Alexithymia is a neuropsychiatric symptom conceptualized as difficulty identifying and describing feelings. Although associated with other non-motor symptoms, mainly neuropsychiatric, alexithymia may present as an isolated symptom in persons with Parkinson's Disease (PwP). The objective of the study is to identify determinants of alexithymia and its association with quality of life (QoL) in Parkinson's disease.
MethodsSubjects with Parkinson's disease were recruited. The following instruments were applied: Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS), Non-Motor Symptoms Scale (NMSS), Montreal Cognitive Assessment (MoCA), Toronto alexithymia scale (TAS-20) and Parkinson's Disease Questionnaire (PDQ-8). Matched healthy controls were screened using TAS-20. Clinical and demographical variables were compared between alexithymic and non-alexithymic. Regression models were used to find determinants of alexithymia. Impact of alexithymia on QoL was estimated with a linear regression model.
Results98 patients were included. 56.1% PwP and 28.8% controls were alexithymic (p<0.001). Education level (OR 0.86) and NMSS urinary score (OR 1.09) determined alexithymia as well as TAS-20 score. Alexithymia was an independent determinant of QoL.
ConclusionsAlexithymia is a prevalent independent non-motor symptom in PwP with impact on QoL. Low education level and urinary symptoms are important determinants of alexithymia.
La alexitimia es un rasgo neuropsiquiátrico conceptualizado como la dificultad para identificar y describir sentimientos. Aunque está asociado con otros síntomas no motores, principalmente neuropsiquiátricos, sigue siendo una característica independiente de las personas con enfermedad de Parkinson (PcP). El objetivo del estudio es identificar los determinantes de la alexitimia y su asociación con la calidad de vida en la enfermedad de Parkinson.
MétodosSe reclutaron sujetos con enfermedad de Parkinson. Se aplicaron los siguientes instrumentos: escala unificada de la enfermedad de Parkinson de la Sociedad de Trastornos del Movimiento (MDS-UPDRS), escala de síntomas no motores (NMSS), evaluación cognitiva de Montreal (MoCA), escala de alexitimia de Toronto (TAS-20) y cuestionario de la enfermedad de Parkinson (PDQ-8). Se incluyeron controles sanos pareados, los cuales se evaluaron usando la TAS-20. Las variables clínicas y demográficas se compararon entre pacientes alexitímicos y no alexitímicos. Se utilizaron modelos de regresión para estimar los predictores de alexitimia. El impacto de este rasgo neuropsiquiátrico en la calidad de vida se estimó con un modelo de regresión lineal.
ResultadosSe incluyeron 98 pacientes. El 56,1% de PcP y el 28,8% de los controles fueron alexitímicos (p < 0,001). El nivel educativo (OR 0,86) y la puntuación urinaria del NMSS (OR 1,09) determinaron la alexitimia, así como la puntuación del TAS-20. La alexitimia fue un determinante independiente de calidad de vida.
ConclusionesLa alexitimia es un síntoma independiente no motor prevalente con impacto en la calidad de vida. El bajo nivel educativo y los síntomas urinarios son determinantes importantes de esta condición.
The neuropsychiatric profile of persons with Parkinson's Disease (PwP) is diverse and complex.1 Interest in neuropsychiatric symptoms and its association with quality of life (QoL) has increased.2
Alexithymia is a complex neuropsychiatric symptom conceptualized as the difficulty identifying and describing feelings, as well as externally oriented thinking and limited imaginative capacity.3 Alexithymia is not listed as a mental disorder in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), and prevalence has been estimated to be 10% in general population.4 As personality trait has been reported as relevant among patients with various neurological disorders, in particular other neurodegenerative diseases such as Alzheimer's disease.3 Earlier reports observed that the prevalence of alexithymia was twice as high in PwP when compared to general population.5 Interestingly, this increased prevalence maintained irrespective of cognitive decline or pharmacological therapy in de novo patients.6 The association of alexithymia with other neuropsychiatric symptoms in PwP is quite complex and only scarcely explored. A recent review suggests that, although tightly associated with depression, anxiety and apathy; alexithymia remains can present as an isolated characteristic of PwP.7 Association with other non-motor symptoms has not been addressed to the best of our knowledge.
From a pathophysiological viewpoint it has been hypothesized neurodegeneration at basal ganglia, vastly connected with the dorsal prefrontal and orbitofrontal areas may explain impairment in emotional experience processing.5,7 Neuroimaging studies in persons with alexithymia have implicated other areas such as the insula and the cingulate cortex.8,9
In addition, alexithymia may cause psychological distress leading to social and emotional dysfunction and psychiatric disorders that might result in a poor QoL, disability and caregiver burden.10
To date, no study has been carried out to explore the association of alexithymia with a broader spectrum of non-motor symptoms and with QoL in PwP. The objective of this study was to identify clinical determinants of alexithymia and its association with QoL in Parkinson's disease.
Material and methodsA total of 98 consecutive PwP were included into the study. All participants attended the Movement Disorders Clinic at the National Institute of Neurology and Neurosurgery in Mexico City. All patients met diagnostic criteria according to the Movement Disorders Society11 and were evaluated by neurologist with experience in movement disorders. Known diagnosis of depression or anxiety was not an exclusion criteria. Subjects who were not able to provide the data were excluded including those with dementia as assessed (Montreal Cognitive Assessment<21).
Complete clinical history was carried out and clinical and demographic data collected. Disease severity was graded according to Hoehn and Yahr scale (HY). All information regarding antiparkinsonian treatment was recorded. Levodopa (L-dopa) equivalent daily dose (LEDD) was calculated.
The Movement Disorders Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) was used to assess non motor experiences of daily living (Part I), motor experiences of daily living (Part II), motor examination (Part III) and motor complications (Part IV).12 MDS-UPDRS part I was used to assess for the presence of psychotic symptoms, depressed and anxious mood, apathy, and sleep problems. This scale is well validated and correlation with other specific clinical scales has been performed.13
Non-motor symptoms were assessed using the Non-Motor Symptoms Scale (NMSS).14 This instrument evaluates 30 items grouped to 9 relevant domains: cardiovascular (2 items); sleep/fatigue (4 items); mood/cognition (6 items); perceptual problems/hallucinations (3 items); attention/memory (3 items); gastrointestinal tract (3 items); urinary (3 items); sexual function (2 items); and miscellaneous items evaluating pain, olfactory alterations, weight loss, and excessive sweating. The score for each item is obtained by multiplying the severity score (from 0 to 3) times the frequency score (from 1 to 4).
Cognition was evaluated with the Montreal Cognitive Assessment (MoCA). The MoCA has been validated as a useful screening tool in PwP evaluating several cognitive domains; education level adjustment was performed as recommended and a MoCA score<26 was used as cut-off for mild cognitive impairment.15
QoL was measured using the Parkinson's Disease Questionnaire Short Form (PDQ-8).16 A summarized index (PDQ-8index), ranging from 0 to 100 was calculated. Higher scores reflect lower QoL.
Alexithymia was evaluated using the twenty-item Toronto alexithymia scale (TAS-20).17 This scale has a three-factor structure: Factor 1 (F1) difficulty identifying feelings; Factor 2 (F2) difficulty describing feeling (F2); and Factor 3 (F3) externally-oriented thinking. The TAS-20 is a self-reported scale that is comprised of 20 items. Items are graded using a 5-point Likert scale. 5 items for F1, 7 for F2 and 8 for F3; accounting for a maximum score of 100. The total score is the sum of independent items. Originally cutoff scoring classifies patients as non-alexithymia (score≤51), borderline alexithymia (scores 52–60) and alexithymia (score≥61). For study purposes, participants were classified as alexithymic or non-alexithymic (non-alexithymia and borderline scores). This classification has been previously used by other authors.18 Extensive validations in different languages and cultures,19 including Mexican population have been published.20 The TAS-20 was preferred over the TAS-26 due its better psychometric properties.21
In addition, TAS-20 has been used extensively to evaluate alexithymia in PwP.5,22,23 The TAS-20 was also applied to age-matched controls to estimate the difference in prevalence between groups since this data has not been reported for Mexican population. The control subjects were recruited from the waiting room and had no known neurological or psychiatric disease.
The study protocol was approved by the Institutional Review Board and all participants gave their written informed consent in accordance with the requirements of the Ethics Committee.
Statistical analysisBivariate analyses were conducted to identify differences between PwP with and without alexithymia. Means comparisons were performed using Student's T test. Qualitative variables were contrasted using chi-squared test or Fisher's exact test as appropriate. Correlation coefficients were used to assess the relation between variables of interest and PDQ-8. Variables with statistically significant differences at the bivariate level were used for the multivariate analysis. For logistic regression, the presence of alexithymia was defined as dependent variable. On the other hand, TAS-20 total score was used as dependent variable in a linear regression model. Multicollinearity was assessed using variation inflation factors (VIF). Hosmer–Lemeshow test was used for goodness of fit of the logistic regression, whereas analysis of residuals was used for the lineal regression. Variance explained by the models was assessed using the Nagelkerke square R or square R, respectively. The relationship between alexithymia and QoL was also explored using a linear regression model with PDQ-8index as dependent variable. Independent variables were selected from the correlation matrix.
A p value of <0.05 was considered significant. Statistical analyses were performed using SPSS, version 17 (SPSS, Inc., Chicago, IL).
ResultsA total of 98 PwP (55.1% male) were recruited for the study. The mean age was 62.7±12.3 years. The mean disease duration 10±5 years, and mean education level 10±5.1 years. In terms of disease severity, 70.4% of patients had mild disease (HY Stage 1 or 2), 20.4% moderate (HY Stage 2), and 9.2% had severe disease (HY Stage 4 or 5). All patients were on antiparkinsonian treatment, 87.8% on L-dopa and 52% on dopamine agonist (DA).
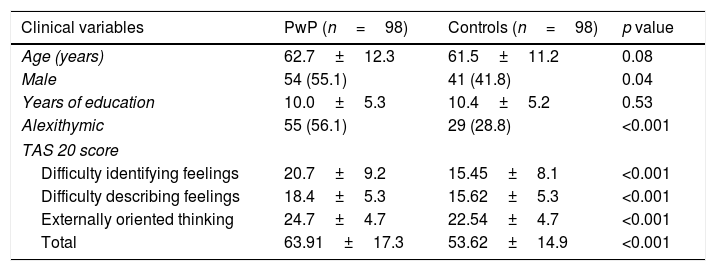
A full comparison between PwP and controls is shown in Table 1. Overall, alexithymia was more common in PwP and was reflected in all TAS-20 factors.
Comparison of clinical variables between PwP and controls.
| Clinical variables | PwP (n=98) | Controls (n=98) | p value |
|---|---|---|---|
| Age (years) | 62.7±12.3 | 61.5±11.2 | 0.08 |
| Male | 54 (55.1) | 41 (41.8) | 0.04 |
| Years of education | 10.0±5.3 | 10.4±5.2 | 0.53 |
| Alexithymic | 55 (56.1) | 29 (28.8) | <0.001 |
| TAS 20 score | |||
| Difficulty identifying feelings | 20.7±9.2 | 15.45±8.1 | <0.001 |
| Difficulty describing feelings | 18.4±5.3 | 15.62±5.3 | <0.001 |
| Externally oriented thinking | 24.7±4.7 | 22.54±4.7 | <0.001 |
| Total | 63.91±17.3 | 53.62±14.9 | <0.001 |
Data are mean±SD; or absolute numbers and percentages. PwP: person with Parkinson's Disease; TAS-20: twenty-item Toronto alexithymia scale.
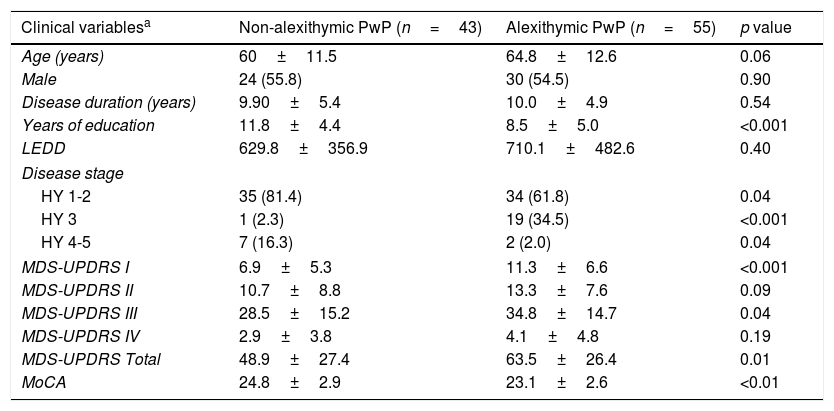
When comparing within the PD group, alexithymic PwP had less years of education, and worse MDS-UPDRS parts I and III scores. A detailed comparison of clinical variables is provided in Table 2. No differences were found between groups regarding the use of L-dopa (p=0.76) or DA (p=0.84).
Comparison of clinical variables between alexithymic and non-alexithymic PwP.
| Clinical variablesa | Non-alexithymic PwP (n=43) | Alexithymic PwP (n=55) | p value |
|---|---|---|---|
| Age (years) | 60±11.5 | 64.8±12.6 | 0.06 |
| Male | 24 (55.8) | 30 (54.5) | 0.90 |
| Disease duration (years) | 9.90±5.4 | 10.0±4.9 | 0.54 |
| Years of education | 11.8±4.4 | 8.5±5.0 | <0.001 |
| LEDD | 629.8±356.9 | 710.1±482.6 | 0.40 |
| Disease stage | |||
| HY 1-2 | 35 (81.4) | 34 (61.8) | 0.04 |
| HY 3 | 1 (2.3) | 19 (34.5) | <0.001 |
| HY 4-5 | 7 (16.3) | 2 (2.0) | 0.04 |
| MDS-UPDRS I | 6.9±5.3 | 11.3±6.6 | <0.001 |
| MDS-UPDRS II | 10.7±8.8 | 13.3±7.6 | 0.09 |
| MDS-UPDRS III | 28.5±15.2 | 34.8±14.7 | 0.04 |
| MDS-UPDRS IV | 2.9±3.8 | 4.1±4.8 | 0.19 |
| MDS-UPDRS Total | 48.9±27.4 | 63.5±26.4 | 0.01 |
| MoCA | 24.8±2.9 | 23.1±2.6 | <0.01 |
PwP: person with Parkinson's Disease; HY: Hoehn and Yahr stage; LEDD: Levodopa equivalent daily dose; MDS-UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale; MoCA: Montreal Cognitive Assessment; TD: tremor dominant; PIGD: postural instability and gait disorder.
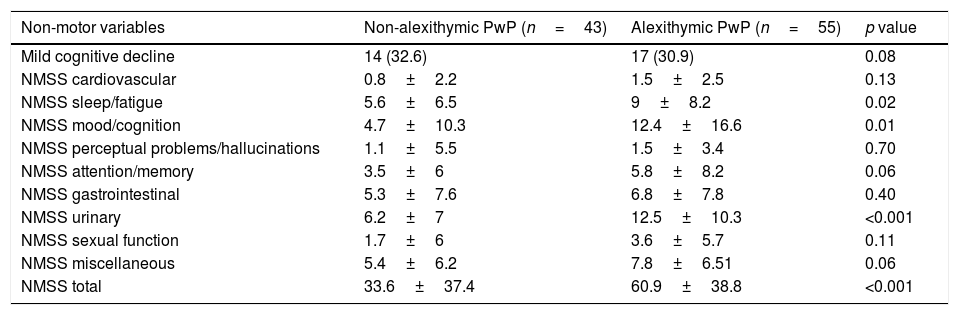
Regarding non motor symptoms, no difference in mild cognitive impairment was found. Concerning the severity of non-motor symptoms, NMSS items evaluating sleep/fatigue, mood/cognition, and urinary symptoms had higher mean scores in alexithymic PwP. A full comparison of non-motor symptoms is shown in Table 3.
Comparison of non-motor symptoms between alexithymic and non-alexithymic PwP.
| Non-motor variables | Non-alexithymic PwP (n=43) | Alexithymic PwP (n=55) | p value |
|---|---|---|---|
| Mild cognitive decline | 14 (32.6) | 17 (30.9) | 0.08 |
| NMSS cardiovascular | 0.8±2.2 | 1.5±2.5 | 0.13 |
| NMSS sleep/fatigue | 5.6±6.5 | 9±8.2 | 0.02 |
| NMSS mood/cognition | 4.7±10.3 | 12.4±16.6 | 0.01 |
| NMSS perceptual problems/hallucinations | 1.1±5.5 | 1.5±3.4 | 0.70 |
| NMSS attention/memory | 3.5±6 | 5.8±8.2 | 0.06 |
| NMSS gastrointestinal | 5.3±7.6 | 6.8±7.8 | 0.40 |
| NMSS urinary | 6.2±7 | 12.5±10.3 | <0.001 |
| NMSS sexual function | 1.7±6 | 3.6±5.7 | 0.11 |
| NMSS miscellaneous | 5.4±6.2 | 7.8±6.51 | 0.06 |
| NMSS total | 33.6±37.4 | 60.9±38.8 | <0.001 |
NMSS: Non-Motor Symptoms Scale.
Statistically significant correlations were found for PDQ-8 with all of the following: TAS-20 score (r=0.36), NMSS mood/cognition (r=0.54), NMSS perceptual/hallucinations (r=0.43), NMSS sexual (r=0.32), NMSS sleep fatigue (r=0.30), NMSS Urinary (r=0.27), NMSS cardiovascular (r=0.46), MDS-UPDRS part I (r=0.50), MDS-UPDRS part II (r=0.61), MDS-UPDRS part III (r=0.51) and MDS-UPDRS part IV (r=0.35).
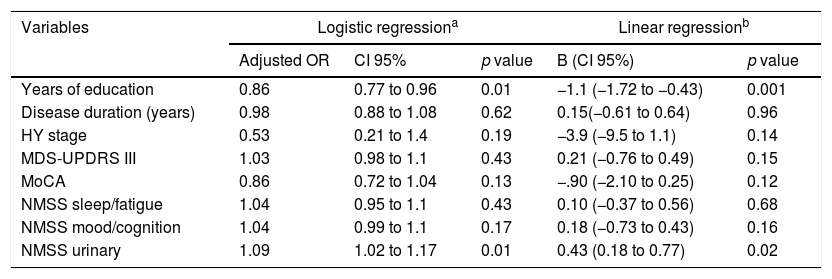
For the multivariate analysis the following variables were considered: years of education, disease duration, HY stage, MDS-UPDRS part III, MoCA, NMSS sleep/fatigue, NMSS mood cognition, NMSS urinary. MDS-UPDRS part I was excluded due to multicollinearity with other NMMS domains. In the logistic regression model, years of education and NMSS urinary remained as determinants for alexithymia in PwP. Hosmer-Lemeshow test showed goodness of fit (p=0.08) and Nagelkerke square R was 0.40. Also, in the linear regression analysis years of education and NMSS urinary were determinants for alexithymia severity. Together these variables explained 30% of TAS-20 score variance. Full data derived from both multivariate analyses are shown in Table 4.
Multivariate analyses for presence and severity of alexithymia in persons with Parkinson's disease.
| Variables | Logistic regressiona | Linear regressionb | |||
|---|---|---|---|---|---|
| Adjusted OR | CI 95% | p value | B (CI 95%) | p value | |
| Years of education | 0.86 | 0.77 to 0.96 | 0.01 | −1.1 (−1.72 to −0.43) | 0.001 |
| Disease duration (years) | 0.98 | 0.88 to 1.08 | 0.62 | 0.15(−0.61 to 0.64) | 0.96 |
| HY stage | 0.53 | 0.21 to 1.4 | 0.19 | −3.9 (−9.5 to 1.1) | 0.14 |
| MDS-UPDRS III | 1.03 | 0.98 to 1.1 | 0.43 | 0.21 (−0.76 to 0.49) | 0.15 |
| MoCA | 0.86 | 0.72 to 1.04 | 0.13 | −.90 (−2.10 to 0.25) | 0.12 |
| NMSS sleep/fatigue | 1.04 | 0.95 to 1.1 | 0.43 | 0.10 (−0.37 to 0.56) | 0.68 |
| NMSS mood/cognition | 1.04 | 0.99 to 1.1 | 0.17 | 0.18 (−0.73 to 0.43) | 0.16 |
| NMSS urinary | 1.09 | 1.02 to 1.17 | 0.01 | 0.43 (0.18 to 0.77) | 0.02 |
OR: odds ratio; HY: Hoehn and Yahr stage; MDS-UPDRS: Movement Disorders Society Unified Parkinson's Disease Rating Scale; MoCA: Montreal Cognitive Assessment; NMSS: Non-Motor Symptoms Scale.
Regarding QoL, alexithymic PwP had higher mean PDQ-8index when compared with non-alexythimic (25.1±16 vs 14.8±12.6, p<0.001). In the multivariate analysis, a linear regression model was built with PDQ-8index as dependent variable, and the highly correlated variables as independent variables. In this model only and TAS-20 score (B coefficient of 0.67 [95%CI 0.04–0.13], p=0.04) and MDS-UPDRS part II remained as QoL determinants. This model explained 45% of PDQ-8index variance.
DiscussionReports of prevalence of alexithymia in PwP are varied. In our study 56% of our sample was alexithymic. This number is considerably higher to that found by previous studies, were prevalence ranged from 18 to 31.6%.23,24 Overrepresentation of other neuropsychiatric symptoms in Mexican PwP has previously been noticed for psychosis, mood/apathy and impulse control disorder,25 suggesting a possible sociocultural influence.
Interestingly prevalence of alexithymia was also higher the healthy controls, when compared to other studies. Prevalence in healthy control varies, but generally is reported between 5 and 15%.26–28 Several reasons may explain this finding. First, variations in the performance of TAS-20 attributed to transcultural differences were shown in a small study comparing between European and American healthy control subjects (including Mexican patients).20 Second, the low mean education level of our sample. In general, patients with higher education have been shown to be less alexithymic.29 Third, alexithymia has been shown to increase with aging.30 The discreet underrepresentation of men is unlikely to contribute to the high prevalence of alexithymia in controls. Studies have found that this trait is more prevalent in men, at least in part as a product of gender role socialization.31 Lastly, even though controls were asked for previous neuropsychiatric diagnosis, we cannot assess if symptoms not directly screened may have contribute to the observed prevalence.
Determinants of alexithymia in PwP have scarcely been explored. The actual independence of alexithymia from other neuropsychiatric symptoms has even been questioned. Many studies have linked depression to alexithymia outside PD.32,33 A meta-analysis conducted in over 3000 subjects only found a moderate relationship between depression and TAS-total score.34 On the other hand, PwP severity of depressive symptoms has also been associated with alexithymia.26 Nonetheless, other studies have demonstrated that alexithymia in PD is independent from depression,35 suggesting alexithymia might be a non-motor symptom by itself. In our study mean NMSS mood/cognition scores were higher in alexithymic PwP, but this variable failed to predict alexithymia. Another potentially relevant factor to be considered is represented by cognitive impairment. Alexithymic PwP have been shown to perform worst on tasks requiring visual-spatial,23 non-verbal processing,36 and executive function.37 In our study cognitive impairment as defined by the MoCA did not differ between PD groups. Nevertheless, it is important to mention that the total MoCA score did differ between groups with alexithyimic PwP scoring lower by 1.7±0.6 points.
In this regard, years of education was found to be a protector variable to both alexithymia and its severity. This has been previously reported by other authors.38,39 Interestingly this association maintained despite the relative low education years of our study population in comparison to other studies, suggesting that this trend maintains even at low levels of education.
Few studies have explored the relationship between motor symptoms and alexithymia. In our study an association was found between severity of motor symptoms and severity of alexithymia. Costa el al. reported that the severity of motor symptoms did not differ between alexithymic and non-alexithymic PwP,23 although it has been reported that postural instability and gait disorder subtype is associated with more difficulty in identifying and describing feeling.39
Perhaps one of the least expected finding in our study was the association of urinary symptoms and alexithymia. To the best of our knowledge, this is the first study to report a positive correlation between these two symptoms. Although not directly explored, a connection between urinary symptoms and alexithymia can be inferred from potentially shared neurobiological mechanisms. The frontal cortex plays an important role in planning, response suppression and regulation of micturition for appropriate social behavior. Together with basal ganglia dysfunction, which appear to suppress micturition; prefrontal, anterior cingulate and insula cortex dysfunction seem to contribute to urinary symptoms in PwP.40 On the other hand, studies suggest that alexithymia may be related to altered activity of frontal regions particularly in right anterior cingulate. These finding have been supported by functional imaging studies.41 Lastly, in our study urinary symptoms were assessed subjectively using the NMSS urinary item which evaluates urgency, frequency and nicturia. Although this scale is listed as “suggested” by the International Parkinson and Movement Disorder Society, clinimetric properties for urinary symptoms have not been evaluated separately.42 Therefore, this instrument may not be truly ideal for grading urinary symptoms. Moreover, urinary symptoms may have been related to the age of the sample, among other factors. Few conclusions can be carried out and further studies using objective urodynamic evaluations are needed to confirm our findings.
In our study alexithymia showed to be an independent determinant of QoL in PwP. Recently, Klietz et al reported that MDS-UPDRS II, BDI and TAS-26 score showed a significant correlation with the QoL assessed with the PDQ-843; our findings confirm this association.
Interestingly this association remained independent of other non-motor symptoms which are well known QoL determinants. The other associated factor with QoL was MDS-UPDRS part II which assess motor aspects of activities of daily living. This finding suggest that increased awareness is needed to develop screening strategies and further therapeutic approaches to impact QoL in PwP.
Our study has limitations. An observational design is not ideal for identifying risk factors and our findings need to be further supported by prospective studies. Also, it should be mentioned that while controls were assessed for previous known neurological and psychiatric diseases, and excluded accordingly, a thoroughly screening was not performed. On the other hand, neuropsychiatric symptoms in PwP were assessed as part of the MDS-UPDRS part I but a formal diagnostic neuropsychological assessment for individual symptoms was not performed. Neither the MDS-UPDRS part I nor the neuropsychiatric-related items in the NMSS can be considered a substitute for a comprehensive assessment and this limitation should be considered when interpreting our findings.
Cognitive impairment was only assessed using Level I criteria (impairment on a scale of global cognitive abilities validated for use in PD) but a more thorough evaluation is warranted in order to validate our findings. In summary, further studies using validated rating scales assessing not only the presence but also the severity of each related symptom is still needed. Lastly, a referral bias was present with underrepresentation of PwP in more severe stages of the disease, therefore our results may not be reproducible in advanced stages of the disease.
ConclusionsIn conclusion alexithymia is prevalent in PD and stands as an independent non-motor symptom in PwP. Alexithymia and its severity were determined by lower education level and urinary symptoms. This personality trait may be worth screening in PwP since impact to QoL is significant.
FundingNone.
Conflict of interestThere is no conflict of interest to declare.