Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder worldwide. Although the precise pathogenesis of PD remains unclear, several studies demonstrate that oxidative stress, inflammation, low levels of antioxidants, and the presence of biomolecules that generate reactive oxygen species can disrupt the blood–brain barrier (BBB) as an essential feature of the disease.
AimsThis study aimed to test whether agonism to cannabinoid receptor type 2 (CB2) through the administration of β-caryophyllene (BCP) could correct BBB permeability in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) parkinsonism induction model.
MethodsWe conducted a molecular assessment of proteins (immunochemistry and western blot), BBB permeability, and related biomarkers of PD (lipid peroxidation) in the MPTP mouse model of the disease.
ResultsExpression of zonula occludens (ZO-1) and occludin tight junction (TJ) proteins was dampened in the striatum and substantia nigra pars compacta of mice, while lipid peroxidation and BBB permeability increased in the striatum in the MPTP-treated group, and these effects were reversed under BCP administration. This phytocannabinoid was able to restore protein expression and immunoreactivity of tyrosine hydroxylase (TH), ionized calcium-binding adapter molecule 1 (Iba-1), and glial fibrillary acidic protein (GFAP), as well as nuclear factor-erythroid 2-related factor (NRF2) translocation to the nucleus, and NADPH quinone oxidase 1 (NQO1) expression in mice treated with MPTP.
ConclusionThese results highlight the role of CB2 as a therapeutic target for PD, suggesting that its activation may ameliorate PD-related BBB disruption and oxidative stress, reducing the selective death of dopaminergic neurons.
La enfermedad de Parkinson (EP) es la segunda enfermedad neurodegenerativa más prevalente a nivel mundial. A pesar de que se desconoce el mecanismo patogénico exacto de la enfermedad, algunos estudios han demostrado que el estrés oxidativo, la inflamación, bajos niveles de antioxidantes y la presencia de biomoléculas que generan especies reactivas de oxígeno pueden dañar la barrera hematoencefálica (BHE), lo que representa un rasgo esencial de esta patología.
ObjetivosEvaluamos si el efecto agonista del beta-cariofileno sobre el receptor cannabinoide de tipo 2 (CB2) podría corregir la permeabilidad de la BHE en un modelo de parkinsonismo inducido por 1-metil-4-fenil-1,2,3,6 tetrahidropiridina (MPTP).
MétodosRealizamos un análisis molecular de proteínas (inmunoquímica y western blot), y determinación de la permeabilidad de la BHE y de biomarcadores de EP (peroxidación lipídica) en un modelo murino de la enfermedad inducida por MPTP.
ResultadosLa expresión de proteínas de la zonula occludens (ZO-1 y ocludina) disminuyó en el cuerpo estriado y la sustancia negra pars compacta de los ratones, mientras quela peroxidación lipídica y la permeabilidad de la BHE aumentaron en el grupo de animales tratados con MPTP. La administración de beta-cariofileno revirtió estos efectos. Este fitocannabinoide restableció la expresión de proteínas y la inmunorreactividad de la tirosina hidroxilasa, la molécula adaptadora de unión al calcio ionizado 1 (Iba-1) y la proteína ácida fibrilar glial (GFAP), así como la translocación nuclear de NEF2 y la expresión de NADPH quinona oxidorreductasa 1 en ratones tratados con MPTP.
ConclusionesNuestros resultados ponen de manifiesto el papel de CB2 como diana terapéutica en EP. Su activación podría mejorar la alteración de la BHE y el estrés oxidativo asociados con la EP, reduciendo la muerte selectiva de neuronas dopaminérgicas.
Parkinson's disease (PD) is the second most prevalent neurodegenerative disorder worldwide.1 It causes loss of control of movement and other non-motor symptoms such as cognitive decline sleep disorders and even contributes to other diseases such as depression.2,3 Classically, it is characterized by progressive degeneration and selective death of nigrostriatal dopaminergic neurons, particularly the substantia nigra pars compacta (SN). Although the precise pathogenesis of PD remains unclear, several studies demonstrate that mitochondrial dysfunction, oxidative stress, inflammation, low levels of antioxidants, and the presence of biomolecules that generate reactive oxygen species can disrupt the blood–brain barrier (BBB) as an essential feature of the disease.4,5
For its part, oxidative stress affects all components of cells, including lipids, DNA, carbohydrates, and proteins (intra and extracellular), amongst them the proteins of the tight junctions (TJs) essentials for the BBB.6 Membrane lipid peroxidation is also a key pathogenic event in tissues, resulting from an imbalance between reactive oxygen species (ROS) generation and endogenous cellular antioxidant defense.7 One of the factors that can play an essential role in increasing the permeability of the BBB is lipid peroxidation. Work carried out by ElAli (2011) demonstrated that lipid peroxidation induces the activation of RhoA, a small GTPase known to phosphorylate TJ proteins and further destabilize BBB.8
On the other hand, neuroinflammation also plays a crucial role since the increase in pro-inflammatory cytokines causes an increase in the permeability of the BBB, which may be caused by the decrease in the expression and functionality of TJ proteins.9 MPTP model of Parkinson's disease is a tool capable of generating motor symptoms in mice accompanied by dopaminergic cell loss and glial response,10,11 along with dampening in the expression of BBB associated proteins.12–14 Inflammation resulting from MPTP treatment could significantly contribute to disruption or increase in permeability of the BBB by accelerating dopaminergic cell death and allowing peripheral inflammatory cells such as ED1+ and CD3+ cells to infiltrate the brain parenchyma.15,16 Various experimental evidence shows that treatment with MPTP increases the permeability of the BBB, probably through a decrease in the expression of TJs: zonula occludens (ZO-1) and occludin in the striatum (STR).12–14
Multiple investigations have shown a significant decrease in the amount of occludin in the STR, which was associated with increased BBB permeability in MPTP-treated mice.17,18 Increased permeability of BBB in the STR was also observed in models of hemiparkinsonism induction with 6-hydroxydopamine (6-OHDA)19 leading to downregulation of occluding.20 In vitro studies have shown that α-synuclein fibs lead to a decrease in the expression of occludin and ZO-1, which allows the BBB to become more permeable, probably due to an increase in pro-inflammatory processes, accompanied by a decrease in the expression levels of tight junction proteins such as ZO-1 and occludin in the STR.21
Several cellular targets have been identified as potential therapeutic interventions for PD treatment. Recent studies have suggested that the cannabinoid signaling system plays a protective role in PD. In particular, the cannabinoid receptor type-2 (CB2R), expressed predominantly by circulating immune cells and resident microglia, plays an essential role in the immune response to injury. Further, work by Viveros-Paredes in 2017, demonstrated that CB2R agonism protects nigrostriatal dopaminergic neurons against MPTP-induced neurotoxicity by inhibiting the pro-inflammatory responses to MPTP-induced dopaminergic cell damage.22 The CB2R has an essential regulatory function in the normal physiology of the BBB, and in the protection against the damage generated by a state of inflammation caused by parkinsonism, which translates into the loss of integrity and composition of the BBB.9
However, it is unknown until now whether agonism to the CB2R through the administration of β-caryophyllene (BCP) may inhibit the permeability of the BBB. For this reason, present work aimed to evaluate the effect of oral administration of BCP on the permeability of the BBB in a parkinsonism induction model.
Materials and methodsAnimal preparationAll experiments were carried out on male C57BL/6J mice (25–30g), obtained from UAM Laboratories (Mexico City). Mice were kept on a 12:00h light–dark cycle, with food and water available ad libitum. All experimental procedures were consistent with ethical policies stipulated by the Ethical Research Committee of Centro de Investigación Biomédica de Occidente (R-2015-1305-8) and were realized according to the official Mexican Norms NOM-062-ZOO-1999 and NOM-033-ZOO-1995 as well as National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Experimental design and treatmentAnimals were randomly divided into four groups n=20/group, 80 mice in total.
The first group received saline solution once a day for five consecutive days via i.p. and served as a control group. Under the same schedule, the second group received MPTP hydrochloride (30mg/kg, i.p.; Sigma-Aldrich, St. Louis, MO, USA) for 5 consecutive days. The third group received BCP (10mg/kg, i.g.; kindly donated by Jürg Gertsch, Institute of Biochemistry and Molecular Medicine, University of Bern, Switzerland) once a day for five consecutive days. The fourth group received MPTP as described above, and once this treatment was finished, the mice received BCP exactly as group three.
Three days after the last administration, the animals of each experimental group were sacrificed (perfusion and decapitation) to carry out the following experimental procedures:
ImmunohistochemistryFive animals from each group were used for immunohistochemical studies of TH, GFAP and Iba-1 (same brand and catalog numbers and dilutions above-mentioned). The mice were anesthetized with i.p. injection of ketamine (100mg/kg) and xylazine (15mg/kg), and each of them was intracardially perfused with 4% paraformaldehyde in 0.1M PBS. After perfusion, the animal's brains were removed, left in fresh PFA fixative for 24h, washed 3 times with 0.1M PBS and finally, coronal vibratome slices (Leica VT1000E; Leica Microsystems, Wetzlar, Germany) were obtained. For morphological analysis of the STR and SN, coronal slices were cut starting at bregma 1.10mm to −0.10mm and from −2.92mm to −3.64mm, respectively, according to Paxinos and Franklin atlas (2013). We collected six sets consisting of six tissue slices from each individual brain, each slice was 35μm wide and 175μm apart from another in every set to prevent over counting of cellular bodies. We selected slices at the same level in all animals to achieve a uniform analysis using the basic principle of fractionation from anterior to posterior areas. The selected slices were washed 3 times for 5min in 0.1M PBS and they were then placed in sodium citrate buffer (pH 6.4) for 10min at 37°C, and then washed immediately with 0.1M PBS. The endogenous peroxidases in the tissues were inactivated for 20min in a solution of 3% H2O2 at room temperature and the tissues were again washed in 0.1M PBS and incubated for 1h at room temperature in blocking solution: 0.1M PBS, 0.1% Triton-X-100, and 10% horse serum. Subsequently, they were incubated for 24h at 4°C with the respective primary antibodies then, the slices were washed with 0.1M PBS and then incubated for 2h with the biotinylated respective secondary antibodies 1:1000 in PBS. After incubation, the slices were washed with 0.1M PBS and they were incubated for 45min in the dark with the avidin–biotin complex (Vector Laboratories, Vectastain ABC kit PK-6100). This solution was then removed, the slices were washed with 0.1M PBS and antibody binding was revealed with diaminobenzidine (Vector Laboratories, DAB SK4100) prepared according to the manufacturer's instructions. The slices were mounted on slides and the tissue slices were placed in xylene for one minute to be fully dehydrated and then mounted under a glass coverslip with synthetic resin. For the quantification of TH in SN and GFAP-Iba1 in both STR and SN regions, the average number of labeled cells per field was evaluated while visualizing it on the microscope for Z axis perception. In the case of the STR, TH immunoreactivity was evaluated as normalized intensity in pixels maintaining the same exposure time, aperture field and light intensity in each acquisition. Images were obtained from the same areas in each tissue sample using a light microscope at a 20x magnification (Leica LDM6 Microscope).
Lipid peroxidationA fraction of the animals (n=5/group) were sacrificed by decapitation, and the SN and STR were rapidly removed and frozen at -80°C until further analyses. The tissues were sonicated in a lysis buffer and protease inhibitor cocktail on an ice bath. Then homogenates were incubated on ice for 30min and centrifuged at 10,000 x g for 30min at 4°C. These samples were used to measure lipid peroxidation since oxidizing agents can alter lipids’ structure, creating lipid peroxides. This primarily results in the formation of malondialdehyde (MDA), which reacts with thiobarbituric acid (TBA). This reaction produces a colorful compound; therefore, the levels of substances reactive to thiobarbituric acid were determined by TBARS spectrophotometrically at 532nm in STR and SN brain homogenates according to the parameters of the TBARS assay kit (item No. 10009055).
Western blottingThe protein concentration was estimated on the homogenate previously described according to the Lowry method using bovine serum albumin as a standard, and 30μg of protein from each sample was separated on a 10% SDS-polyacrylamide gel and transferred to nitrocellulose membranes. The membrane was blocked with non-fat milk (10%: Svelty) in Tween at 0.05% on PBS (TPBS). The membranes were incubated for 24h with the corresponding primary antibodies: anti-ionized calcium-binding adapter molecule 1 (Iba-1, 1:500; Abcam, ab178846), Cambridge, UK), anti-tyrosine hydroxylase (TH, 1:1000; Abcam, ab112), anti-occludin (1:500; Biorbyt, orb412121) anti-glial fibrillary acidic protein (GFAP, 1:1000; Biocare Medical, CM 065 A,C) anti-zonula occludens-1 (ZO-1, 1:500; Thermo Fisher, 61–7300) and anti NAPDH Quinone Oxidase 1 (NQO1, 1:10000 Abcam, Ab80588). After five washes in TPBS, membranes were incubated for 2h with the corresponding secondary antibodies: biotinylated goat anti-rabbit IgG antibody (1:500 dilution in PBS: Vector Laboratories, BA1000) for the first 3, biotinylated rabbit anti-mouse antibody (IgG1:500 dilution in PBS: Vector Laboratories, BA2000) and biotinylated goat anti-rat antibody (IgG1:500 dilution in PBS: Vector Laboratories, BA5000) for the last two respectively. After five washes with TPBS, the membranes were incubated for one hour with the avidin–biotin complex (Vector Laboratories, Vectastain ABC kit PK-6100). After a final three TPBS washes, antibody binding was visualized with diaminobenzidine (Sigma, D5905). Protein expression was evaluated using the ImageJ software (Wayne Rasband, National Institutes of Health, USA, version 1.50e). The data obtained were normalized to the corresponding expression of β-actin (anti β-actin antibody, 1:5000, MA1-140 Thermofisher) as an internal control for each sample and the data were reported as arbitrary units of intensity.
For cytoplasmic and nuclear extraction, other third of the animals were used (n=5/group) [in the particular case of the nuclear factor-erythroid factor 2-related factor (Nrf2) protein], the “NE-PER Nuclear and Cytoplasmic Extraction Reagents” kit (catalog No. 78833 Thermo Scientific) was used. For this, 20mg recovered from the STR region were placed in microcentrifuge tubes, washed with PBS 1X, and centrifuged at 500rpm/5min; the supernatant was removed later to homogenize the tissues with 200μL of CER I, mixing vigorously by a vortex for 15s to re-suspend each pellet. They were incubated for 10min on ice, and 11μL of CER II were added, mixing by vortex for 5s. They were incubated again for 1min on ice and vortexed again for 5s, then centrifuged for 5min at maximum speed. The supernatants, which form the cytoplasmic fraction, were immediately recovered and stored at −20°C until further analysis. The remaining pellets were re-suspended with 100μL of NER, mixing vigorously with a vortex for 15s; the samples were placed on ice and continued mixing with a vortex for 15s every 10min, for a total of 40min. Subsequently, the samples were centrifuged at maximum speed for 10min, and the supernatant, belonging to the nuclear fraction, was immediately transferred to new tubes and stored at −20°C for further analysis. For the extraction of cytoplasmic and nuclear protein fractions the internal control genes were β-actin and Lamin (anti-Lamin, 1:1000; Abcam, ab8980) respectively.
BBB assessmentFluid-phase BBB permeability was assessed as described before23 using sodium fluorescein (NaF) at a 1-day post of finishing the treatments. The rest of the mice (n=5/group) were injected with 10% NaF diluted on a saline solution at a dose of 2.5ml/kg via i.p. (Cat. F6377, Lot. 061M0048V, Sigma-Aldrich, MO, USA). Subsequently, 30min after systemic fluorescein diffusion, the animals were anesthetized using a lethal dose of sodium pentobarbital, cardiac blood was collected followed by transcardial perfusion with 15ml of heparin (1000U/L) in PBS. STR and SN was dissected and weighed. To determine BBB permeability, the tissue was homogenized in 1/10 dilution in PBS and centrifuged at 16,060g for 15min at 4°C; supernatants were recovered and mixed with 95% ethanol to precipitate residual proteins and stabilize the fluorescent signal. NaF fluorescence was measured in a fluorescent plate reader (Ex: 485nm; Em: 525nm).
Statistical analysisThe Kolmogorov–Smirnov test was performed to determine the normality of the data. Given the fact that data distributed normally the parametric statistical tests were chose. Subsequently, a comparison between multiple groups was explored with the ANOVA test and a Tukey post hoc test (GraphPad Prism 7 software). For this study, results were considered statistically significant if p≤0.05.
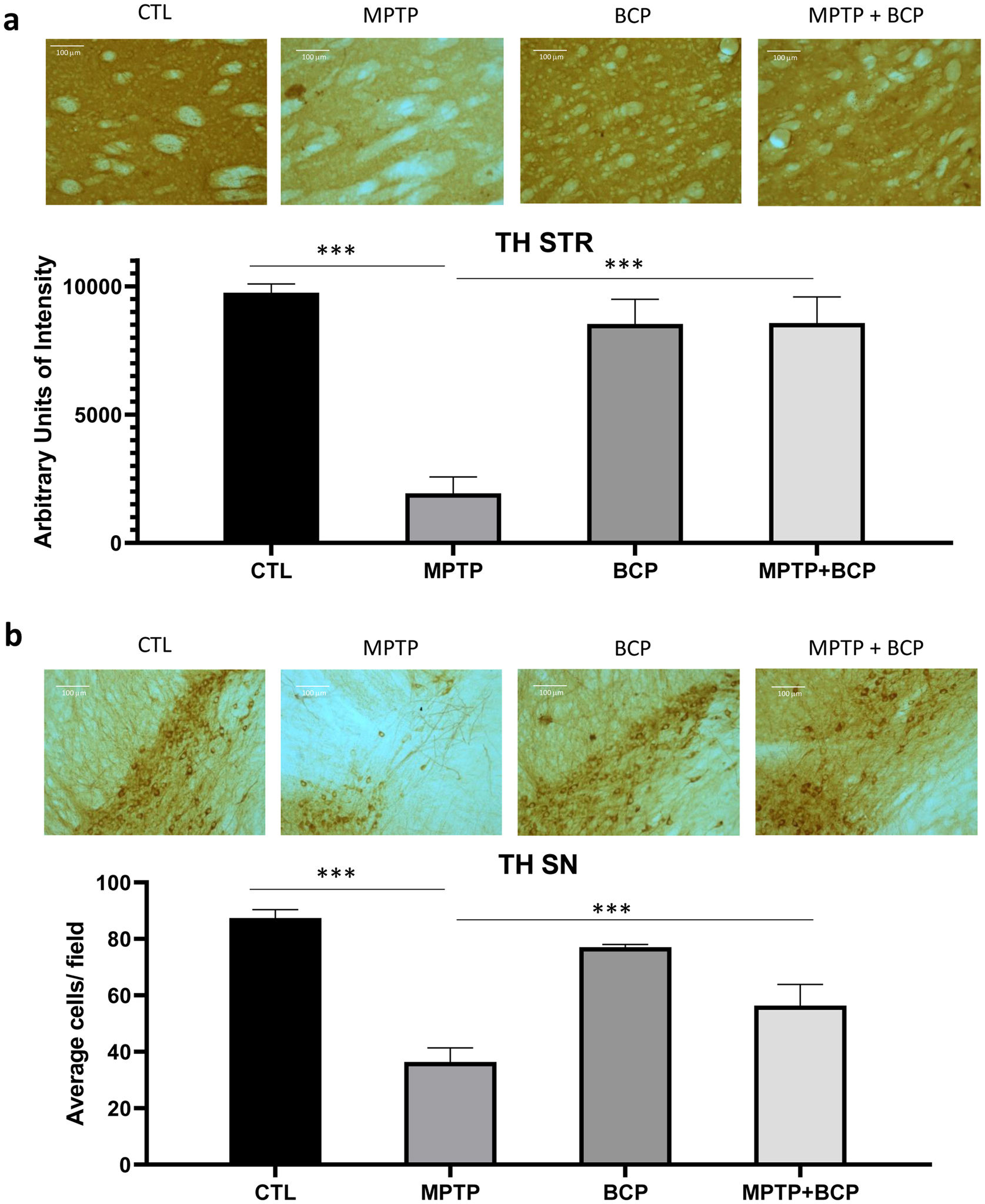
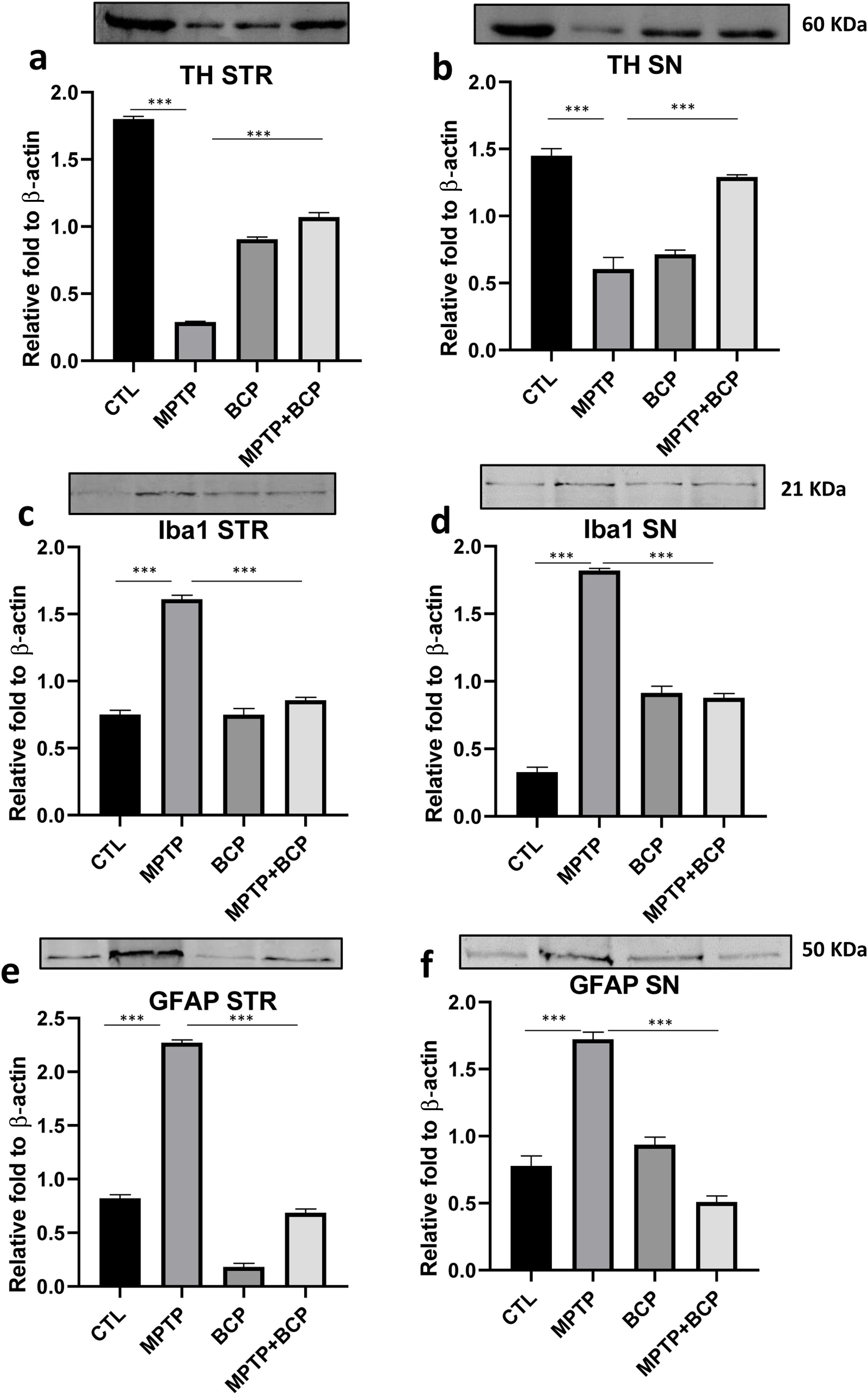
ResultsBCP inhibits MPTP-induced TH immunoreactivity loss in STR and SNThe analysis of immunochemistry revealed that administered MPTP animals showed a decrease in TH-immunoreactive cells than saline control mice in the STR (p≤0.0001) and SN (p≤0.0001). In contrast, the treatment of BCP in combination with MPTP recovered the TH protein immunoreactivity in the STR and SN (p≤0.001) compared to the MPTP-injected mice (Fig. 1a and b).
Representative images and comparative graphs of immunoreactivity for the TH protein in STR (a) and immunoreactive cells for SN (b). The columns represent the mean±SD of intensity relative to negative control for STR (a) and average number of immunoreactive cells per field for SN (b), n=5/group. (***) Indicates statistically significant differences, p≤0.001; ANOVA, post hoc Tukey. Photomicrogrpahs 20×.
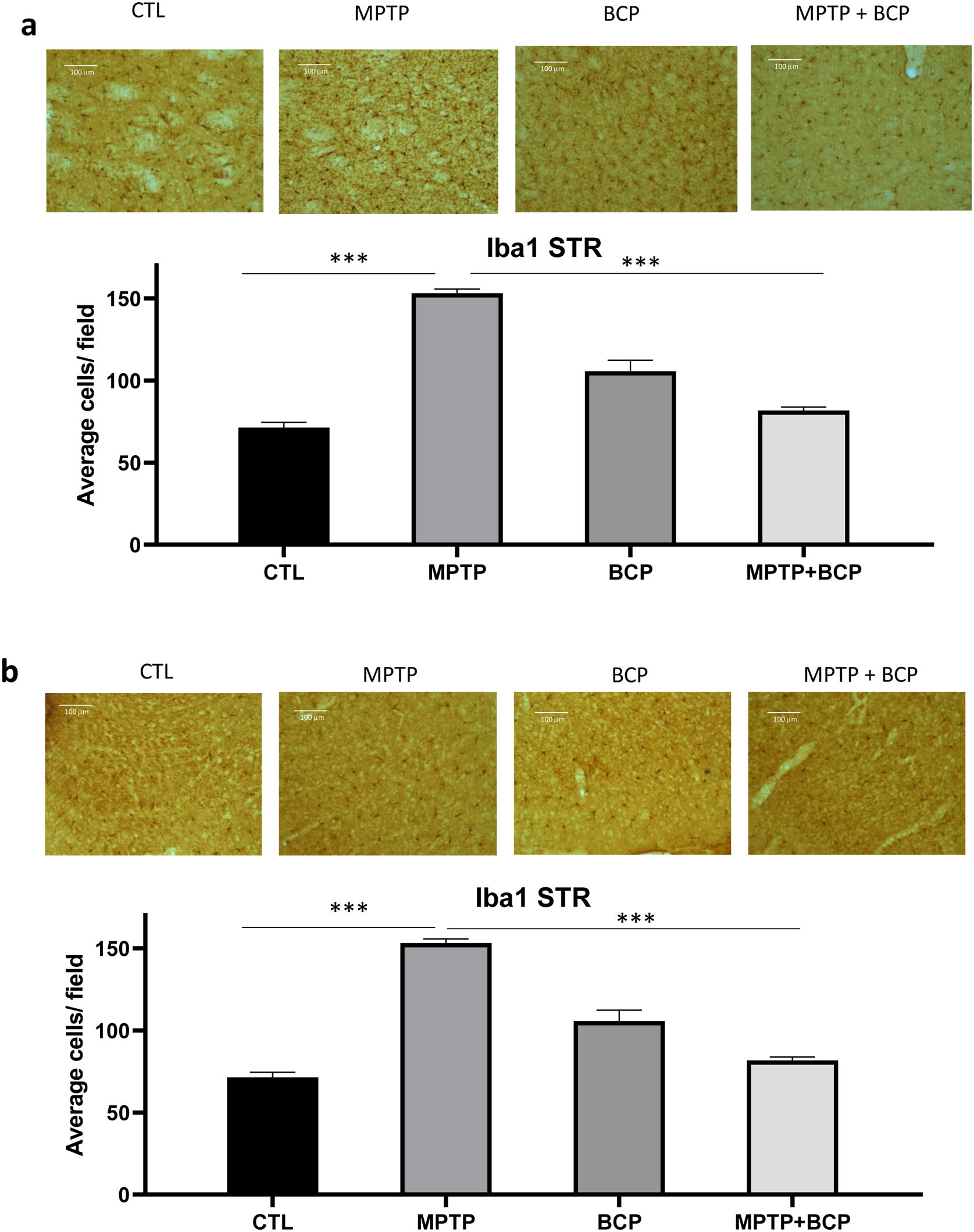
Ionized calcium-binding adapter molecule 1 (Iba-1) is a microglia marker, which was detected by immunochemistry in the STR and SN. The immunoreactivity of Iba-1 in the STR and SN of the MPTP-treated group was significantly higher (p≤0.001) than that of the control group. However, in mice treated with BCP and MPTP, a decrease (p≤0.001) of Iba-1 immunoreactive cells was observed compared to the MPTP group in STR and SN (Fig. 2a and b).
Representative images and comparative graphs of immunoreactive cells for the Iba1 protein in STR (a) and SN (b). The columns represent the mean±SD of average number of immunoreactive cells per field for STR (a) and SN (b), n=5/group. (***) Indicates statistically significant differences, p≤0.001; ANOVA, post hoc Tukey. Photomicrogrpahs 20×.
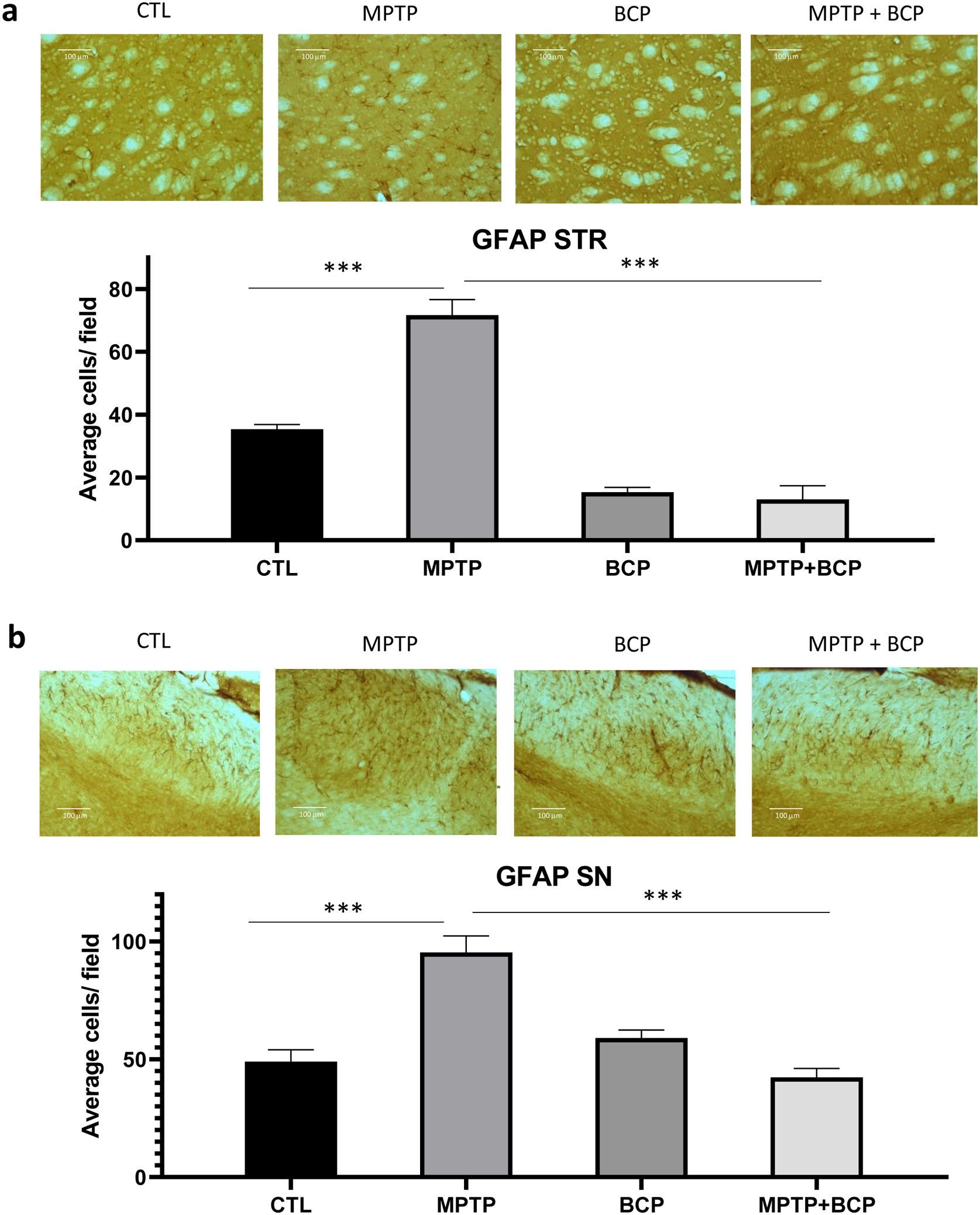
Similar to Iba-1, an increase in immunoreactivity of GFAP was observed in the group treated with MPTP (p≤0.001) compared to the control group. For its part, the immunochemistry analysis revealed that the administration of BCP in combination with MPTP led to a reduction in the number of immunoreactive cells GFAP (p≤0.001) compared to the MPTP-treated group in both brain regions (Fig. 3a and b).
Representative images and comparative graphs of immunoreactive cells for the GFAP protein in STR (a) and SN (b). The columns represent the mean±SD of average number of immunoreactive cells per field for STR (a) and SN (b), n=5/group. (***) Indicates statistically significant differences, p≤0.001; ANOVA, post hoc Tukey. Photomicrogrpahs 20×.
The analysis of western blot revealed that administered MPTP animals showed a decrease in TH-immunoreactive cells than saline control mice in the STR (p≤0.0001) and SN (p≤0.0001). In contrast, the treatment of BCP in combination with MPTP recovered the TH protein expression in the STR and SN (p≤0.001) compared to the MPTP-injected mice (Fig. 4a and b). For his part the expression of Iba-1 in the STR and SN of the MPTP-treated group was significantly higher (p≤0.001) than that of the control group. However, in mice treated with BCP and MPTP, a decrease (p≤0.001) of Iba-1 expression was observed compared to the MPTP group in STR and SN (Fig. 4c and d). Accordingly, an increase in expression of GFAP was observed in the group treated with MPTP (p≤0.001) compared to the control group. For its part, the western blot analysis revealed that the administration of BCP in combination with MPTP led to a reduction in the expression of GFAP (p≤0.001) compared to the MPTP-treated group in both brain regions (Fig. 4e and f).
Representative images and comparative graphs of western blot for the proteins TH (a and b), Iba-1 (c and d) and GFAP (e and f) in STR and SN respectively. The columns represent the mean±SD of intensity relative to β-actin, n=5/group. (***) Indicates statistically significant differences, p≤0.001; ANOVA, post hoc Tukey.
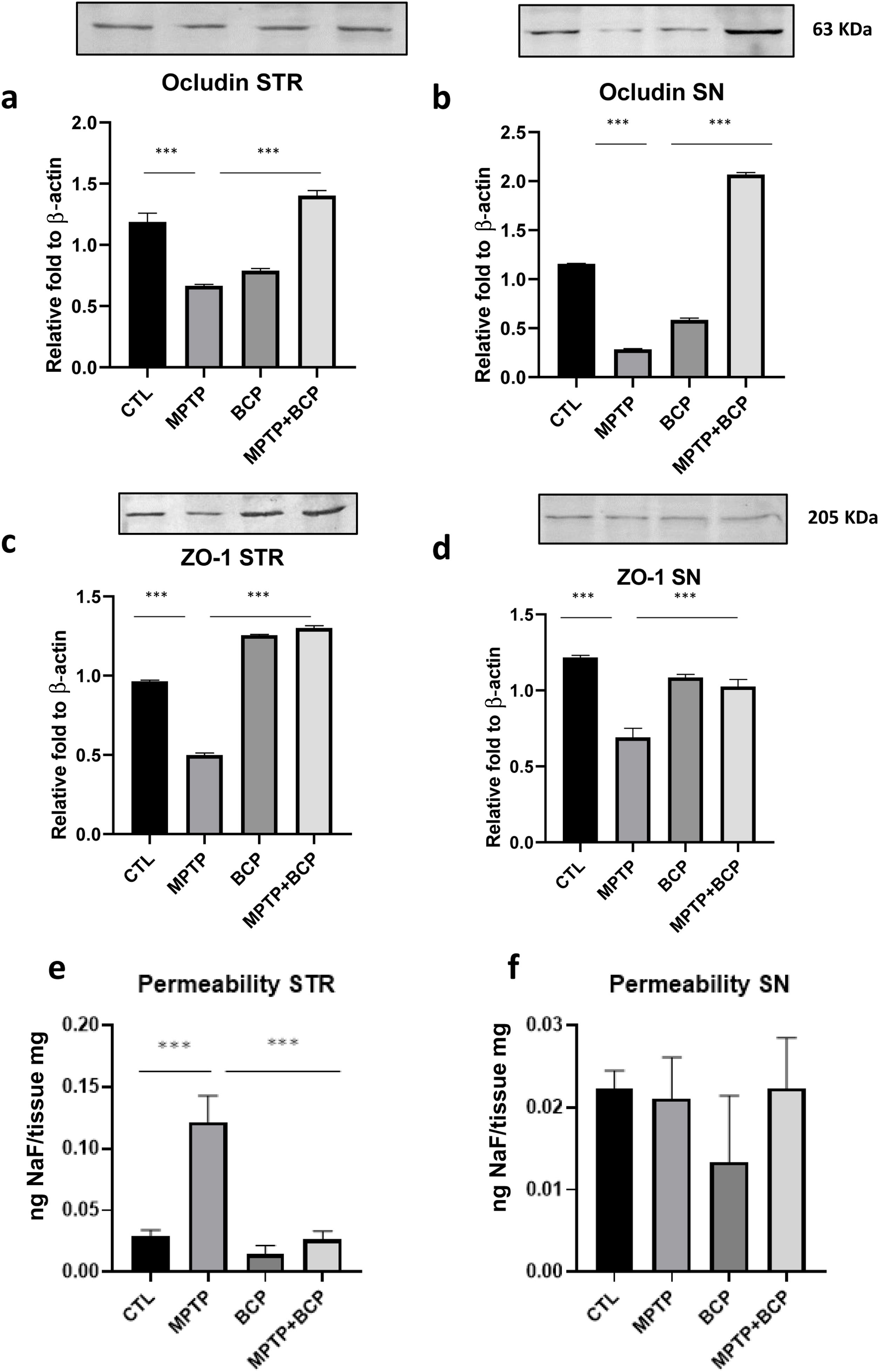
To investigate the effect of BCP on TJ proteins, western blot analysis was performed to detect the protein expression levels in the STR and SN. As shown in Fig. 5, the expression levels of occludin and ZO-1 in the MPTP group were significantly lower compared with those in the control group; conversely, the administration of BCP along with MPTP reversed the effect induced by MPTP by increasing the expression of TJ-associated proteins (Fig. 5a–d) (p≤0.001).
Comparative graphs and representative images of western blotting for the proteins occludin (a and b) and ZO-1 (c and d), as well as permeability (e and f) in STR and SN respectively. The columns represent the mean±SD of intensity relative to β-actin, n=5/group in panels a–d. For the panels e and f the columns represent the mean±SD of ng of NaF/mg of tissue, for the fluorescent measurements, n=5/group in panels. (***) Indicates statistically significant differences, p≤0.001; ANOVA, post hoc Tukey.
BBB permeability was assessed by NaF uptake into the STR and SN. NaF uptake was significantly increased in the MPTP group (p≤0.001) compared to the control group in STR. However, treatment with BCP after MPTP reduced (p≤0.001) the injury-induced increase in NaF uptake in the same region (Fig. 5e). No differences were found for the permeability in the SN region (Fig. 5f).
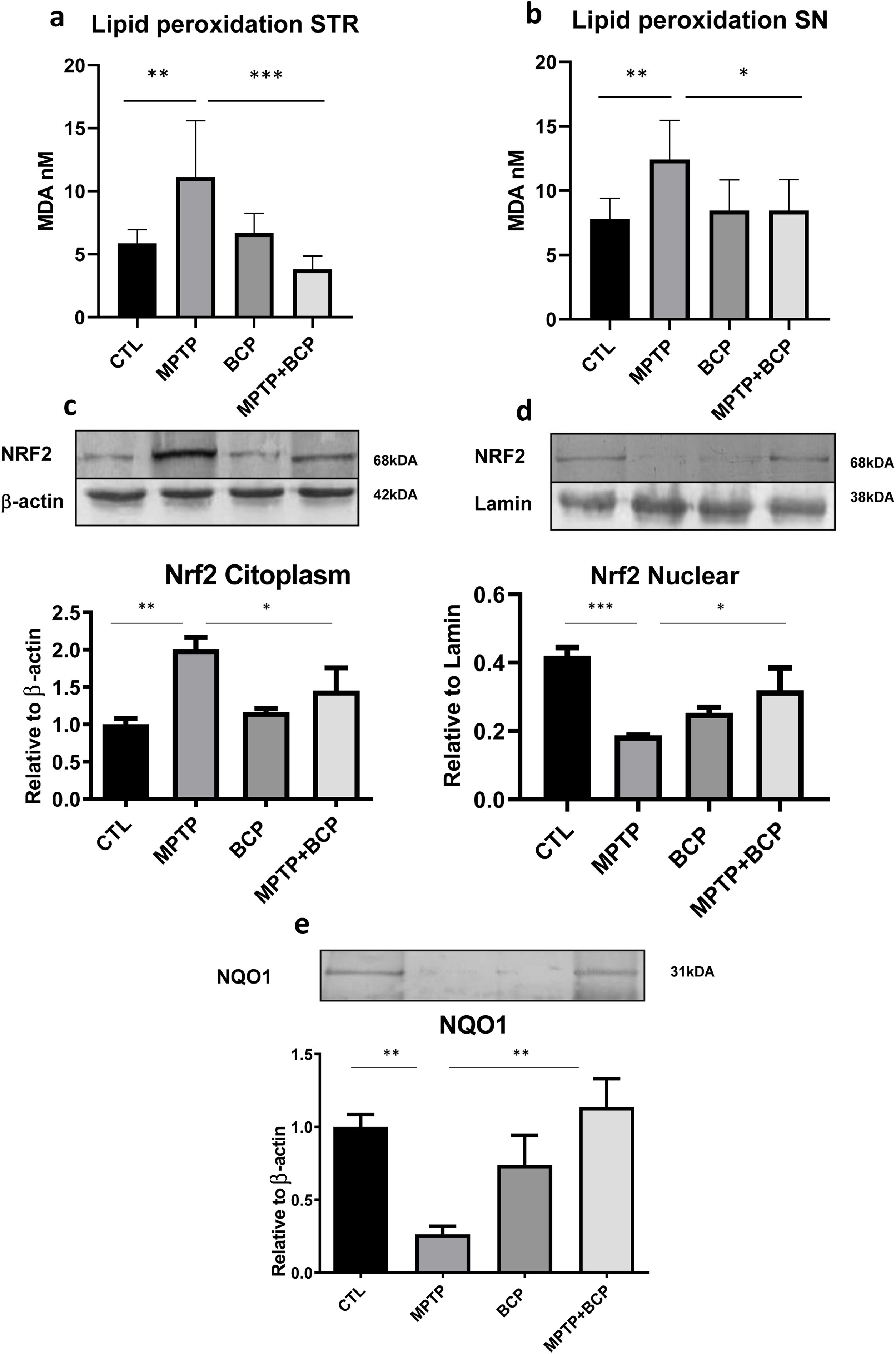
Effect of BCP on the MPTP-induced lipid peroxidation in miceOn the other hand, the results of lipid peroxidation expressed as malondialdehyde (MDA) levels demonstrated that the administration of MPTP increased the lipoperoxidation of the membranes compared to the control group in the STR (p≤0.01) and SN (p≤0.01). However, treatment with BCP in combination with MPTP caused a decrease in peroxidation compared to the MPTP group in the STR (p≤0.0001) and SN (p≤0.05). The group treated only with BCP did not differ significantly versus the control group (Fig. 6a and b).
Lipid peroxidation for the STR (a) and SN (b) and expression of Nrf2 in the STR, cytoplasmic fraction (c) nuclear fraction (d) and cytoplasmatic expression of NQO1 in the same region (e). The columns represent the mean±SD of MDA nM, n=5/group for panels a and b. The rest of the panels c–e show a representative image of the membrane bands in the upper image. The lower image of shows the bar graph where the mean±SD (n=5) of the differences between the different groups is represented. (*) Indicates statistically significant differences, p≤0.05, (**) p≤0.01 (***) p≤0.001; ANOVA, post hoc Tukey.
Due the fact that STR region had significant differences in both permeability and MDA concentrations we further investigated the mechanisms that could integrate these results at transcription factor levels targeting Nrf2 and its influence in NQO1 as a relevant antioxidant enzyme. The results obtained for the expression of Nrf2 in the cytoplasmic fraction show that the group of mice treated with MPTP had an increase in the expression of Nrf2 compared to the control group (p≤0.01). It was observed that the animals treated with BCP in combination with MPTP expressed the Nrf2 protein to a lesser extent, being a statistically significant difference compared to the group treated with MPTP (p≤0.05) (Fig. 6c). Regarding the nuclear fraction, a decrease of Nrf2 expression in the group of mice treated with MPTP (p≤0.001) compared to the control group was observed. However, treatment with BCP in combination with MPTP caused an increase in the levels of expression of the protein Nrf2, compared to the group treated with MPTP (p≤0.05) (Fig. 6d).
NQO1 expression in the STRThe results of the present work on the expression of NQO1 in the STR show a decrease in the expression levels in the group treated with MPTP compared to the control group (p≤0.01). On the contrary, in the group of mice treated with BCP in combination with MPTP, an increase in the expression levels of this enzyme is observed with respect to the MPTP group (p≤0.01) (Fig. 6e).
DiscussionCellular levelDopaminergic neuronsWorks done by Gómez-Gálvez in 2015 demonstrated the neuroprotective effect of treatment with HU-308 (a CB2R agonist) in a model of LPS injury in C57BL/6 mice, which manages to reverse the reduction of TH in the SN.24 This study suggests that when there is an inflammatory process, there is an increase in the expression of CB2R, which is the consequence of an endogenous protective response.12 It has also been found that mice that lack these receptors are much more vulnerable to damage by LPS concerning the percentage of loss of dopaminergic neurons in SN.24 On the other hand, the work carried out by García M. in 2015 demonstrates the existence of neuronal CB2R, employing an immunohistochemical analysis for TH cells positive in the SNpc, and that, in turn, this receptor tends to increase its expression in regions where there is some damage. Once it has been shown that CB2R is found in dopaminergic neurons, it is possible to assume that the binding of the ligand to its receptor triggers various intracellular mechanisms that generate an antioxidant effect.25–27
MicrogliaDecreased immunoreactivity of microglia cells observed in the present study is corroborated by previous studies such as the one carried out by Huang in 2017. The activation of microglial cells in the SN and STR regions was determined after a dosing regimen with MPTP. Using immunofluorescence, the authors demonstrated elevated levels of the microglia marker (Iba-1). Furthermore, a remarkable number of microglial cells were still in an activated state in the SN.10 It is known that the MPTP-mediated model of parkinsonism is characterized by the generation of an inflammatory process and oxidative stress, with a state change from M0 to M1 in microglial cells. This is partly responsible for mediating damage and oxidative stress, as well as dopaminergic cell death, through the production of pro-inflammatory cytokines such as tumoral necrosis factor alpha (TNF-α), interleukin (IL)-1β, and IL-6 in the MPTP model.28,29 In a study by Javed in 2016, the Iba-1 immunofluorescence technique was performed, and it was observed that rats treated with BCP had a notable decrease in immunofluorescence to this marker. In addition, administration of AM630 (a CB2R antagonist) before BCP administration to rotenone-pretreated animals showed an increase in activated microglia. CB2R activation can induce a phenotype switch from M1 to M2 mediated by anti-inflammatory actions through the production of IL-10 (a critical cytokine in the establishment of the M2 phenotype), inhibit the production of the previously mentioned pro-inflammatory cytokines TNF-α, IL-1β and IL-6, as well as reduce oxidative stress that contributes to the neuroprotective effect.7 Similarly, another study showed that WIN 55212-2 and JHW treatment reduced microglia expression to control levels in the SN, something that was reversed after JTE (CB2R antagonist) administration, similarly, a genetic study also showed that CB2R knockout mice −/− show greater sensitivity to MPTP, which leads to the death of the experimental subjects.30
Administration of BCP also generated an increase in immunoreactivity to Iba-1 in the SN; this may be because, under normal conditions, there is a basal level of CB2R; however, the administration of BCP could cause supersaturation and desensitization, generating the activation of intracellular cell arrest mechanisms such as β-arrestins, which internalize CB2R and degrade it as a final step. In addition, it has been proven that these mechanisms generate cellular stress, something that is reflected in our results.26,27
AstrocytesWork carried out by Jo in 2018, as well as by Su in 2017, where the administration of MPTP in mice and 6-OHDA in rats, respectively, was used as a model induction of parkinsonism, show the morphological alterations of astrocytes utilizing immunohistochemistry. Likewise, in these studies, this immunoreactivity was observed to be significantly elevated in the SN compared to the control group.31,32 The exacerbated presence of astrocytes that express pro-inflammatory cytokines, such as IL-6, IL-1β, and TNF-α, as well as reactive oxygen species and nitric oxide in the SN, leads to signaling pathways of intracellular factors related to neuronal death. Therefore, astrocytes can contribute significantly to the underlying neurotoxic and neurodegenerative process of PD.32 Another mechanism in which astrocytes are involved is in the expression of mediators such as MPO, a key enzyme involved in generating cytotoxic ROS/RNS.28 For its part, the decrease in the expression of GFAP in the group of mice treated with BCP in conjunction with MPTP is consistent with that cited in the literature by Ojha in 2016 where the phytocannabinoid was studied in a model of rotenone-induced parkinsonism. They observed that BCP promotes immunofluorescence inhibition of astrocytes in the striatum region in mice that exhibited rotenone-induced damage.7 Likewise, in the study carried out by Mendes in 2019, they found more significant expression of GFAP in the group injured by MPTP than the control group. Reactive astrocytes undergo a characteristic morphological change known as astrogliosis due to a hypertrophic state, where they adopt neurotoxic functions and loss of neurotrophic functionality, they also lose many functions of antioxidant pathways and release a variety of chemokines and cytokines such as TNF-α, IL-6, IL-1β, and INFγ, subsequently producing NO through the synthesis of iNOS, molecules that alter neuronal morphology and cause the dopaminergic cells death.33,34 The said molecules may also be responsible for cell apoptosis of endothelial cells, enhancing BBB disruption, in addition to neutrophil and monocyte transmigrations.35 Therefore, the primary underlying mechanism of CB2R activation is attenuation of oxidative/nitrosative stress and neuroinflammation, inhibition of gliosis and release of pro-inflammatory cytokines, and decrease of nigrostriatal degeneration.7 Another protective effect of astrocytes in an anti-inflammatory state by activating CB2R is to allow them to avidly take up extracellular glutamate and mitigate the damaging effects of subthalamic excitotoxic input to the NS.36 In the same way, CB2R agonism can regulate the activity of astrocytes, which can maintain the characteristics of endothelial cells through the synthesis of various factor.
BBB and tight junction proteinsOur results show an increase in the amount of NaF in the group treated with MPTP; simultaneously, this group presents a decrease in the tight junction proteins ZO-1 and occludin. There is evidence described in the literature showing that BBB disruption is probably the result of multiple processes, including endothelial cell death, GAP junction disruption, and decreased coupling of GAP proteins to the cytoskeleton, which agrees with the results obtained.23
This is supported by studies conducted by Chung in 2016, which showed a statistically significant increase in FITC-LA in the MPTP-treated group compared to the control group.28 Likewise, in work carried out by Zhao in 2007, they report that in the group treated with MPTP, there is evidence of a high infiltration of FITC-LA in the STR compared to the control group.37 Similarly, Chen in 2008, found that ZO-1 and occludin proteins in STR are significantly decreased in the MPTP-treated group compared to the control group.12 As in work carried out by Choi in the year 2018, where the evaluation of the ZO-1 protein in the group treated with MPTP is found to be decreased in a statistically significant way compared to the control group.38
The agonism of CB2R by β-caryophyllene significantly decreases the permeability of the BBB, possibly through the following mechanism: activation of CB2R increases the presence of TJ proteins in the endothelium by increasing the expression of ZO-1, occludin, and claudin, which reduces BBB permeability, also contributes to CB2R agonism has anti-inflammatory effects.2,9,39,40
Oxidative stress and Nrf2The cause of dopaminergic neuron death remains uncertain; however, this death may be mainly due to oxidative stress, hence the excessive generation of lipid peroxidation. MDA is one of the primary end products of lipid peroxidation; therefore, the formation of this molecule is a marker for the accumulation of lipid peroxidation, although MDA is known to arise due to cell death. It has been reported that MPTP through its active form (MPP+) can exert neuronal death by interacting free radicals with polyunsaturated lipids that induce lipid peroxidation. Likewise, an increase in MDA has been reported in tissues from PD patients.41,42
Activation of CB2R by BCP can generate intracellular pathways mediated by kinases such as the PI3K/Akt pathway, which can phosphorylate other proteins, including Nrf2, which remains inhibited by the keap1 protein. Once Nrf2 is phosphorylated, it induces its release from keap1 and its translocation to the nucleus, where it can bind Maf and in turn binds the antioxidant response element (ARE) and encode multiple phase II enzymes, including superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST), heme oxygenase-1 (HO-1), catalase, NQO1, among others; thus favoring an antioxidant environment against MPTP-induced damage. Thus, an increase in the activity of the enzyme NQO1 has been reported and its expression is expression is mediated by Nrf2 and can decrease the concentration of ROS, consequently avoiding the oxidation of the lipid membrane and the damage of the genetic material of the cells, thus protecting to dopaminergic cells from oxidative stress damage.29 These previous results are consistent with the levels of MDA observed in the present study, BCP treatment diminished these levels probably through the Nrf2-NQO1 signaling cited or by is naturally occurring antioxidant activity.
Peroxisome proliferator-activated receptor γ PPAR-γActivation of CB2R by β-caryophyllene results in an increase in phosphorylated cAMP response element-binding protein (pCREB). This stimulates sirtuin 1 (SIRT1) deacetylase, which in turn, by increasing PGC-1α activity, is a likely activator of PPAR-γ in the absence of its ligands (ligand-independent activation) and is an essential coactivator of PPAR-γ. This may represent a new explanation for how β-caryophyllene (an CB2R agonist) activates PPAR-γ.43 In addition, this receptor has therapeutic potential because it has anti-inflammatory, anti-apoptotic, and antioxidant activities. When it is activated, it is a mediator of Sirt3, which generates an overexpression of this protein, which belongs to the sirtuin family and plays a significant role in several cellular processes, as well as, in neurological diseases. It is a regulator in cell protection, which contributes to neurovascular recovery, its overexpression causes a decrease in BBB permeability by inhibiting p38 phosphorylation and promotes expression of TJ proteins such as claudins, occludins, and ZO-1. Sirt3 has also been shown to be a proliferative and anti-apoptotic regulatory factor in endothelial cells.44
β-Caryophyllene has an essential cytoprotective role because it has also been seen to increase the expression of PPAR-γ and activate their respective signaling pathways. Previous studies have indicated a robust correlation between PPAR-γ and Nrf2 since an increase in Nrf2 generates an increase in PPAR-γ expression, and nuclear translocalization of PPAR-γ can increase Nrf2 expression. The effect of β-caryophyllene on these pathways is after the activation of CB2R and the PPAR-γ receptor.45–47 In addition, several studies have shown that β-caryophyllene has dual activity at PPAR-γ and CB2R.43 Several studies have shown that the PPAR-γ receptor is a promising therapeutic target in animal models of stroke, traumatic brain injury, Alzheimer's, and PD since it maintains the BBB's functionality and reduces neuroinflammation and oxidative stress, and neuronal damage. Activation of the PPAR-γ receptor inhibits pro-inflammatory cytokines such as TNF-α and nuclear factor kappa-B (NF-κB), decreasing the inflammatory response and restoring TJ protein levels. NF-κB activation also plays a significant role. PPAR-γ activation also increases brain-derived neurotrophic factor (BDNF) expression which is important in the regulation of TJ proteins.48
ConclusionsThe effects of BCP administration were able to inhibit the permeability of the BBB generated by the MPTP model in STR. This effect was probably mediated by the modulation of the expression of the TJ proteins as well as downregulation of oxidative stress. The results of this work allow us to postulate further evaluation of BCP as a BBB permeability inhibitor given the different effects that its administration presents.
FundingThis work was supported by Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCyT) project (#281452) granted to Dr. M.E. Flores-Soto and Scholarship (#759132) granted to A.R. Ramos-Molina.
Conflicts of interestThe authors declare that there is no conflict of interest.
We would like to thank to Instituto Mexicano del Seguro Social and Universidad de Guadalajara for providing facilities and institutional help.