Since the beginning of the COVID-19 pandemic, a wide range of manifestations, particularly respiratory symptoms, have been associated with SARS-CoV-2 infection. However, due to the neurotropic and neuroinvasive potential of the virus, cases have also been observed of such neurological symptoms as anosmia, dysgeusia, headache, cerebrovascular accidents, seizures, Guillain-Barré syndrome, and encephalitis.1–3
We present the case of a patient with posterior reversible encephalopathy syndrome (PRES) associated with SARS-CoV-2 infection.
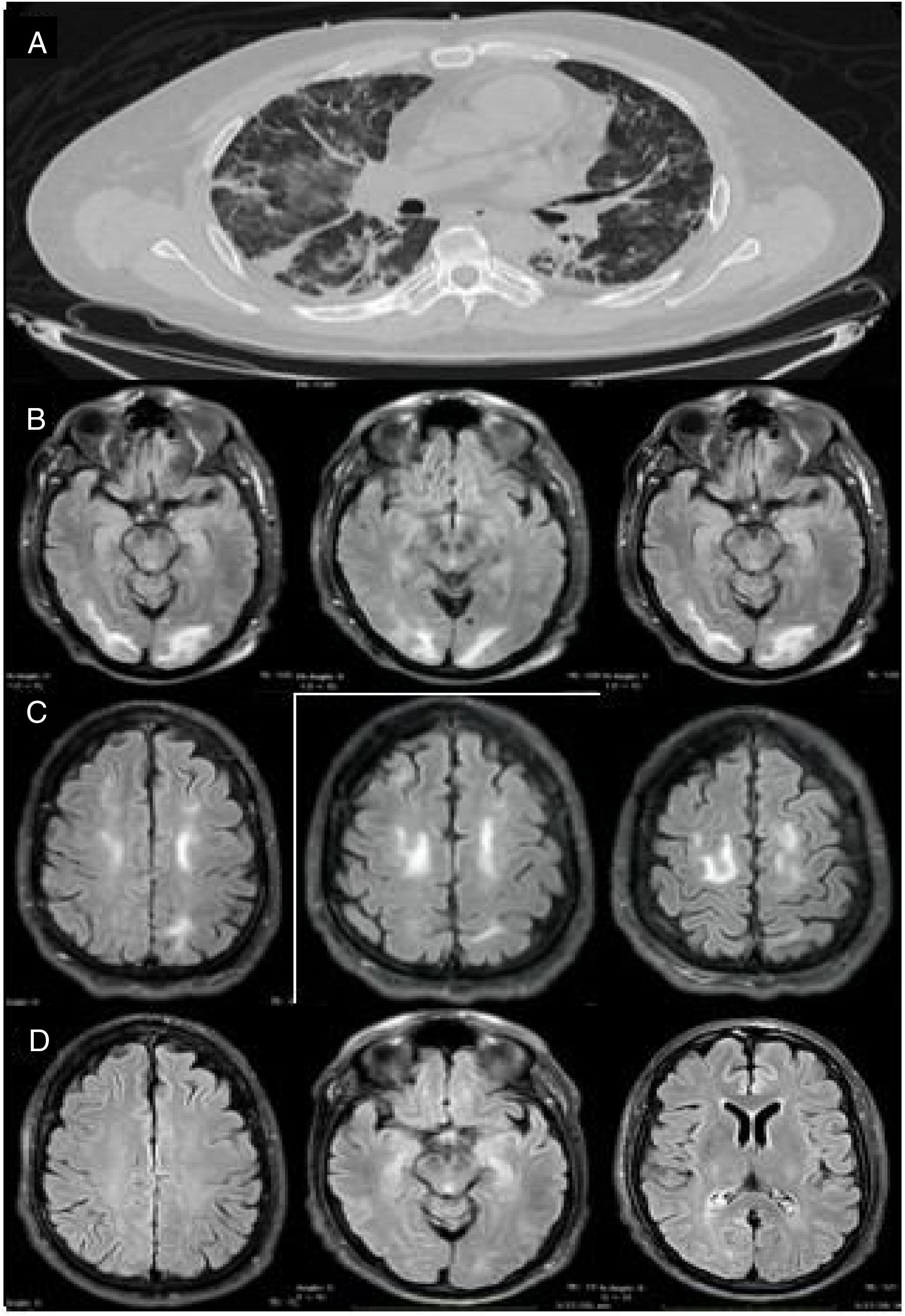
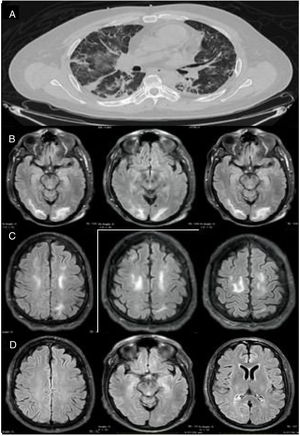
Our patient was a 46-year-old man (clinical characteristics and medical history are shown in Table 1) who visited the emergency department with a 4-day history of muscle pain, cough, hyposmia, dysgeusia, and fever. The initial examination revealed dyspnoea, fever (39.0°C), arterial blood pressure of 130/70 mm Hg, and oxygen saturation of 85%. Chest CT revealed multiple subpleural and peribronchovascular areas of ground-glass opacification, predominantly in the inferior lobes, as well as subpleural consolidation (Fig. 1A). Reverse transcription polymerase chain reaction (RT-PCR) of nasopharyngeal exudate returned positive results for SARS-CoV-2. We started treatment with hydroxychloroquine, azithromycin, and cefuroxime. Three days later, the patient presented exacerbation of dyspnoea, with oxygen saturation of 60%, and required invasive mechanical ventilation (IMV) and admission to the intensive care unit. From admission, he presented poorly controlled blood pressure imbalance, with a mean arterial pressure (MAP) of 120-130 mm Hg. He was extubated 9 days later (22 days after symptom onset), and presented disorientation, psychomotor agitation, and disconnection from his surroundings. A contrast-enhanced MRI scan revealed bilateral subcortical and white matter hyperintensities in the occipital and frontal lobes on T2-weighted and FLAIR sequences; the lesions were hypointense on T1-weighted sequences and showed no diffusion restriction or contrast uptake (Fig. 1 B and C). An EEG study revealed no alterations. However, 48 hours after extubation, the patient required orotracheal intubation; IVM was maintained for a further 7 days. After the second extubation, the patient continued to present disorientation, which improved progressively. He reported no visual impairment, and presented quadriparesis predominantly affecting the lower limbs; quadriparesis improved and the patient was able to walk with assistance at discharge. He was discharged 34 days later, presenting no alterations in cognitive function; lower limb weakness persisted. A follow-up MRI scan performed 15 days after the initial scan revealed resolution of the hyperintense lesions in the frontal and occipital lobes; these findings are compatible with PRES (Fig. 1D).
Characteristics and clinical progression of our patient.
| Variable | |
|---|---|
| Age (years) | 46 |
| Anthropometric data | Weight: 86 kg, height: 163 cm, BMI: 32.40 kg/m2 |
| Vital signs at admission | HR: 115 bpm, RR: 24 bpm, BP: 130/70, temp.: 39°C, O2 sat.: 85% |
| Family history | Mother: SAH and DM2 |
| Medical history | DM2 (since 2004), SAH (since 2019) |
| Toxic substance use | Alcohol (once per month), tobacco (social smoker, once per week) |
| Long-term treatment before admission | Insulin (insulin glargine + lixisenatide) 22 IU/day, furosemide 40 mg/24 h, amlodipine 5 mg/24 h, pravastatin 20 mg/24 h, linagliptin/metformin 2.5/850 mg/8 h |
| Time from symptom onset to PRES onset (days) | 22 |
| MAP (range) | 69-130 mm Hg |
| Laboratory analyses | Admission | Critical phase | Discharge |
|---|---|---|---|
| Leukocyte count (cells/mm3) | 9.10 | 18.8 | 9.3 |
| Platelet count (count/mm3) | 207 000 | 657 000 | 203 000 |
| Creatinine (mg/dL) | 2.50 | 3.71 | 1.09 |
| CRP (mg/dL) | 20.56 | 20.56 | 1.13 |
| ESR (mm/h) | 101 | 104 | 22 |
| Ferritin (ng/mL) | 435.8 | 1581 | 643 |
| D-dimer (ng/mL) | 500.54 | 6161 | 651 |
BMI: body mass index; BP: blood pressure; CRP: C-reactive protein; DM2: diabetes mellitus type 2; ESR: erythrocyte sedimentation rate; HR: heart rate; MAP: mean arterial pressure; O2 sat.: oxygen saturation; PRES: posterior reversible encephalopathy syndrome; RR: respiratory rate; SAH: systemic arterial hypertension; temp.: body temperature.
Imaging studies of our patient. (A) Chest CT (axial plane) showing multiple areas of ground-glass opacification in both lungs. (B) Brain MRI scan (axial plane) showing subcortical and white matter hyperintensities in the occipital lobes. (C) T2-weighted FLAIR brain MRI scan (axial plane) showing white matter hyperintensities in both frontal lobes. (D) Follow-up MRI scan performed 15 days later, revealing complete disappearance of hyperintensities in the frontal and occipital lobes.
PRES is a neurological disorder characterised by epileptic seizures, altered level of consciousness, visual alterations, and headache, associated with typical neuroimaging signs of reversible subcortical vasogenic oedema mainly involving parietal and occipital regions. The syndrome may present with blood pressure imbalances, kidney disease, and neoplasia, and its pathogenesis may involve different factors, including hypertensive encephalopathy, infectious diseases (mainly viral infections), sepsis or septic shock, and treatment with antiviral drugs or immunosuppressants.4
Arterial hypertension, acute kidney disease with or without renal replacement therapy, and severe respiratory infection constitute the common denominator of all published cases of PRES associated with SARS-CoV-2 infection.5–7 Although PRES has classically been associated with endothelial dysfunction secondary to ischaemia, the exact pathophysiological mechanisms are still to be determined, particularly in the context of SARS-CoV-2 infection. Several hypotheses support direct involvement of the underlying infection. It has even been hypothesised that the drugs used to treat the infection, such as hydroxychloroquine (which was used in most published cases), play a direct role in the pathogenesis of PRES, although it is unclear whether this effect constitutes an independent factor for the development of PRES or simply an incidental observation in patients with COVID-19.5 Inflammatory cytokines play a major role in the immune response to viral infection, with SARS-CoV-2 infection being no exception. In vitro studies have shown release of proinflammatory cytokines and chemokines (mainly IL-1β, IL-6, IL-4, IL-10, and tumour necrosis factor) in dendritic cells and epithelial cells of the respiratory tract8; furthermore, increased levels of vascular endothelial growth factor, together with tissue hypoperfusion, cause endothelial damage and increase vascular permeability. This results in a “cytokine storm,” one of the most widely accepted theories today, which constitutes the therapeutic target for treatments against COVID-19. It is yet to be determined whether SARS-CoV-2 infection is an independent factor in the appearance of PRES.
Further research into this topic will provide more homogeneous data on risk factors, progression, and treatment, and help to determine the pathophysiology of SARS-CoV-2 infection and to establish prognostic biomarkers for early detection of the disease.
Please cite this article as: Ordoñez-Boschetti L, Torres-Romero CM, Ortiz De Leo MJ. Síndrome de encefalopatía posterior reversible (PRES) asociado a SARS-CoV-2. Reporte de caso. Neurología. 2020;35:696–698.