Early diagnosis of Alzheimer disease (AD) through the use of biomarkers could assist in the implementation and monitoring of early therapeutic interventions, and has the potential to significantly modify the course of the disease.
DevelopmentThe classic cerebrospinal fluid and approved structural and functional neuroimaging biomarkers are of limited clinical application given their invasive nature and/or high cost. The identification of more accessible and less costly biomarkers, such as blood biomarkers, would increase their use in clinical practice. We review the available published evidence on the main blood biochemical biomarkers potentially useful for diagnosing AD.
ConclusionsBlood biomarkers are more cost- and time-effective than CSF biomarkers. However, immediate applicability in clinical practice is relatively unlikely. The main limitations come from the difficulty of measuring and standardising thresholds between different laboratories and the failure to replicate results. Of all the molecules studied, apoptosis and neurodegeneration biomarkers and the biomarker panels obtained through “omics” approaches, such as isolated or combined metabolomics, offer the most promising results.
El diagnóstico precoz de la enfermedad de Alzheimer mediante la utilización de biomarcadores podría facilitar la instauración y monitorización de intervenciones terapéuticas tempranas con potencial capacidad para modificar significativamente el curso de la enfermedad.
DesarrolloLos biomarcadores clásicos de líquido cefalorraquídeo y de neuroimagen estructural y funcional aprobados tienen una aplicación clínica limitada, dado su carácter invasivo o su elevado coste. La identificación de biomarcadores más accesibles y menos costosos, como los sanguíneos, facilitaría su aplicación en la práctica clínica. Se presenta una revisión bibliográfica de los principales biomarcadores bioquímicos sanguíneos con potencial utilidad para el diagnóstico de la enfermedad de Alzheimer.
ConclusionesLos biomarcadores sanguíneos son coste y tiempo efectivos con respecto a los marcadores de líquido cefalorraquídeo. Sin embargo, la aplicabilidad inmediata de los biomarcadores bioquímicos sanguíneos en la práctica clínica es poco esperable. Las principales limitaciones estriban en la dificultad para la medición y estandarización de los umbrales entre los diferentes laboratorios y en los fallos de replicación de resultados. Entre todas las moléculas estudiadas, los biomarcadores de apoptosis y neurodegeneración, al igual que los paneles de biomarcadores obtenidos mediante aproximaciones ómicas – como la metabolómica de forma aislada o combinada – ofrecen los resultados más prometedores.
Alzheimer disease (AD) is the most frequent neurodegenerative disease, causing 50%–70% of all cases of dementia1–7; the disease is expected to affect up to 115 million people by 2050.8–10 Dementia is currently the third leading cause of death, after cardiovascular diseases and cancer.3
Despite its great impact, the pathogenesis of AD largely remains unknown. The most widely accepted explanation is the amyloid cascade hypothesis,10–13 although disorders affecting calcium, cholesterol, and glucose homeostasis have been described as contributing factors in AD pathogenesis.4 Furthermore, micro- and macrovascular disease may contribute to the development of amyloidosis and neurodegeneration, preceding the development of dementia associated with AD.11
In addition to this, neurodegenerative processes are thought to start up to 20–30 years before symptom onset.3,14 Early detection of AD in presymptomatic stages would represent a great opportunity, enabling therapeutic intervention earlier and with a greater chance of success, since interventions would be performed before synaptic damage and neuronal loss are extended. Therefore, it would be highly beneficial to include new, more accessible, and less costly biomarkers in clinical practice.15
In this study, we present a literature review of the main blood biomarkers with potential usefulness for the diagnosis of AD in clinical practice.
Usefulness and limitations of blood biomarkers in Alzheimer diseaseThe main limitation of the classic AD biomakers (core CSF biomarkers: amyloid beta [Aß], total tau [t-tau] and phosphorylated tau protein [p-tau], and PET imaging of glucose metabolism and amyloid deposition4,16–23) is the invasiveness and high cost of tests, which hinders their application in clinical practice.24
Blood is more accessible and may be used as a source of biomarkers for AD screening and diagnosis. Furthermore, blood biomarkers are more cost- and time-effective than CSF markers.2,18,25,26 Biomarkers should be associated with specific characteristics of the disease and present high levels of sensitivity and specificity for AD, and tests should be repeatable, non-invasive, simple, and inexpensive.9 Given these considerations, blood biomarkers may identify patients at risk of developing AD, progression from mild cognitive impairment (MCI) to AD, and rapid progression of clinically established AD.8
One difficulty of identifying blood biomarkers in AD resides in the fact that this is a slowly progressing disease, and that the degree of loss of integrity of the blood-brain barrier is unknown. However, researchers have described blood-brain barrier dysfunction in patients with AD, which would result in the exchange of proteins and other molecules between the CSF and the blood.16
Another limitation is that blood is a complex fluid with multiple confounding variables; standardised protocols are needed to prepare the sample and analyse it.17,25 The differences reported in specific analyte concentrations may be due to the lack of standardised calibration methods, different dilutions of biological samples, variability in the antibodies used, and the differences in instrument sensitivity and reliability.2,25
The different approaches to identifying blood biomarkers in AD may be classified as those that aim to identify specific molecules (candidates) associated with known pathogenic mechanisms, and those that use omics methods to analyse a serum or plasma profile of molecules without bias.25
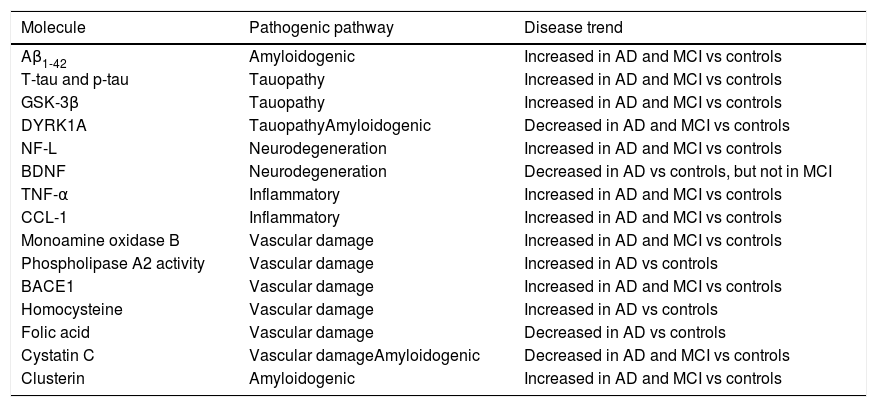
Identification of blood biomarkers of Alzheimer disease using a candidate molecule approachAmyloid beta peptidesBased on the hypothesis that amyloidogenesis plays a central role in AD pathogenesis,9 attempts have been made to identify markers of amyloidosis in the peripheral blood (Table 1).
List of biomarkers identified with candidate molecule studies with possible diagnostic use in AD.
| Molecule | Pathogenic pathway | Disease trend |
|---|---|---|
| Aβ1-42 | Amyloidogenic | Increased in AD and MCI vs controls |
| T-tau and p-tau | Tauopathy | Increased in AD and MCI vs controls |
| GSK-3β | Tauopathy | Increased in AD and MCI vs controls |
| DYRK1A | TauopathyAmyloidogenic | Decreased in AD and MCI vs controls |
| NF-L | Neurodegeneration | Increased in AD and MCI vs controls |
| BDNF | Neurodegeneration | Decreased in AD vs controls, but not in MCI |
| TNF-α | Inflammatory | Increased in AD and MCI vs controls |
| CCL-1 | Inflammatory | Increased in AD and MCI vs controls |
| Monoamine oxidase B | Vascular damage | Increased in AD and MCI vs controls |
| Phospholipase A2 activity | Vascular damage | Increased in AD vs controls |
| BACE1 | Vascular damage | Increased in AD and MCI vs controls |
| Homocysteine | Vascular damage | Increased in AD vs controls |
| Folic acid | Vascular damage | Decreased in AD vs controls |
| Cystatin C | Vascular damageAmyloidogenic | Decreased in AD and MCI vs controls |
| Clusterin | Amyloidogenic | Increased in AD and MCI vs controls |
Aβ1-42: 42-residue isoform of beta amyloid protein; AD: Alzheimer disease; BACE1: β-secretase 1; BDNF: brain-derived neurotrophic factor; CCL-1: chemokine C-C motif ligand 1; DYRK1A: dual specificity tyrosine phosphorylation regulated kinase 1A; GSK-3β: glycogen synthase kinase 3β;MCI: mild cognitive impairment; NF-L: neurofilament light peptide; TNF- α: tumour necrosis factor α.
The Aβ42 isoform, the main component of senile plaques,1 is a product of amyloid precursor protein degradation and may damage DNA via oxidative stress mechanisms. Circulating Aβ originates in both peripheral and central sources. Several biochemical, technical, clinical, demographic, and genetic factors have been observed to affect Aβ levels. For example, given its hydrophobic nature, circulating Aβ binds to plasma proteins and to the walls of the test tubes, causing epitope masking and analytical interference.16
There is a weak correlation between the levels of Aβ in the blood and in the CSF.1 However, serum Aβ1-42 levels are reported to be higher in patients with AD than in controls; this effect is more pronounced in patients with familial AD or associated with Down syndrome.3,17 High plasma Aβ42 levels, low levels of Aβ40, and a reduced Aβ42/Aβ40 ratio in elderly patients may indicate progression from normal cognition to MCI or AD.3
However, due to the involvement of numerous factors, the inability to reproduce many results, and their controversy, it is not currently possible to clearly establish the role of Aβ as a plasma biomarker.3,25
Tau protein and enzymes related to its phosphorylationPhosphorylation of tau protein at different residues regulates the protein’s capacity to form oligomers and aggregates,3,9 which contribute to the formation of neurofibrillary tangles.
Methods based on the ELISA immunoassay technique are not sufficiently sensitive to detect plasma tau protein at low concentrations,1,2 but a new, ultrasensitive immunoanalysis technique does offer this possibility. Studies using this technique report higher tau protein levels in patients with AD than in patients with MCI and controls. The correlation between plasma and CSF tau protein concentrations is negligible.27
The detection of tau protein in the plasma has been associated with greater longitudinal loss of hippocampal volume and cortical thickness in regions specifically affected by AD, such as the entorhinal cortex, the inferomedial temporal lobe, the fusiform gyrus, and the precuneus.26,28,29
T-tau and p-tau protein levels are thought to be correlated with neuropsychological test performance and may discriminate between patients with AD and those with MCI, and between the latter and controls.1,4,8,25,27 However, the large overlap between control and patient values limits its usefulness as a biomarker.25,26
Plasma tau protein may be a non-specific marker of neurodegeneration, as its levels are increased in patients with ischaemic stroke, head trauma, and prion disease.16 However, the association described between plasma tau levels and atrophy in regions specifically affected by AD supports its potential use as a screening marker for early AD.17
Some protein kinases, including glycogen synthase kinase 3β (GSK-3β), have been linked to tau protein hyperphosphorylation.3 Plasma GSK-3β levels are significantly increased in patients with AD and MCI as compared with controls of the same age, which makes the protein a potential biomarker.17
Dual specificity tyrosine-phosphorylation regulated kinase A (DYRK1A) is also involved in tau hyperphosphorylation. This enzyme is associated with amyloidosis and tauopathy, as DYRK1A levels are regulated by Aβ levels. Blood DYRK1A levels are significantly lower in patients with AD than in controls, even in early stages of the disease,9 which makes it a potential biomarker for early diagnosis. DYRK1A has also been associated with dysregulation of neurotrophic pathways, especially that of the brain-derived neurotrophic factor, which has multiple functions in synaptic plasticity and neuronal survival; blood DYRK1A levels are decreased in moderate and advanced stages of AD.9,30
Neurofilament light polypeptidePatients with AD present high CSF concentrations of neurofilament light polypeptide (NF-L), a marker of neuronal damage.26 There is an excellent correlation between plasma and CSF levels of this protein.1,17 Plasma NF-L levels are increased in Aβ-positive patients with AD and MCI and are associated with the degree of cognitive impairment (scores on the Mini–Mental State Examination and the Trail Making Test, part B) and with the neuroimaging alterations observed during diagnosis and progression of AD. However, increased plasma NF-L levels are not specific to AD: they are also observed in other neurodegenerative diseases, and are therefore considered a marker of neurodegeneration.26
Biomarkers based on the inflammatory hypothesis of Alzheimer diseaseAccording to the inflammatory hypothesis, neuroinflammation is not the consequence of neurodegeneration, but a pathogenic factor in the initial stages and progression of the disease, perpetuating neuronal damage, with the activation of microglia and peripheral T cells and involvement of the innate immune system.10 Tau deposition activates microglia and astrocytes. Furthermore, insoluble forms of Aβ act on toll-like receptors, whereas soluble forms are phagocytosed by microglia, activating mitogen-activated protein kinases and stimulating the production of proinflammatory genes and cytokines, perpetuating inflammation. Cytokines, in turn, attract cells from the peripheral immune system and affect the permeability of the blood-brain barrier.
CSF and blood inflammatory mediators, particularly cytokines and chemokines, may be used as biomarkers for the early diagnosis of AD. Among the cytokines that mediate the immune response in the brain of patients with AD, interleukins (IL-1, IL-4, IL-6, IL-10), interferon-γ, and tumour necrosis factor α (TNF-α) are of particular significance.4,22,30
Cytokine I-309 is a glycoprotein secreted by activated T-cells. Its main function is to attract immature B-cells, monocytes, natural killer cells, and dendritic cells with CCR8 receptors. It has been suggested as a possible predictor of progression from MCI to AD.25
AutoantibodiesThe presence of autoantibodies in AD is well established, although their pathogenic role is less clear. Their application as a possible biomarker is of great interest, given their presence in blood and CSF.
Research into anti-Aβ antibodies has not determined their clinical usefulness; the use of blood autoantibody profiling has been proposed, with promising results.16,31,32
There is evidence that levels of certain redox-reactive antiphospholipid autoantibodies are decreased in the CSF of patients with AD, but are not clearly reduced in blood. Levels of these antibodies initially increase in MCI, and subsequently decrease as disease progresses. Therefore, they may constitute a marker of the stage of AD, but not a diagnostic marker.13
Apoptosis biomarkersClusterin is associated with neurodegenerative processes, and is detected at higher concentrations in the blood of patients with AD. Clusterin levels are correlated with the amyloid deposition observed on PET images and with the degree of hippocampal atrophy. Clusterin is thought to act as a chaperone for extracellular proteins, including Aβ; its sequestering action decreases Aβ toxicity.25,33
Platelet biomarkersIncreased β-secretase-1 activity, elevated expression of monoamine oxidase B, and phospholipase A2 activity have been observed in the platelets and brains of patients with AD.3,17
Biomarkers of microvascular damageVascular risk factors have classically been considered to contribute to increased risk of AD. Increased blood levels of atrial natriuretic peptide and adrenomedullin have been detected from prodromal stages. Furthermore, increased adrenomedullin levels are reported in the brains of patients with AD,34 confirming this protein’s role as a potential biomarker associated with the pathogenesis of the disease. In contrast, no differences have been identified in the expression of cell adhesion molecules (VCAM-1 and ICAM-1) or selectins.35
Plasma levels of homocysteine seem to be directly related to Aβ42 levels. Hyperhomocysteinaemia reduces neurogenesis through a mechanism involving the fibroblast growth factor. Moderately high levels of homocysteine are a risk factor for vascular dementia and AD; a significant increase in plasma homocysteine levels has been observed in patients with AD.9
Furthermore, an association has been described between AD and low plasma levels of folic acid. Folic acid is essential for homocysteine metabolism. In AD, DNA repair is inhibited by the oxidative damage induced by Aβ, together with folate deficiency. Folic acid regulates DNA methyltransferase, attenuating production of Aβ. DNA methyltransferase activity is correlated with short-term memory formation and the maintenance of long-term memory. It has been proposed that the combination of folate, haemoglobin, and APOE levels presents greater predictive sensitivity than folate level alone as a diagnostic biomarker of AD. Haemoglobin has been shown to bind to Aβ and to favour its aggregation; therefore, high homocysteine concentration may be considered a risk factor for AD.12
Cystatin C is an endogenous inhibitor of cysteine produced by almost all human cells and available in practically all bodily fluids; it is also considered a potential marker of vascular damage. Cystatin C prevents Aβ aggregation and deposition in a concentration-dependent manner by binding to amyloid precursor protein and to Aβ40 and Aβ42 peptides.36 Decreased serum and CSF cystatin C levels are observed in patients with AD, from the initial stages.
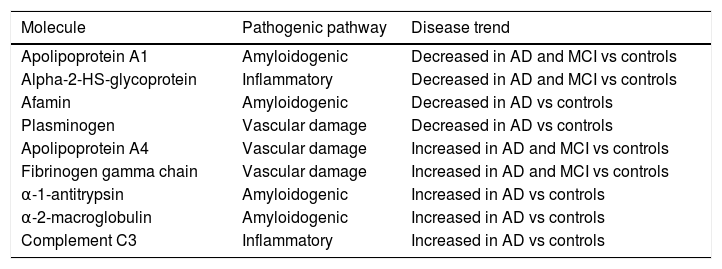
Identification of blood biomarkers in Alzheimer disease by omics techniquesPlasma proteomicsWith the aim of discriminating between patients with AD and healthy individuals, several studies have provided biomarker panels including large numbers of proteins in different combinations, reporting high sensitivity and specificity. However, their results are limited in scope due to the difficulty of replicating them (Table 2).17,25,37
List of biomarkers identified with proteomics studies with possible diagnostic use in AD.
| Molecule | Pathogenic pathway | Disease trend |
|---|---|---|
| Apolipoprotein A1 | Amyloidogenic | Decreased in AD and MCI vs controls |
| Alpha-2-HS-glycoprotein | Inflammatory | Decreased in AD and MCI vs controls |
| Afamin | Amyloidogenic | Decreased in AD vs controls |
| Plasminogen | Vascular damage | Decreased in AD vs controls |
| Apolipoprotein A4 | Vascular damage | Increased in AD and MCI vs controls |
| Fibrinogen gamma chain | Vascular damage | Increased in AD and MCI vs controls |
| α-1-antitrypsin | Amyloidogenic | Increased in AD vs controls |
| α-2-macroglobulin | Amyloidogenic | Increased in AD vs controls |
| Complement C3 | Inflammatory | Increased in AD vs controls |
AD: Alzheimer disease; MCI: mild cognitive impairment.
In comparisons of proteomic profiles in the peripheral blood, levels of apolipoprotein A-1 (which inhibits aggregation of Aβ oligomers by decreasing its extracellular accumulation), α-2-HS-glycoprotein (with anti-inflammatory and neuroprotective effects), afamin (a vitamin E–binding glycoprotein that enables transport of vitamin E across the blood-brain barrier, with a potentially beneficial effect on the damage caused by Aβ or oxidative stress), and plasminogen are reported to be significantly lower in patients than in controls. Levels of apolipoprotein A-4 and fibrinogen γ chains, which are thought to be responsible for vascular anomalies in AD, are significantly higher in patients than in controls.3,16,18 Therefore, they may become biomarkers for the early diagnosis of AD, as is the case of α-1-antitrypsin, α-2-macrogobulin, apolipoprotein E, and complement C3, which were proposed as diagnostic biomarkers of AD after a systematic review with subsequent replication.38
Plasma metabolomicsMetabolomics enables detection of metabolic alterations through simultaneously monitoring of a great variety of metabolites, which contributes to a better understanding of the pathogenesis of the disease. Thus, analysis of lipodomic profiles in patients with sporadic AD has demonstrated significant deficits in 2 important categories of structural lipids (glycerophospholipids and sphingolipids), together with modifications to their metabolism.16,17,39–41
ConclusionsCurrently, there is broad consensus on the need to apply anti-Aβ therapies at early (and possibly preclinical) stages of AD. The slow deposition of Aβ supports the existence of a wide time window to modify Aβ accumulation, but the clinical identification of this process requires the use of biomarkers. Non-invasive, cost-effective biomarkers, such as blood biomarkers, would be the ideal choice.
Historically, the sensitivity and specificity of blood biomarkers have been lower than those of CSF biomarkers. The great difficulty of reproducing and validating blood biomarker results with sufficient sensitivity and specificity has limited their use in clinical practice to date. However, mounting evidence supports the existence of a type of blood biosignature in AD.15 Blood biomarkers of degeneration (including tau protein and NF-L in particular), apoptosis (clusterin), and proteomics and metabolomics panels are probably the most promising biomarkers. In contrast, the usefulness of inflammatory, platelet, or microvascular damage biomarkers is currently more debatable.
Identifying reliable blood biomarkers for diagnosing AD continues to be limited by technical issues: it is difficult to standardise a blood biomarker to be used worldwide, considering differences in sampling, the populations studied, etc. However, the existence of global initiatives to overcome such limitations has made it probable that, in a near future, standardised blood biomarkers will be used in clinical practice. Given the limitations of individual blood biomarkers in terms of sensitivity, specificity, and predictive values, their combination in biomarker panels seems to be the most realistic option for their application in clinical practice.
Furthermore, new fields of knowledge are emerging as sources of potential diagnostic tools; an example is the case of epigenetic biomarkers, including DNA methylation marks and some non-coding RNAs, such as microRNA.
In the future, studies following omics approaches will lead to new levels of knowledge and analysis. For example, by simultaneously applying the different omics technologies to the same set of samples, or with new data analysis methods combining bioinformatic, statistical, and artificial intelligence techniques, we may identify new biological pathways that are altered in AD. The identification of new patterns of biomarkers and affected molecular cascades may lead to the discovery of future therapeutic targets for treating AD.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Altuna-Azkargorta M, Mendioroz-Iriarte M. Biomarcadores sanguíneos en la enfermedad de Alzheimer. Neurología. 2021;36:704–710.