Advances in the development of drugs with novel mechanisms of action have not been sufficient to significantly reduce the percentage of patients presenting drug-resistant epilepsy. This lack of satisfactory clinical results has led to the search for more effective treatment alternatives with new mechanisms of action.
DevelopmentThe aim of this study is to examine epidemiological aspects of the use of cannabis-based products for the treatment of epilepsy, with particular emphasis on the main mechanisms of action, indications for use, clinical efficacy, and safety. We conducted a narrative review of articles gathered from the PubMed, EMBASE, and Google Scholar databases and from the reference sections of relevant publications.
ConclusionsIn recent years there has been growing interest in the use of cannabis-based products for the treatment of a wide range of diseases, including epilepsy. The cannabis plant is currently known to contain more than 100 terpenophenolic compounds, known as cannabinoids. The 2 most abundant are delta-9-tetrahydrocannabinol and cannabidiol. Studies of preclinical models of epilepsy have shown that these cannabinoids have anticonvulsant properties, and 100% purified cannabidiol and cannabidiol-enriched cannabis extracts are now being used to treat epilepsy in humans. Several open-label studies and randomised controlled clinical trials have demonstrated the efficacy and safety of these products.
Los avances en el desarrollo de medicamentos con mecanismos de acción novedosos no han sido suficientes para modificar de manera significativa el porcentaje de pacientes con epilepsia refractaria. Esa falta de resultados clínicos satisfactorios nos ha llevado a buscar alternativas terapéuticas más eficaces y con mecanismos de acción diferentes a los convencionales.
DesarrolloEl objetivo de este artículo es profundizar en los aspectos epidemiológicos relacionados con el uso de productos a base de cannabis para el tratamiento de la epilepsia, haciendo énfasis en los principales mecanismos de acción, las indicaciones de uso, la eficacia clínica y la seguridad. Para lo anterior, se realizó una revisión narrativa mediante la búsqueda de artículos en PubMed, EMBASE, Google Scholar y a través de la revisión exhaustiva de la bibliografía relevante.
ConclusionesEn los últimos años ha crecido el interés relacionado con el uso de cannabis medicinal para el tratamiento de diferentes enfermedades, incluyendo la epilepsia. En la actualidad, sabemos que las plantas de cannabis contienen más de 100 compuestos terpenofenólicos que se han denominado cannabinoides. Los 2 más abundantes son el delta-9-tetrahidrocannabinol y el cannabidiol. Diferentes modelos preclínicos de epilepsia han demostrado que estos cannabinoides tienen propiedades anticonvulsivas, por ello se ha comenzado a utilizar cannabidiol purificado al 100% y extractos de cannabis enriquecidos con cannabidiol para el tratamiento de la epilepsia en humanos. La eficacia y la seguridad de estos productos han quedado demostradas en diferentes estudios abiertos y ensayos clínicos controlados y aleatorizados.
The lack of satisfactory results in the treatment of some neurological diseases has led to a search for more effective medications with new action mechanisms. Within the search for new drugs, cannabis-based products have emerged as a safe and effective treatment alternative.1 Today, cannabinoids are considered a complementary tool for the symptomatic management of various chronic neurological diseases when other first-line therapies have failed.1,2 Current evidence supports the use of cannabis-based products to treat refractory epilepsy; chronic neuropathic pain; spasticity and bladder dysfunction associated with multiple sclerosis; movement disorders including tremor, dystonia, and Tourette syndrome; headache; and some sleep disorders associated with neurological diseases.2,3 For decades, the prescription of cannabinoids for therapeutic purposes has been subject to restrictions and controls under specific regulatory frameworks, which has limited the development, clinical research, and commercialisation of cannabinoids for medicinal use.4 However, recent years have seen significant advances worldwide in favour of the safe use and responsible prescription of medicinal cannabis for various clinical conditions.4 Furthermore, new regulations have allowed for the exponential growth of clinical research into the use of cannabinoids for several neurological diseases.3–5 The objective of this article is to examine epidemiological aspects of the use of cannabis-based products to treat epilepsy, with an emphasis on their main action mechanisms, indications, clinical efficacy, and safety. To that end, we conducted a narrative review of the relevant literature on the use of cannabinoids to treat epilepsy, based on a search of the PubMed, EMBASE, and Google Scholar databases.
Epidemiological aspectsEpilepsy is one of the most frequent neurological diseases, affecting approximately 70 million people worldwide.6 While epilepsy is a global disease, it is unequally distributed, with almost 80% of affected individuals living in populations with limited economic resources.6–8 Epilepsy is treatable, with high rates of treatment response: approximately 70% of patients control their disease with antiepileptic drugs and live normal lives.8 More than 25 drugs are currently available for seizure control; however, despite the multiple therapeutic alternatives and the development of new drugs, 25%-35% of patients do not respond to treatment.9 This situation is known as refractory epilepsy, and constitutes a frequent, chronic, and economically costly condition requiring integrated multidisciplinary management.9 Refractory epilepsy predisposes to numerous neuropsychiatric comorbidities and has a considerable impact on patients’ quality of life and increases the rates of morbidity and mortality associated with the disease.9,10 Advances in the development of drugs with novel action mechanisms have been insufficient to significantly reduce the percentage of patients with refractory epilepsy; this has been demonstrated in different prospective cohorts evaluating treatment response over periods longer than 30 years.11 These studies have found that up to 36% of patients continue to present seizures despite treatment with several appropriately selected drugs (Table 1).11 The lack of satisfactory clinical outcomes has led to a search for more effective treatments with different action mechanisms than those of conventional treatments.
Percentage of responders in 3 cohorts of patients recently diagnosed with epilepsy.
| Assessment period | Year of analysis | No. patients | Total (%) | Study |
|---|---|---|---|---|
| 1982-1997 | 1999 | 470 | 64 | Kwan and Brodie (2000) |
| 1982-2001 | 2003 | 780 | 64.4 | Mohanrah and Brodie (2006) |
| 1982-2006 | 2008 | 1098 | 68.4 | Brodie et al. (2012) |
| 1982-2012 | 2014 | 1795 | 63.8 | Chen et al.11 (2018) |
Summary of the 4 large prospective cohort studies evaluating treatment response in patients recently diagnosed with epilepsy. Despite numerous advances in the development of new antiepileptic drugs, the percentage of patients with refractory epilepsy has not significantly decreased in recent years.
In recent years, there has been growing interest in the treatment of epilepsy with medicinal cannabis. Cannabis plants contain more than 100 terpenophenolic compounds, termed cannabinoids.12 The 2 most abundant compounds are delta-9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD).12 Various preclinical models of epilepsy have suggested that cannabinoids act on systems other than the endocannabinoid system, opening the door to potential new treatment strategies and novel action mechanisms.13 Several recent case reports have described a significant improvement in seizure control with the use of different cannabis-based products.13 Randomised controlled trials have begun to confirm the considerable expectations that have arisen from these isolated case reports. To date, 3 clinical trials have evaluated the efficacy of CBD for controlling certain epileptic syndromes (Dravet syndrome and Lennox-Gastaut syndrome).14 Although the results are promising, several studies have demonstrated that CBD presents complex pharmacokinetics, variable bioavailability, and a high rate of drug-drug interactions; as a result, researchers have searched for different therapeutic and pharmacological alternatives.14 For example, some studies have used CBD-enriched cannabis extracts, which usually have a CBD:THC ratio of 20:1; this approach is justified by various preclinical and pharmacological studies, which suggest that the entourage effect of all the plant’s components results in greater efficacy than that of isolated chemical compounds.13 In some countries, including Israel, studies into the efficacy of these products for controlling refractory epilepsy have found that more than 50% of patients achieve a significant improvement in seizure frequency; furthermore, the authors observed subjective improvements in behaviour, alertness, communication, and some motor skills.15
Action mechanism and pharmacological considerationsThe action mechanism, pharmacokinetics, and pharmacodynamics of cannabinoids are complex.13,14 Cannabis plants contain more than 100 phytocannabinoids and terpenes that act through complex molecular pathways and signalling networks.14 The most abundant and most studied compounds in different cannabis plants are Δ9-THC and CBD. These molecules act through G protein–coupled receptors, which belong to the endocannabinoid system and are classified as CB1 (mainly expressed by neurons in the central and peripheral nervous systems) and CB2 (mainly expressed by immune system cells).16 CB1 receptors reduce neuronal excitability and the release of neurotransmitters by modulating the opening of potassium channels and blocking calcium channels.14,16,17 CB2 receptors are involved in immune modulation.17 Δ9-THC is a partial CB1/CB2 receptor agonist and has a potent anti-inflammatory effect; however, as it is responsible for the majority of the plant’s psychotropic, cognitive, and behavioural effects, little effort has been made to develop THC as an antiepileptic drug. Furthermore, some preclinical studies have found THC to have proconvulsant properties.18 Most studies into epilepsy have focused on CBD and its analogue, cannabidivarin.19 Unlike Δ9-THC, CBD has very low affinity for CB1 and CB2 receptors (which would explain its reduced psychotropic activity); as a result, it has been suggested that the compound’s antiepileptic activity does not involve the endocannabinoid system.19 While its exact action mechanism in patients with epilepsy is currently unknown, several hypotheses have been suggested. For instance, it modulates the ENT transporter, the GPR55 receptor, and the TRPM8 channel, which are involved in neuronal hyperexcitability.18,19 It also modulates the activation of the 5HT1a serotonergic receptor, some glycine receptors, and the TRPA1 channel, which contribute to the regulation of intracellular calcium concentrations.18,19 Furthermore, CBD is a potent inhibitor of certain hepatic enzymes (CYP3A4, CYP2C9, and CYP2C19); it consequently inhibits the metabolism of some antiepileptic drugs using the same enzyme system, potentiating their antiepileptic properties; this is the case for clobazam, topiramate, zonisamide, and eslicarbazepine.20 The oral bioavailability of CBD is very low (less than 10%), which is partially explained by its high first-pass metabolism in the gut and liver.14 The elimination half-life of CBD ranges from 18 to 32 hours, enabling administration once or twice per day.13,19 Some studies suggest that the combined effects of all the plant’s components are associated with better efficacy than the isolated compounds (CBD and Δ9-THC). This phenomenon is known as the entourage effect.21 This indicates that the clinical effects of cannabis are secondary to complex interactions between different cannabinoids, rather than the action of an isolated compound.14,21 This hypothesis is supported by several studies that have demonstrated that CBD potentiates some of the beneficial properties of Δ9-THC and reduces its psychotropic effects, improving tolerability.14,22 CBD seemingly has the capacity to counteract some of the functional effects of CB1 receptor activation in the central nervous system.22 This may explain why users of cannabis with a high CBD:THC ratio experience few psychotropic effects compared to those who consume cannabis with a lower proportion of CBD.19
Clinical efficacyEvidence from 34 studies of 6 animal species suggests that Δ9-THC acts as an anticonvulsant in 61% of cases, a proconvulsant in 2.9%, presents a mixed response in 2.9%, and has no significant effect in 32.4% of cases.13,23 CBD and its homologue, cannabidivarin, are anticonvulsant in 80.5% of cases and have no significant effect in 19.5% of cases. Unlike Δ9-THC, CBD and cannabidivarin have not been shown to present proconvulsant properties in any preclinical trial.13,23 Based on these studies of animal models, pure CBD and CBD-enriched cannabis extracts have begun to be used to treat epilepsy in human patients.23
Clinical efficacy of cannabidiol-enriched cannabis extractsPublished cases of the use of CBD-enriched cannabis extracts report diverging results (both anticonvulsant and proconvulsant effects); however, most authors describe subjective improvements in seizure frequency.24–28 One of the best-known cases, which generated considerable media coverage, was that of Charlotte Figi, an American child with Dravet syndrome and refractory epilepsy who presented a significant improvement in seizure control after using a CBD-enriched cannabis extract. Several surveys have also been conducted to collect data from the family members and caregivers of patients with epilepsy. One of the most important of these studies was published in 2015 by Hussain et al.29 The survey was distributed to the parents of children aged 3-10 years who had epilepsy (infantile spasms and Lennox–Gastaut syndrome) and were using a CBD-enriched cannabis extract. Eighty-five percent of respondents reported a reduction in seizure frequency, and 14% reported complete seizure freedom.29 Mean treatment duration was 6.8 months, and the approximate CBD dose was 4.3 mg/kg/day. Many participants also reported improvements in sleep, alertness, and mood.29 In a recent survey conducted in Australia, 137 of 976 respondents were using or had used cannabis products to treat epilepsy.30 These products were effective for seizure control in 71% of children and 89.5% of adults; furthermore, nearly half of respondents reported a reduction in the concomitant use of antiepileptic drugs.30 In that study, only 6.5% of respondents reported that they used cannabis following recommendation by a physician, and the majority of the products used were obtained from illegal suppliers and no clear information on their composition was available.30 In another recent study conducted in Mexico, a survey was distributed to 53 parents of paediatric patients with epilepsy aged between 9 months and 18 years.31 The majority of patients had Lennox–Gastaut syndrome or unspecified refractory epilepsy. Eighty-one percent of parents reported a reduction in seizure frequency with CBD-enriched cannabis extracts, with 16% reporting complete seizure freedom.31
The main limitation of these studies is the variability in the types of epilepsy analysed and differences in the chemical compounds used, dosage, route of administration, and manufacture. Furthermore, home-made cannabis extract products are relatively easy to procure in some countries, resulting in considerable variation in quality control and the chemical consistency of the products.23 Given these limitations, it is unsurprising that many preparations are not subject to appropriate quality validation and that the content of individual cannabinoids in some marketed products differs considerably from the values printed on their label; some may even contain potentially harmful contaminants.32 The United States Food and Drug Administration evaluated 18 cannabis-based products that are freely marketed for controlling epilepsy, finding that 8 contained no CBD, 9 contained less than 1% CBD, and one contained 2.6% CBD.33 A recent Australian study reported similar results.34 That study evaluated the composition of cannabis extracts used by 65 patients with refractory epilepsy and found that, contrary to the expectations of the patients’ families, most of the samples studied contained low concentrations of CBD.34 Therefore, it is important to use standardised products that comply with regulatory, healthcare, and legal controls in each country. In Israel, for example, a CBD-enriched cannabis extract is marketed with CBD:THC ratios of 2:1, 5:1, and 20:1, with the latter being used most frequently. This product is standardised and meets local legal and healthcare requirements.15 A recent study evaluated the efficacy of this product for controlling epilepsy.15 The authors assessed 74 patients with refractory epilepsy using the CBD-enriched cannabis extract with a CBD:THC ratio of 20:1. The dose of THC did not exceed 0.5 mg/kg/day, and the CBD dose ranged from 1 to 20 mg/kg/day; however, 81% of patients received doses below 10 mg/kg/day, which was attributed to the fact that the majority of patients held the product under the tongue for several minutes, improving bioavailability and reducing first-pass metabolism. Approximately half of patients’ parents reported a > 50% reduction in seizure frequency. Improvements were also observed in behaviour, alertness, language, communication, motor skills, and sleep.
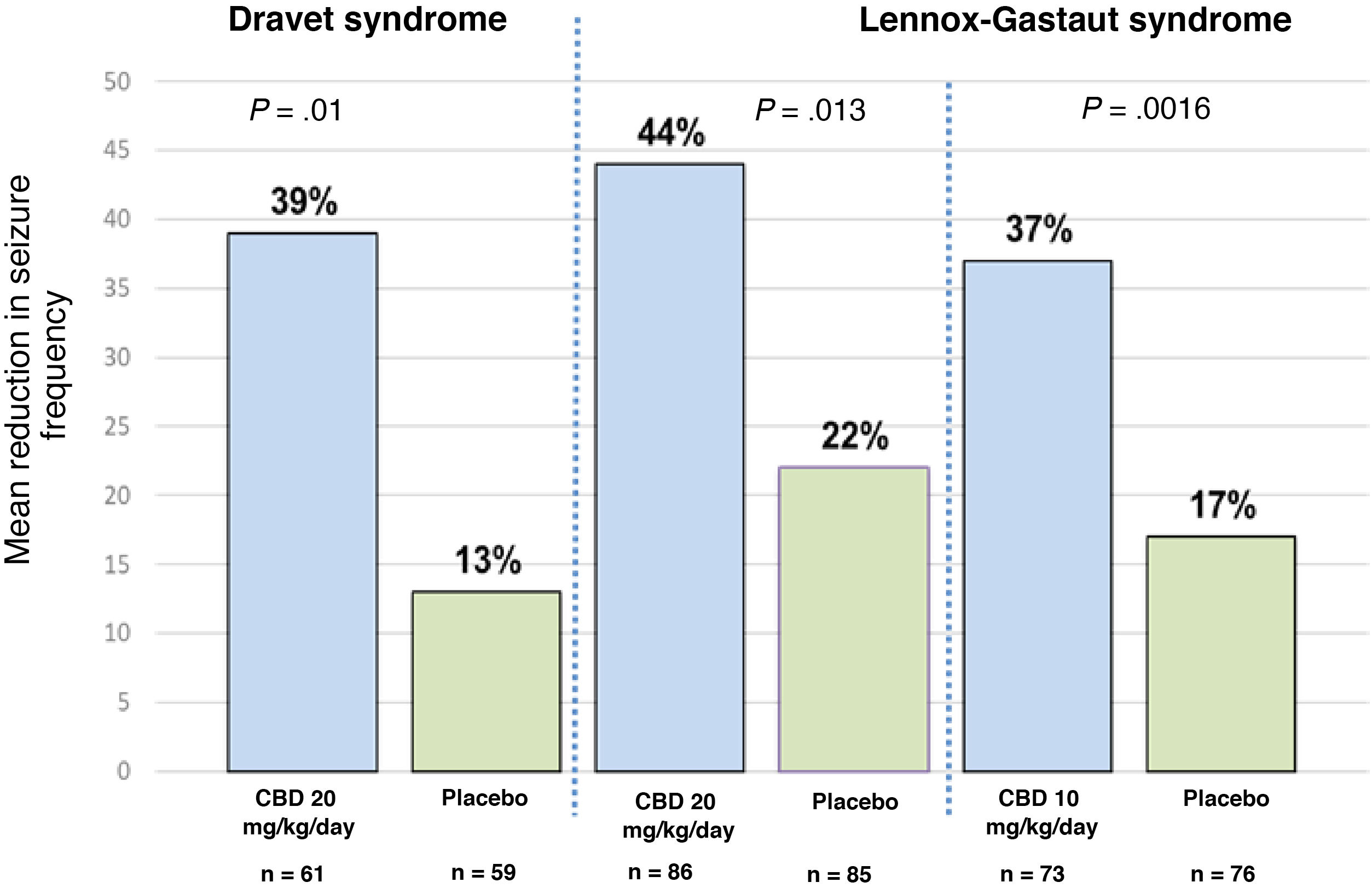
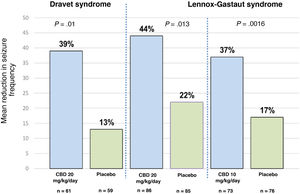
Clinical efficacy of pure cannabidiolThree randomised controlled clinical trials have evaluated the use of pure CBD.23 One of these studies was conducted in patients with Dravet syndrome, and the other 2 included patients with Lennox-Gastaut syndrome.35–37 The former study included patients aged between 2.3 and 18 years; 43% of participants achieved a > 50% reduction in seizure frequency and 5% achieved complete seizure freedom.35 The study of patients with Lennox-Gastaut syndrome included patients aged between 2 and 55 years, with 46% achieving a > 50% reduction in seizure frequency and 6% achieving seizure freedom.36,37Fig. 1 summarises the main findings of these studies. One of the main limitations of the study of patients with Dravet syndrome is the fact that up to 66% of patients receiving CBD were also using clobazam35; this correlation may have considerable clinical relevance, in the light of recent evidence indicating that levels of n-desmethylclobazam (the active metabolite of clobazam) can increase by up to 500% after CBD use. Therefore, we are unable to determine whether the improvement in seizure frequency was a result of the direct action of CBD or simply due to the increased plasma levels of coadjuvant drugs, principally clobazam, based on the data reported in these trials.38
Mean reduction in seizure frequency in the 3 clinical trials evaluating the efficacy of CBD as an adjuvant therapy in patients with Dravet syndrome and Lennox-Gastaut syndrome. For Dravet syndrome, seizure frequency refers to convulsive seizures. For Lennox-Gastaut syndrome, seizure frequency refers to drop attacks. P-values refer to comparisons between the CBD group and the placebo group in each trial. References 35–37 provide further information on these results.
It remains to be established whether the efficacy of CBD is independent of the concomitant use of other drugs. Regarding this point, a recently published study demonstrated a new pharmacodynamic mechanism by which CBD and clobazam increase the activation of the GABA receptor, contributing to the anticonvulsant effect of the combination of both drugs.39 Another recent study also demonstrated that the therapeutic response to a CBD-enriched cannabis extract was independent of the use of clobazam, and that clobazam contributed significantly to the sedative effects of CBD.40
Adverse reactionsStudies into the safety and effectiveness of CBD-enriched cannabis extracts report adverse reactions in 35%-45% of patients15,29–31; these rates are lower than those reported in clinical trials of pure CBD (adverse reactions in up to 79% of patients).35–37 The majority of adverse effects are mild and transient, and are largely explained by drug-drug interactions, particularly with clobazam, topiramate, zonisamide, and eslicarbazepine.38,39Table 2 lists the most frequent adverse reactions reported in the literature.
The most frequently reported adverse reactions to cannabinoids in patients with epilepsy.
| Drowsiness and fatigue, particularly in patients concomitantly using clobazam |
| Changes in appetite (increase or decrease) |
| Changes in body weight (increase or decrease) |
| Gastrointestinal symptoms: nausea, vomiting, diarrhoea, constipation, etc. |
| Irritability and behavioural changes |
| Increased transaminase levels and thrombocytopaenia, particularly in patients concomitantly using valproic acid. Most studies report an improvement in transaminase levels after the dose of valproic acid or the cannabis extract is reduced. Isolated cases of hyperammonaemia have been reported. |
| Some studies describe increased seizure frequency; however, most authors consider this change to constitute part of the natural history of the disease, rather than an effect of the product. |
Adverse reactions reported both for pure cannabidiol and for cannabidiol-enriched cannabis extracts.
Cannabinoids are considered a complementary tool for the symptomatic management of various neurological diseases when other first-line therapies have failed. Two therapeutic options are available to treat refractory epilepsy: pure CBD and CBD-enriched cannabis extracts. While both products contain the same active ingredient, it is not appropriate to extrapolate the results of clinical trials of pure CBD to CBD-enriched cannabis extracts. The 2 products are functionally distinct, with each presenting specific pharmacokinetic properties. CBD-enriched cannabis extracts constitute a model of botanical synergy in which the clinical effect is thought to be the result of complex interactions between all the components of the plant, rather than an isolated chemical product, as would be the case with pure CBD. It should be noted that this therapeutic difference is hypothetical: the exact action mechanism is not known in either case, although the majority of preclinical models demonstrate anticonvulsant properties. Furthermore, the current evidence on CBD-enriched cannabis extracts is insufficient for therapeutic decision-making; there is a need for controlled clinical trials to confirm the clinical efficacy and safety of these products. Nonetheless, the case reports, targeted surveys, and open-label trials published to date indicate that they may represent a safe, effective treatment for refractory epilepsy. Unlike CBD-enriched cannabis extracts, robust scientific evidence is available for the use of pure CBD: the results of 3 randomised controlled clinical trials support its use for the treatment of refractory epilepsy in patients with Dravet syndrome and Lennox-Gastaut syndrome. Many open questions must be addressed in specific studies. For instance, it is yet to be established whether the action mechanism of CBD is independent of drug-drug interactions, particularly with clobazam; we also currently lack information about its long-term safety, especially with regard to brain development and during pregnancy. Despite these questions, cannabinoids constitute a promising alternative for the treatment of patients with refractory epilepsy.
Conflicts of interestDr Camilo Espinosa-Jovel has consulted for the following pharmaceutical companies: BIOPAS Laboratoires, Khiron Life Sciences Corp., UCB Pharma, and Abbott.