Cerebrotendinous xanthomatosis (CTX) is a rare autosomal-recessive lipid storage disease caused by mutations in the CYP27A1 gene. Clinical manifestations of CTX are so broad and diverse that diagnostic delay or under-diagnosis happened frequently, especially in the early disease phase.
We present the case of a 31-year-old Chinese woman presented with gait disturbance for more than 1 year. She developed recurrent diarrhea since birth and suffered from frequent coughing and fever since the age of 4. She had learning difficulties and was poor at sports throughout childhood. At 10 she developed progressive vision loss, and had operation due to bilateral cataracts 6 years later. Cholecystectomy was performed for cholecystitis with cholecystolithiasis at the age of 27. Recently, she was found short-tempered and difficult to communicate.
Her physical examination demonstrated bilateral ptosis, decreased myodynamia of lower limbs (grade 4), ataxia, knee and ankle hyperreflexia, positive ankle clonus and positive bilateral pathological signs. The protuberance on her achilles tendons are visible and palpable on both sides. Her Mini-Mental State Examination score was 24.
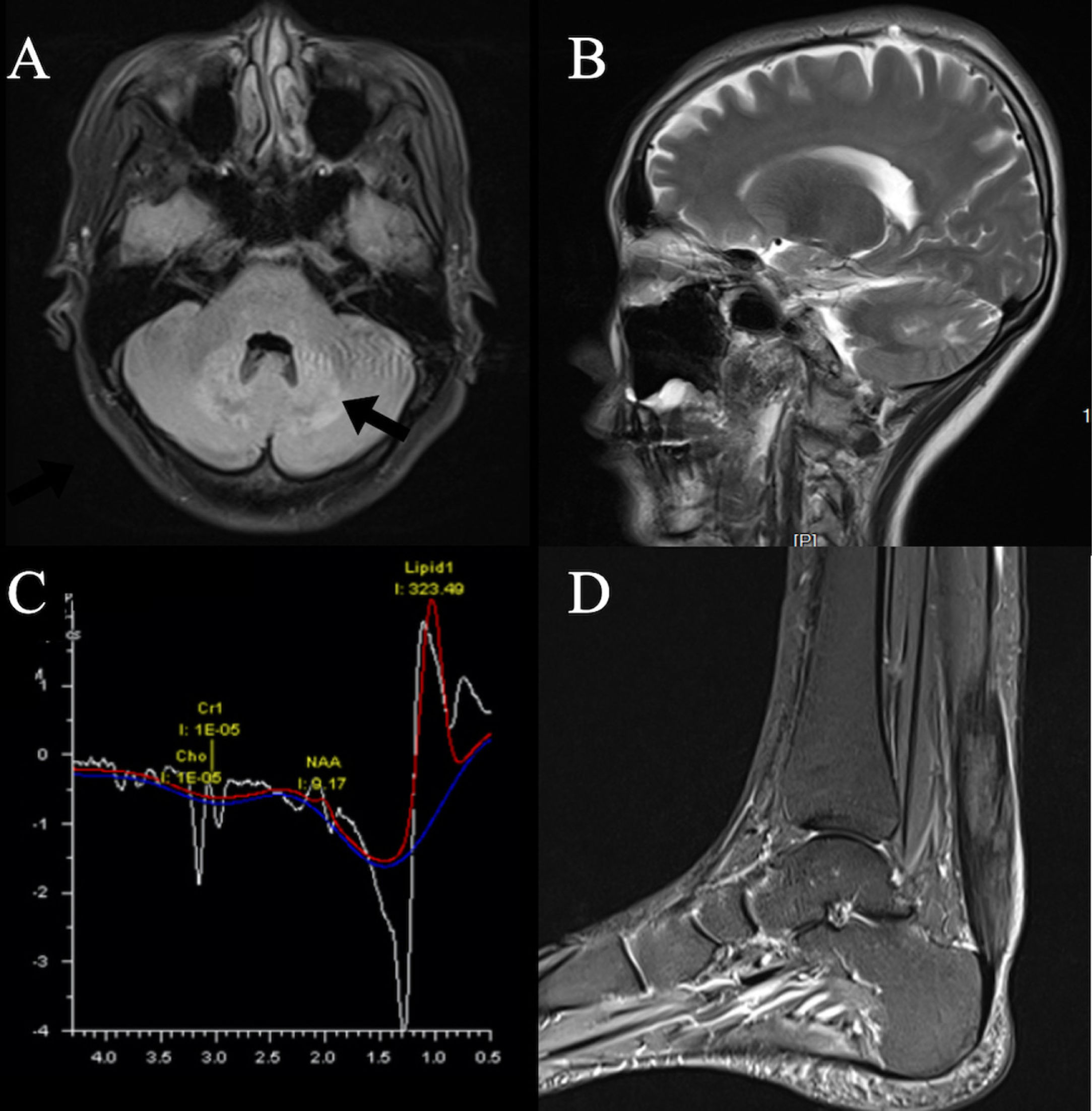
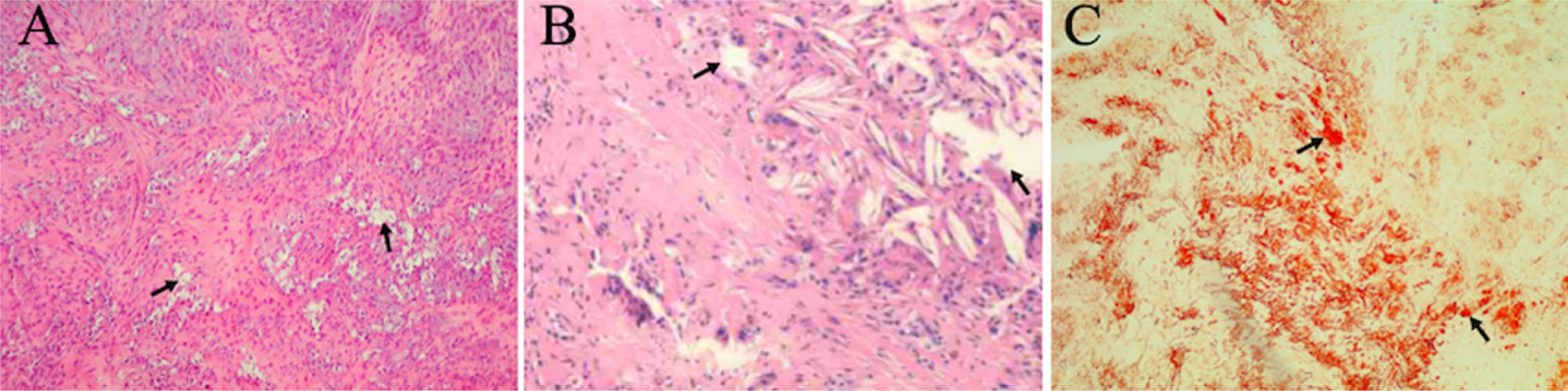
Our patient's serum lipid level was normal while both chenodeoxycholic acid (CDCA) and ursodeoxycholic acid (UDCA) were decreased. The brain MRI revealed hyper-intensity lesions on bilateral cerebellar dentate nucleus and deep medulla (Fig. 1A). MRS showed that NAA and Cho peak decreased, and lipid peak and lactate peak increased at bilateral cerebellar dentate nucleus (Fig. 1C). PD-weighted images of left ankle displayed thickening of the left Achilles tendon with abnormal lesions (Fig. 1D). Foam-like cellular tissue with scattered lymphocyte infiltration was observed after a biopsy of the left Achilles tendon (Fig. 2). Sanger sequencing showed two heterozygous mutations in the CYP27A1 gene. According to ACMG guidelines, c.379C>T can be graded as pathogenic variants, while c.1263+3G>C can be graded as variants of uncertain significance (VUS).
Neuroimaging features. Axial (A) and Sagittal (B) T2 Flair images display bilateral hyperintensity of the dentate nucle (black arrow). MRS (C) revealed increased lipid and lactate peaks and decreased choline and N-acetyl aspartate peaks in bilateral cerebellar dentate nucleus. Left ankle MRI PD-weighted images (D) display uneven hyperintensity of the left Achilles tendon.
Treatment was initiated since April 2021 with UDCA supplementary treatment and lipid-lowering treatment. At the most recent follow-up, the patient became calmer and her ptosis disappeared due to a possible neural functional recovery, while her gait remained the same as before.
Decreased activity of the mitochondrial enzyme 27-sterol-hydroxylase encoding by the CYP27A1 gene leads to impaired bile acid synthesis, resulting in reduced production of cholic acid and bile acids (especially CDCA).1,2 Consequently, increased cholesterol metabolites, such as cholestanol, accumulate mainly in the brain, lenses, and tendons.3 Our patient's serum lipid level is within normal limits; however, she still had early signs of premature atherosclerosis and tendon xanthomas. The reduction in the ability of reverse cholesterol transport and the anti-atherosclerotic function of extrahepatic sterol 27-hydroxylase may explain the contradiction.4,5
At present, CYP27A1 is the only gene known to be associated with CTX. In this case, the paternal mutation c.379C>T is a known pathogenic mutation, while the maternal mutation is c.1263+3G>C, which has not been reported yet, and the clinical significance of the variant remains undefined.
Chronic unexplained infantile diarrhea and juvenile cataracts are considered as an important cue for early diagnosis, which often precede the appearance of tendon xanthomas and neurological symptoms.6 Lens nuclei from CTX patients had a greater cholestanol content compared with the senile lens nuclei used as a control.7 Recurrent respiratory tract infections since the patient's preschool period was unequivocal, which has not been considered as an early symptom of CTX yet.
During the second or third decade of life, xanthomas usually start to appear on the Achilles tendon.8,9 Xanthoma is a characteristic feature but not essential for CTX diagnosis.10 However, the diagnosis of CTX will not be considered by current doctors until obvious tendon xanthoma is noticed.
The most common neuropsychiatric symptoms of CTX are mental retardation, pyramidal and cerebellar symptoms. Learning difficulties and poor athletic ability are often neglected. Behavioral and personality changes are easily concealed by adolescent rebellion. Therefore, it is particularly important to take developmental delays, mental retardation, learning difficulties, behavioral disorders, and mood disorders beginning in childhood or adolescence into consideration for early diagnosis of CTX.
At present, CTX is considered to be a treatable metabolic disease. The long-term efficacy of CDCA treatment was demonstrated in a study in 1984.11 There have been continuous doubts about it recently, especially for patients with existing significant neurological symptoms. Although the cholesterol level returns to normal after the use of CDCA treatment, the deterioration of the disease is inevitable.12 Long-term follow-up studies have shown that the age at which patients were diagnosed and started CDCA treatment is closely related to their prognosis, and early diagnosis and treatment are essential for CTX.
With this case of CTX where a new CYP27A1 mutation was found, we aim to provide more clues for clinicians in diagnosis and treatment, and help to expand the phenotypic and genotypic spectrum of this rare disease. In addition to neurologist, pediatricians and ophthalmologists as the first contact doctors should raise their awareness to CTX as well.
Authors’ contributionsAll authors contributed significantly to the creation of this manuscript; each fulfilled criteria as established by the ICMJE.
Consent-to-disclosePatient Consent-to-disclose Form was signed by the patient after fully understanding.
FundingF.Y. Qian reports that this study was supported by the National Natural Science Foundation of China (82001336) and Nanjing Municipal Health Science and Technology Development Special Fund Project (YKK19161).
Y.J. Guo reports that this study was supported by the National Natural Science Foundation of China (81471187).
Conflict of interestThe authors declare that they have no conflict of interest.