep electrodes, was found to decrease during treatment, and was linked to patient response; this suggests that the effect of vagus nerve stimulation may be linked to this mechanism. Furthermore, during deep brain stimulation of the anterior thalamic nucleus, the authors observed increased resting-state network connectivity in responders; the working hypothesis to explain this was that this network increases the threshold for seizure propagation.73
Caution should be exercised when extrapolating these findings to other types of epilepsy or epileptic syndromes. Several region-specific seizure-onset patterns are known, but they may involve different cellular, network, and synchronisation mechanisms.46,59 For example, increased functional connectivity has been detected in specific cortical regions in untreated patients with juvenile myoclonic epilepsy. This interictal abnormality is increased in the preictal state. Nodal graph statistics revealed abnormal neuronal dynamics in the cortical area identified as the seizure-onset zone in this type of generalised epilepsy.74
The complex relationships between epilepsy, epilepsy aetiology, antiepileptic agents, and epilepsy duration are yet to be fully understood. However, analysis of epileptic networks may be more relevant in non-lesional epilepsies75 or in such critical conditions as status epilepticus.76
Author contributionsAll authors made substantial contributions to the study and approved the manuscript for publication.
FundingNone.
Conflicts of interestThe authors have no financial or commercial relationships that could create conflicts of interest with regard to this article.
None.