At present, there is a lack of economic assessments of second-line treatments for relapsing-recurring multiple sclerosis. The aim of this study was to compare the efficiency between fingolimod and natalizumab in Spain.
MethodsA cost minimisation analysis model was developed for a 2-year horizon. The same relapse rate was applied to both treatment arms and the cost of resources was calculated using Spain's stipulated rates for 2012 in euros. The analysis was conducted from the perspective of Spain's national health system and an annual discount rate of 3% was applied to future costs. A sensitivity analysis was performed to validate the robustness of the model.
ResultsIndirect comparison of fingolimod with natalizumab revealed no significant differences (hazard ratio between 0.82 and 1.07). The total direct cost, considering a 2-year analytical horizon, a 7.5% discount stipulated by Royal Decree, and a mean annual relapse rate of 0.22 was € 40914.72 for fingolimod and € 45890.53 for natalizumab. Of the total direct costs that were analysed, the maximum cost savings derived from prescribing fingolimod prescription was € 4363.63, corresponding to lower administration and treatment maintenance costs. Based on the sensitivity analysis performed, fingolimod use was associated with average savings of 11% (range 3.1%–18.7%).
ConclusionsFingolimod is more efficient than natalizumab as a second-line treatment option for relapsing-remitting multiple sclerosis and it generates savings for the Spanish National Health System.
Actualmente, existe una ausencia de evaluaciones económicas en el manejo de la esclerosis múltiple remitente-recurrente en segunda línea. El objetivo del trabajo fue comparar la eficiencia de fingolimod y natalizumab en España.
MétodosSe desarrolló un modelo de minimización de costes en un horizonte temporal de 2 años. La tasa de recaída aplicada fue la misma para los 2 tratamientos y los recursos utilizados fueron calculados a partir de los costes vigentes en España en euros de 2012. Se aplicó la perspectiva del Sistema Nacional de Salud, con un descuento anual del 3% de los costes futuros. Se desarrolló un análisis de sensibilidad para comprobar la consistencia del modelo.
ResultadosLa comparación indirecta de fingolimod con natalizumab no fue significativa (hazard ratio entre 0,82 y 1,07). Los costes directos para el horizonte temporal de 2 años, considerando una tasa media anual de recaídas de 0,22 y el descuento por Real Decreto del 7,5%, fueron de 40.914,72 € para fingolimod y 45.890,53 € para natalizumab. Del total de costes directos valorados, el máximo ahorro por paciente derivado del uso de fingolimod fue de 4.363,63 € y correspondió a la reducción en los costes de administración y mantenimiento. En función del análisis de sensibilidad considerado, fingolimod generó ahorros medios del 11% (rango 3,1%-18,7%).
ConclusionesFingolimod es un tratamiento eficiente de segunda línea para el tratamiento de la esclerosis múltiple remitente-recurrente comparado con natalizumab, generando ahorros para el Sistema Nacional de Salud.
Multiple sclerosis (MS) is a chronic inflammatory and degenerative neurological disease affecting mainly young and working-age adults.1 The disease limits their quality of life2 and that of their caregivers.3 This situation results in direct and indirect costs for the Spanish National Health System (SNS), which increase significantly with the deterioration that occurs in MS.4,5 According to studies carried out in Spain, total mean cost per patient as of 2012 ranged from €29037 to €38596; direct costs made up between 60% and 73.8% of the total.4,6 A positive correlation between total cost and scores on the Expanded Disability Status Scale has also been observed.4,6
The latest data published in the Atlas of MS1 estimate that more than 1.3 million people have the disease. Europe is the continent with the largest number of diagnosed cases (63000), accounting for some 48% of the total number of MS cases worldwide. The highest incidence and prevalence rates are therefore found in Europe, with a prevalence of 80 cases per 100000 persons and an incidence of 3.8 cases per 100000 person-years (an increase in incidence has been observed, above all in women).1
Data from Spain show a prevalence of between 42 and 125 cases per 100000 persons7,8 and an incidence rate ranging from 2.1 to 5.3 cases per 100000 person-years.7 This disease affects women more than men, with a female/male ratio of 2.3 to 1.1 Likewise, it is estimated that 85% to 90% of cases are due to relapsing-remitting multiple sclerosis (RRMS).9
In recent years, MS prevalence has increased in Spain, as well as in other regions of the world.8 It is also believed that numerous latent cases still lack an appropriate diagnosis,10 a situation which entails a high consumption of healthcare resources and thus affects the care system's sustainability.9 For this reason, we anticipate that finding the most appropriate treatment for managing MS will remain a concern for healthcare administrators in the coming years.
The number of current studies of new treatments for MS is increasing, signalling the end of a period of low therapeutic innovation.9 Natalizumab and fingolimod are the main treatments currently used to manage RRMS in patients who do not respond to conventional immunomodulatory agents or those experiencing high disease activity since diagnosis. Natalizumab is an injectable solution11 and fingolimod, approved in 2010, was the first orally administered treatment for MS.12
Direct comparison of the efficacy of the two drugs is not currently possible due to the lack of published studies comparing them.13 Also, when making an indirect comparison based on results from the clinical trials (FREEDOMS14 for fingolimod and AFFIRM15 for natalizumab), doctors must recall that there are differences in patient follow-up and baseline characteristics, and in the design of the clinical trials for each drug.16 Patients in the FREEDOMS study14 were 18 to 55 years old, had one or more relapses in the 2 years prior to the study, and could have been treated with a beta-interferon or glatiramer acetate (provided that treatment had been suspended 3 months before the study). In the AFFIRM study,15 patients were 18 to 50 years old and experienced one or more relapses in the 12 months prior to the study. One of the criteria delimiting the study population was that patients previously treated with beta-interferon or glatiramer acetate were excluded.
A recent meta-analysis by Del Santo et al.13 indirectly compared the efficacy of the drugs currently available for treating MS. The study compared the efficacy of fingolimod and natalizumab based on the FREEDOMS14 and AFFIRM studies15 and the variable ‘relapse-free subjects at 12 months’. Results showed no significant differences in efficacy between the two drugs. Zintzaras et al.17 also compared available treatments for MS and found no significant differences between fingolimod and natalizumab, which resembled the results of Del Santo et al. study.13
In the Spanish context, the Group for Innovation, Assessment, Standardisation and Research in the Selection of Drugs (GENESIS Group) evaluated fingolimod and concluded that the two drugs are equally valid treatment options. Therefore, the decision to choose one option over the other must be based mainly on efficacy criteria. The study also suggested a cost minimisation analysis as the most suitable comparative pharmacoeconomic study for these two drugs.
All the above may lead us to think that there is currently no evidence showing one treatment to be superior to the other for management of MS. The aim of our study was to assess the cost-efficiency of natalizumab and fingolimod as second-line treatments for RRMS in the context of the Spanish SNS.
MethodsCost minimisation analysisBased on available evidence,13,17 the assessment of the GENESIS Group18 and the absence of significant differences in fingolimod and natalizumab efficacy, we chose a cost minimisation analysis as the most suitable pharmacoeconomic study to evaluate monetary differences between the 2 treatments.
Using Microsoft Excel® 2010, we developed a pharmacoeconomic model to perform the cost minimisation analysis for fingolimod and natalizumab.
The analysis was performed from the Spanish SNS perspective and thus included only direct healthcare costs. The direct costs considered were drug costs, administration and maintenance costs, complementary test costs, and relapse costs. All costs evaluated were given in 2012 prices in euros.
Following the recommendations of López-Bastida et al.19 for evaluating healthcare technologies, we used a time horizon of 2 years and applied an annual discount rate of 3% for estimated costs in the second year.
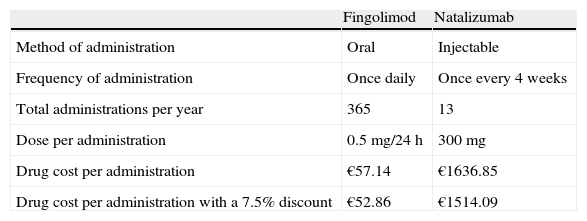
Drug costThe treatment options in the comparison were fingolimod (oral route) and natalizumab (IV route). We considered the number of doses mentioned in their respective summaries of product characteristics11,12 and assumed full treatment adherence for both drugs as the baseline scenario. Drug cost was estimated from Spanish official unit prices published in the General Council of Official Pharmacy Associations database and expressed as ex-factory prices,20 VAT excluded. Our scenario included the 7.5% reduction in ex-factory prices stipulated by Royal Decree-Law 8/201021 (Table 1).
Drug cost and type of administration.
| Fingolimod | Natalizumab | |
| Method of administration | Oral | Injectable |
| Frequency of administration | Once daily | Once every 4 weeks |
| Total administrations per year | 365 | 13 |
| Dose per administration | 0.5mg/24h | 300mg |
| Drug cost per administration | €57.14 | €1636.85 |
| Drug cost per administration with a 7.5% discount | €52.86 | €1514.09 |
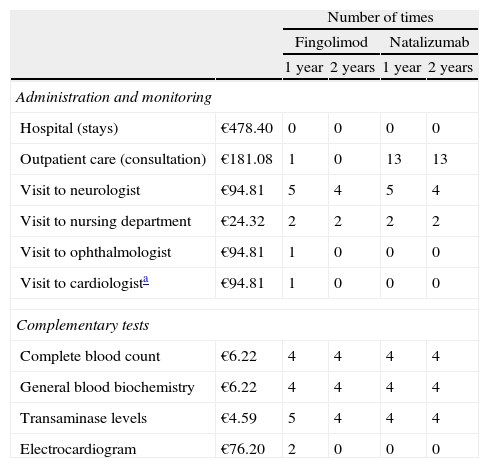
The amount of resources used in administering and maintaining each treatment, as well as necessary tests for each treatment option, was calculated based on an expert clinician's opinion (Table 2). Unit costs associated with these resources were obtained from the eSalud database of healthcare costs.22
Cost and volume of resources used in administration, maintenance, and complementary tests.
| Number of times | |||||
| Fingolimod | Natalizumab | ||||
| 1 year | 2 years | 1 year | 2 years | ||
| Administration and monitoring | |||||
| Hospital (stays) | €478.40 | 0 | 0 | 0 | 0 |
| Outpatient care (consultation) | €181.08 | 1 | 0 | 13 | 13 |
| Visit to neurologist | €94.81 | 5 | 4 | 5 | 4 |
| Visit to nursing department | €24.32 | 2 | 2 | 2 | 2 |
| Visit to ophthalmologist | €94.81 | 1 | 0 | 0 | 0 |
| Visit to cardiologista | €94.81 | 1 | 0 | 0 | 0 |
| Complementary tests | |||||
| Complete blood count | €6.22 | 4 | 4 | 4 | 4 |
| General blood biochemistry | €6.22 | 4 | 4 | 4 | 4 |
| Transaminase levels | €4.59 | 5 | 4 | 4 | 4 |
| Electrocardiogram | €76.20 | 2 | 0 | 0 | 0 |
In estimating relapse costs, we considered both those from relapse rates from pivotal studies and active comparison studies. This was because the design of pivotal studies and the different baseline characteristics for fingolimod and natalizumab studies make indirect comparisons difficult to conduct.18 The relapse rate featured here (0.22) corresponded to the average of the rates for each of the drugs vs. placebo as provided in the respective summaries of product characteristics.11,12
The relapse cost given here was based on the cost obtained in the Kobelt et al. study6 (€3181.77) calculated within the framework of the Spanish SNS using data from 1848 patients and updated to 2012 costs by applying the consumer price index.23
Sensitivity analysisWe conducted a sensitivity analysis that considered distinct scenarios for analysing indirect cost differences between the 2 treatments. In one of these scenarios, the current discount of 7.5% on ex-factory prices stipulated by the Royal Decree-Law21 was not applied. We also took into account the relapse rates given in the summaries of product characteristics for each of the drugs.11,12 To model treatment adherence according to the route of administration (injectable or oral), and its repercussion on the number of doses and the relapse rate, we estimated 90% adherence in the case of natalizumab and 80% in the case of fingolimod (based on experts’ clinical opinions). Costs were calculated for a time horizon of 10 years. We considered a specific analysis scenario for heart-risk patients on fingolimod that included a visit to a cardiologist during the first year and an additional analysis. This scenario assumes that monitoring should be repeated in patients in whom treatment is discontinued for at least one day in the first 2 weeks of treatment, for more than 7 days in the third and fourth weeks of treatment, or for more than 2 weeks after the first month of treatment. In the case of natalizumab treatment, we evaluated how outcomes change in patients who test positive for anti-virus JC antibodies and require a magnetic resonance imaging scan. Lastly, we assumed a conservative resource-use scenario for fingolimod based on a recent Spanish study whose results are still preliminary. This study examined, for example, the cost of vaccinating 5% of patients against varicella,24 and the cost of providing only 12 natalizumab injections instead of the 13 indicated in the summary of product characteristics.11
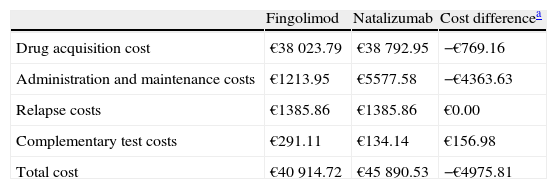
ResultsResults for the 2-year time horizon, assuming the 7.5% discount stipulated by the Royal Decree,21 showed lower costs for fingolimod than that for natalizumab. Fingolimod showed a total cost per patient of €40914.72 at 2 years, while natalizumab showed a total cost per patient of €45890.53. Fingolimod reduced direct costs by 10.8%, resulting in total savings to the Spanish SNS of €4975.81 per patient (Table 3).
Indirect costs for the 2-year time horizon.
| Fingolimod | Natalizumab | Cost differencea | |
| Drug acquisition cost | €38023.79 | €38792.95 | −€769.16 |
| Administration and maintenance costs | €1213.95 | €5577.58 | −€4363.63 |
| Relapse costs | €1385.86 | €1385.86 | €0.00 |
| Complementary test costs | €291.11 | €134.14 | €156.98 |
| Total cost | €40914.72 | €45890.53 | −€4975.81 |
The largest savings generated by fingolimod affected administration and maintenance costs and amounted to €4363.63 per patient. This was due to the lower number of visits to the outpatient clinic among patients treated with fingolimod. Drug cost-related savings amounted to €769.16 per patient. Relapse costs per patient for both treatments came to €1385.86. In patients treated with fingolimod, costs of complementary tests were higher, reaching €156.98 per patient.
For both treatments, the costs with the greatest impact on the total were drug costs, which represented 92.9% for fingolimod and 84.5% for natalizumab. The 2 drugs presented a similar distribution of direct cost except for administration and maintenance costs, which accounted for 12.2% of the total cost in the case of natalizumab and only 3% of the total for fingolimod.
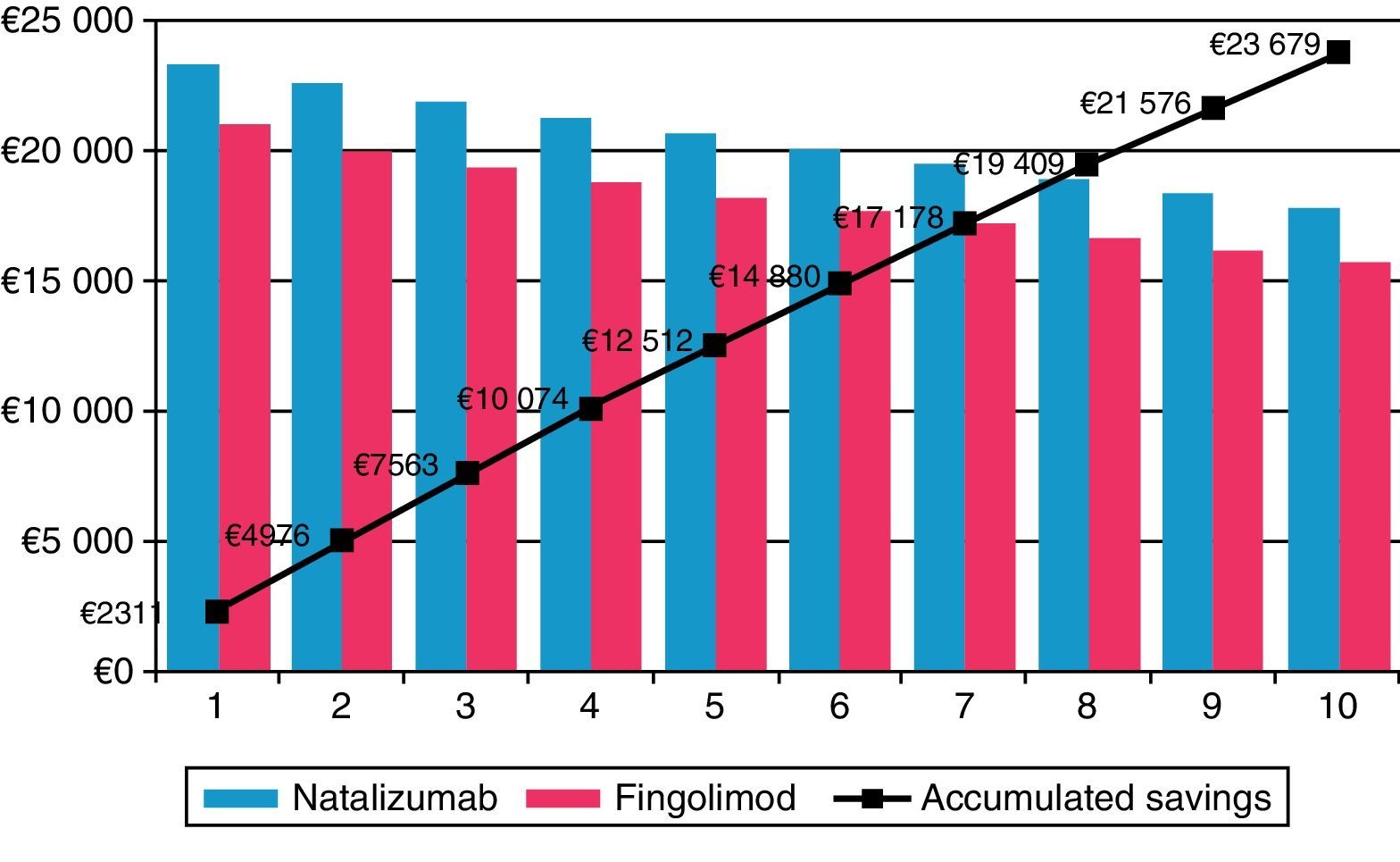
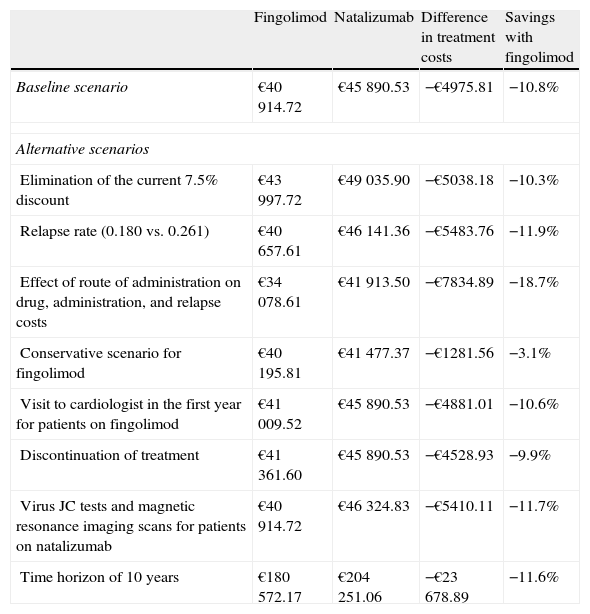
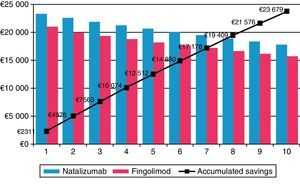
Fingolimod was the most economical option in all the different scenarios considered in the sensitivity analysis (Table 4). In the scenario in which the 7.5% discount stipulated by Royal Decree was not applied,21 fingolimod remained the most cost-effective option, reducing costs by €5038.18 (a savings of 10.3%) compared to natalizumab. When the time horizon was extended to 10 years, cost differences between fingolimod and natalizumab increase to €23678.89 (savings of 11.6%) (Fig. 1). Savings obtained with fingolimod were positively correlated to time. The maximum difference in costs was found when considering the impact of treatment compliance not only on the decrease in natalizumab consumption and administration but also in the change in relapse rate among non-compliant patients with a treatment efficacy equivalent to that of placebo (0.805). The savings amounted to €7834.89 (18.7%). Assuming one additional visit to a cardiologist for patients treated with fingolimod and at risk for heart disease, costs were €4881.01 lower than that in the case of natalizumab (savings of 10.6%). In addition, considering cases of discontinuation of treatment and having to monitor patients again (outpatient admission, electrocardiogram and additional visit to a cardiologist), we obtained savings of 9.9%. For a scenario in which a JC virus test would be necessary in patients treated with natalizumab, plus a magnetic resonance imaging scan in patients testing positive for the virus, cost differences between natalizumab and fingolimod amounted to €5410.11 (savings of 11.8% for fingolimod). In the scenario considered conservative for fingolimod, the difference in direct costs between that drug and natalizumab remained, although it was smaller (€1281.56, savings of 3.1%).
Sensitivity analysis.
| Fingolimod | Natalizumab | Difference in treatment costs | Savings with fingolimod | |
| Baseline scenario | €40914.72 | €45890.53 | −€4975.81 | −10.8% |
| Alternative scenarios | ||||
| Elimination of the current 7.5% discount | €43997.72 | €49035.90 | −€5038.18 | −10.3% |
| Relapse rate (0.180 vs. 0.261) | €40657.61 | €46141.36 | −€5483.76 | −11.9% |
| Effect of route of administration on drug, administration, and relapse costs | €34078.61 | €41913.50 | −€7834.89 | −18.7% |
| Conservative scenario for fingolimod | €40195.81 | €41477.37 | −€1281.56 | −3.1% |
| Visit to cardiologist in the first year for patients on fingolimod | €41009.52 | €45890.53 | −€4881.01 | −10.6% |
| Discontinuation of treatment | €41361.60 | €45890.53 | −€4528.93 | −9.9% |
| Virus JC tests and magnetic resonance imaging scans for patients on natalizumab | €40914.72 | €46324.83 | −€5410.11 | −11.7% |
| Time horizon of 10 years | €180572.17 | €204251.06 | −€23678.89 | −11.6% |
In our study, fingolimod was the most efficient option for RRMS management, as it generated lower direct costs. The higher cost generated by the larger number of necessary complementary tests was balanced by the lower costs for drug acquisition, administration, and disease management. Drug acquisition cost accounted for most of the total cost (92.9%), with a cost profile and breakdown similar to those found by other studies conducted on first-line treatments in Spain.6,25
For the reasons listed above, we chose a cost minimisation analysis and used the results of the indirect comparisons made by Del Santo et al.13 and Zintzaras et al.17 as our reference. However, we must stress that further head to head studies or direct comparison trials are needed to confirm that there are no significant differences in efficacy between the two drugs, whether as initial or second-line treatment. Also, as stated by the authors, there are methodological limitations on the meta-analysis.13,17
The European Medicines Agency26 has determined that fingolimod is similar to natalizumab in terms of efficacy and adverse effects. Healthcare authorities from countries such as the Netherlands27 and Sweden27 have accepted cost minimisation analysis in the comparison of the two treatments. Researchers such as Heisen et al.27 have adopted this approach in their studies, as there is still no evidence showing significant differences between the two drugs.
The National Institute for Health and Clinical Excellence (NICE)28 and the Scottish Medicines Consortium29 have recommended fingolimod for treating RRMS since it is regarded as a cost-effective option for the United Kingdom and Scotland healthcare systems. NICE estimated that the most plausible cost-effectiveness ratio for fingolimod compared with the weighted average of the comparators was likely to be in the range of £25000–£35000 per quality-adjusted life year gained. In Spain, a recent preliminary study also compared the 2 drugs in terms of cost-effectiveness, although there are no studies to date showing significant differences in fingolimod and natalizumab efficacy.24 This study also showed that treating RRMS patients with fingolimod after first-line treatment had failed was more economical than treating with natalizumab.
A general limitation applying to the selection of any pharmacoeconomic study is the lack of direct comparison studies9,13,17 measuring the efficacy of fingolimod and natalizumab means. This situation leads us to exercise more caution about results and stress the need for future studies that confirm the robustness of these findings. It should be mentioned that in the pivotal studies of natalizumab,15 the decrease in the relapse rate vs. placebo was higher than that in the fingolimod trial.14 But, as mentioned in the introduction, differences in population characteristics, trial follow-up, and design prevent any naive indirect comparisons between the two drugs, since their reliability would be low.16
It should be noted that our study only contemplates resource use and direct costs related to the disease and relevant for current healthcare decision makers. However, we know that indirect costs borne by RRMS patients can represent between 30% and 40% of the total cost.4,6
In the recent study by Heisen et al.27 the cost minimisation analysis of fingolimod and natalizumab was carried out within the framework of the Dutch healthcare system. The study showed that savings from using fingolimod rather than natalizumab remained present over time, which coincides with results in our study. Authors estimated that the cost of natalizumab would have to be reduced by a factor of 3 in order to obtain neutral results for this drug. Authors also pointed to the potential risks of considering a cost-effectiveness analysis in the comparison of the two drugs. This is the case of the study performed by O’Day et al.30 which found that natalizumab was a more cost-effective alternative than fingolimod in the USA (mean cost of $86461 for natalizumab and $98748 for fingolimod) based on relapse rates given in the summaries of product characteristics.11,12 One of the main explanations presented by the authors is that relapse rates were obtained from studies with divergent definitions of relapse and different baseline characteristics among patients.27
Over the last few years, efforts to innovate in MS drugs have focused mainly on improving efficacy and on increasing ease of use and administration flexibility for patients. Regarding this second objective, as the NICE guidelines28 highlight, fingolimod is an important breakthrough due to its oral route of administration. In our study, the greatest savings were observed in the item ‘administration costs’ (78.2%). The higher cost of administration for natalizumab was essentially due to the cost of having to administer the drug in outpatient clinics.
One of the disadvantages of oral drugs is that they are not so closely controlled, which may result in poorer treatment adherence and its subsequent effect on efficacy. This can lead to higher costs as the number of relapses may increase. The sensitivity analysis contemplated a potentially higher level of adherence to natalizumab and a lower level of adherence to fingolimod, which translated into lower drug acquisition costs and increased relapse costs. Nevertheless, this did not alter our conclusions. A personalised study of treatment suitability for each patient is necessary in order to achieve increased treatment efficacy with orally administered drugs. Some authors have mentioned that keeping patients more informed about their MS treatment is sufficient for increasing adherence to that treatment.31 Olascoaga et al.2 recommend conducting quality of life questionnaires before proposing a specific treatment, since they found that depression was related to lower treatment adherence. Meanwhile, route of administration did not significantly influence the patient's level of adherence.
In conclusion, according to results from the fingolimod and natalizumab cost minimisation analysis performed for the Spanish SNS, fingolimod generated lower direct costs as a second line treatment for RRMS in a Spanish setting. Consequently, this translates into greater savings for the Spanish SNS.
FundingThis study was financed by Novartis Farmacéutica S.A. (Spain).
Conflicts of interestThis study was financed by Novartis Farmacéutica S.A. (Spain). The authors declare that the sponsoring and financing entity did not participate in the analysis of the results or the presentation of conclusions. Dr. G. Izquierdo and Dr. A. García-Ruiz have received professional fees for participating in the project. M. Brosa and C. Crespo work for an independent consultancy company which received financial support from Novartis Farmacéutica S.A. Granell is employed by Novartis Farmacéutica S.A.
The authors would like to thank N. López, from the consultancy company Oblikue Consulting, and M. Riera, from Novartis Farmacéutica S.A., for their valuable contributions to the development and completion of this project.
Please cite this article as: Crespo C, Izquierdo G, García-Ruiz A, Granell M, Brosa M. Análisis de minimización de costes entre fingolimod y natalizumab en segunda línea de tratamiento de esclerosis múltiple remitente-recurrente. Neurología. 2014;29:210–217.
Preliminary results from this study were presented at the 16th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in Berlin.