Primary intracranial pressure disorders include idiopathic intracranial hypertension and spontaneous intracranial hypotension. Remarkable advances have been made in the diagnosis and treatment of these 2entities in recent years. Therefore, the Spanish Society of Neurology's Headache Study Group (GECSEN) deemed it necessary to prepare this consensus statement, including diagnostic and therapeutic algorithms to facilitate and improve the management of these disorders in clinical practice.
This document was created by a committee of experts belonging to GECSEN, and is based on a systematic review of the literature, incorporating the experience of the participants, and establishes practical recommendations with levels of evidence and grades of recommendation.
Los trastornos primarios de la presión intracraneal incluyen la hipertensión intracraneal idiopática y la hipotensión intracraneal espontánea. El diagnóstico y tratamiento de ambas entidades ha presentado un avance destacable en los últimos años; por lo que desde el Grupo de Estudio de Cefaleas de la Sociedad Española de Neurología (GECSEN) consideramos necesaria la elaboración de este documento de consenso con la inclusión de algoritmos diagnósticos y terapéuticos para mejorar su manejo en la práctica diaria.
Este documento ha sido redactado por un comité de expertos del GECSEN tras realizar una revisión sistemática de la bibliografía, incorporando la experiencia de los participantes y estableciendo unas recomendaciones prácticas con niveles de evidencia y grados de recomendación.
Headache attributed to disorders of intracranial pressure (ICP), whether due to increases or decreases in cerebrospinal fluid (CSF) pressure, is included in the third edition of the International Classification of Headache Disorders (ICHD-3)1 in the group of headaches attributed to non-vascular intracranial disorder.
This group includes 2 entities caused by primary disorders of ICP, idiopathic intracranial hypertension (IIH) and spontaneous intracranial hypotension (SIH), which have received increasing attention in recent years.
The Spanish Society of Neurology’s Headache Study Group (GECSEN, for its Spanish initials) developed this consensus statement on disorders of ICP based on a qualitative, systematic review of the literature, gathering evidence published in the PubMed, Cochrane Library Plus, and MeSH databases between 1990 and 2023. We also evaluated clinical practice guidelines and the most recent consensus statements, and drew on the clinical experience of the members of the study group. This review is intended to provide clinicians with a series of practical recommendations for appropriate diagnostic and therapeutic management of these entities, which should be adapted to the experience and characteristics of each healthcare centre. Levels of evidence (LE) and grades of recommendation (GR) of therapeutic approaches were evaluated according to the criteria described in GECSEN’s most recent clinical practice guidelines for headache (Table 1).2
Levels of evidence and grades of recommendation.
| Level I | Controlled prospective clinical trials with masked outcome assessment in a representative populationSystematic reviews of controlled clinical trials carried out in a representative populationBoth types require the following characteristics:
|
| Level II | Prospective cohort studies in a representative population with masked outcome assessment and meeting all criteria from (a) to (e)Prospective controlled clinical trials with masked outcome assessment in a representative population, but not meeting one of the criteria from (a) to (e) |
| Level III | All other controlled trials in a representative population in which outcome assessment was independent from the treatment administered |
| Level IV | Uncontrolled trials, case series, case reports, or expert opinions |
| Grade A | Recommendation definitively effective, ineffective, or dangerous. Requires at least one conclusive level 1 study or 2 consistent level 2 studies |
| Grade B | Recommendation likely to be effective, ineffective, or dangerous. Requires at least one conclusive level 2 study or several level 3 studies |
| Grade C | Recommendation that may be effective, ineffective, or dangerous. Requires at least 2 conclusive level 3 studies |
| GECSEN | Recommendation potentially effective, ineffective, or dangerous. This recommendation does not meet minimum requirements for a grade C, but it reflects consensus among contributors to this consensus statement. |
The CSF is essential to the functioning of the central nervous system. Changes in its composition, flow, or pressure can cause a broad range of neurological signs and symptoms. It is distributed in the ventricular system and the cranial and spinal subarachnoid space, and has 2 main functions: it reduces the effective weight of the brain from 1500 g to approximately 50 g, preventing mechanical lesions; and acts as a medium for the transport of nutrients and waste products.
Healthy adults have a CSF volume of between 125 and 150 mL. Eighty percent of CSF is produced in the choroid plexus of the lateral, third, and fourth ventricles, and the rest is produced in the ependyma, arachnoid mater, and brain tissue.3
The dynamic circulation of CSF is driven by its secretion, at a rate of approximately 25 mL per hour. Old detritus is displaced by this constant flow of CSF, ensuring a stable environment.
CSF produced in the lateral ventricles flows through the foramina of Monro to the third ventricle, then through the aqueduct of Sylvius to the fourth ventricle. From the fourth ventricle, CSF drains through the foramina of Luschka and Magendie into the subarachnoid space, near the pontine cistern.
Absorption of CSF mainly occurs via the arachnoid granulations, projections from arachnoid cells into the dural venous sinuses. These sinuses drain directly into the venous circulation. Another, minor, pathway of CSF clearance is through the subarachnoid space of the olfactory bulbs, which connects to a space surrounding the olfactory nerves (therefore, it is very close to the olfactory mucosa and nasal cavity). From there, it travels to local lymph nodes. A third clearance pathway is through the glymphatic system, a recently discovered system of channels made up of astrocytes surrounding the arteries of the pia mater. Its function is to provide a route for the entry of CSF, which is exchanged with the interstitial fluid of the brain and spinal cord. Thus, small quantities of CSF enter the nervous tissue, while the same amount of interstitial fluid exits the subarachnoid space and is cleared via the dural venous sinuses.4,5
Measurement of CSF pressure and normal rangeMeasurement of CSF pressure is a fundamental part of the lumbar puncture procedure. Pressure should be measured with the patient in a lateral decubitus position, using a small-calibre spinal needle, with the aim of minimising CSF leakage. Normal opening pressure ranges from 100 to 250 mm CSF, measured with a manometer connected to the needle, with zero at the level of the spinous processes. The CSF pressure value recorded with the manometer represents the venous pressure transmitted from the right side of the heart through the venous sinuses; therefore, pressure may fluctuate with breathing or Valsalva manoeuvres.6 To prevent this, we recommend asking the patient to symmetrically extend the lower limbs after the puncture is successfully performed, preventing compression of the abdomen. If lumbar puncture cannot be performed with the patient in a lateral decubitus position, it may be performed with the patient seated, although this is not recommended as there is a risk of herniation or syncope, and CSF pressure will be higher; conversion tables are recommended to estimate equivalent CSF pressure in the decubitus position.6
Idiopathic intracranial hypertensionEpidemiologyThe annual incidence of IIH is estimated at 1-2 cases/100 000 person-years,7 although there are discrepancies between epidemiological studies performed in different geographical regions.7–9 Among women aged 15-44 years, incidence rises to 3.3-28 cases/100 000 person-years, reaching even higher rates among women with obesity.8 As weight gain is the main risk factor for this disease, the rise in rates of obesity in the population is expected to cause a progressive increase in the incidence and prevalence of IIH.10,11
IIH may also occur in men, children, elderly people, and those without overweight/obesity; in these cases, it is particularly important to rule out secondary causes. In paediatric patients, the difference between sexes is less clear, and obesity rates do not differ from those observed in healthy controls.12 The difference between sexes is also less marked after 45 years of age, due to the high prevalence of obesity. Men with IIH present a similar age distribution, with obesity continuing to be the most significant risk factor, despite the lower prevalence than in women.13 Some authors suggest the possibility of a genetic component, as up to 5% of patients present family history, a higher rate than the prevalence of IIH among patients with obesity.14
AetiopathogenesisThe mechanism underlying development of IIH is not fully understood. Numerous factors may be involved, with alterations in CSF dynamics and increased venous sinus pressure playing an important role. Furthermore, the clear associations with female sex and obesity have led some authors to suggest that hormonal and metabolic factors may play a role.
Changes to CSF dynamics and increased venous pressureObstruction of CSF reabsorption in the arachnoid granulations and/or lymphatic drainage areas is currently one of the main hypotheses proposed to explain the development of IIH.15 CSF reabsorption depends on the pressure gradient between the venous sinuses and the subarachnoid space. Therefore, increased venous pressure would result in decreased reabsorption rates.16 Increased venous sinus pressure has gained increasing attention as a pathophysiological factor. However, it is currently unclear whether this is the primary aetiology of the disease, or a consequence thereof.15
CSF drainage through the arachnoid granulations requires a pressure gradient of 3-5 mm Hg between the subarachnoid space and the venous sinuses. Therefore, increased pressure in the venous sinuses could prevent CSF reabsorption. Venous sinus stenosis, particularly in the transverse sinus, is often detected in patients with IIH. However, a retrospective study found no significant association between the degree of stenosis and CSF opening pressure16; furthermore, some patients presented an improvement in stenosis after correction of ICP.17
Metabolic and hormonal factorsWeight gain is associated with increased rates of development or recurrence of IIH, regardless of presence of obesity, and the disease improves with weight loss.15 However, higher body mass index (BMI) seems to be insufficient to explain this association.
Obesity is increasingly perceived as an inflammatory disease, and the CSF of patients with IIH shows increased levels of such proinflammatory mediators as leptin, CCL2, IL-2, and IL-7.18,19 Increased leptin levels in patients with excess body fat may promote hypersecretion of CSF in the choroid plexus epithelium due to increased activity of Na+/K+-ATPase.17 An association has also been reported between IIH and androgen excess, with increased levels of testosterone and androstenedione being associated with earlier disease onset.16
Research is underway into the potential role of such incretins as glucagon-like peptide-1 (GLP-1) in CSF regulation, as GLP-1 may act on the choroid plexus; animal models have found administration of a GLP-1 receptor agonist to reduce IIH due to Na+/K+-ATPase inhibition. Finally, other metabolic factors, such as the angiotensin-aldosterone and the corticosteroid systems, are also involved.17
Clinical manifestationsNinety percent of patients present headache, which tends to be the reason for consultation and affects their quality of life.17 Pain characteristics are nonspecific, although pain may be accompanied by warning symptoms (progressive headache, precipitation by Valsalva manoeuvres, and worsening with changes in posture). Patients typically describe holocranial headache, predominantly affecting the frontal or retro-orbital area, with pulsatile or pressing characteristics, associated with nausea and occasionally vomiting; headache presents characteristics of migraine in up to 70% of cases.16,20 Pain predominantly occurs in the morning, responds poorly to analgesic treatment, and is typically exacerbated by Valsalva manoeuvres. Up to one-third of patients are thought to present medication overuse headache.16,19,20 Pain intensity is moderate-severe, and is not necessarily correlated with ICP values.7,10 In fact, up to 60% of patients present persistent headache despite normalisation of ICP.11,19
Visual symptoms affect over 70% of patients.19 The most frequent is repeated episodes of uni- or bilateral blurring of vision lasting less than one minute (known as transient visual obscurations), triggered by changes in posture, Valsalva manoeuvres, exposure to intense light, or eye movement. These episodes are explained by transient ischaemia of the optic nerve, and are more frequent in patients with more severe papilloedema. Patients may describe photopsia triggered by changes of position or Valsalva manoeuvres. Peripheral vision loss is observed in 40% of patients, although it generally goes unnoticed and is detected with visual field testing.14 Some patients present acute, progressive vision loss that leads to rapid onset of treatment due to the risk of blindness.19 Diplopia on the horizontal plane (either intermittent or continuous) is observed in approximately 30% of patients, and is caused by uni- or bilateral involvement of the sixth cranial nerve.14,20,21
More than half of patients report tinnitus. Tinnitus is unilateral, is characterised as a “murmuring,” and is usually relieved by compression of the ipsilateral jugular vein.14,20 It is thought to be caused by turbulent flow at the transverse sinus due to stenosis. Other symptoms of intracranial hypertension include olfactory nerve involvement, facial palsy, neck pain, and lower back pain.14,20,21 In a cohort of 31 patients with IIH, cognitive deficits persisted for 3 months after normalisation of ICP.22 Up to 25% of patients may be asymptomatic, with IIH being diagnosed after incidental detection of bilateral papilloedema.21
DiagnosisDiagnostic criteriaTables 2 and 3 show the modified Dandy criteria for the diagnosis of IIH23 and the ICHD-3 criteria for headache attributed to IIH,1 respectively.
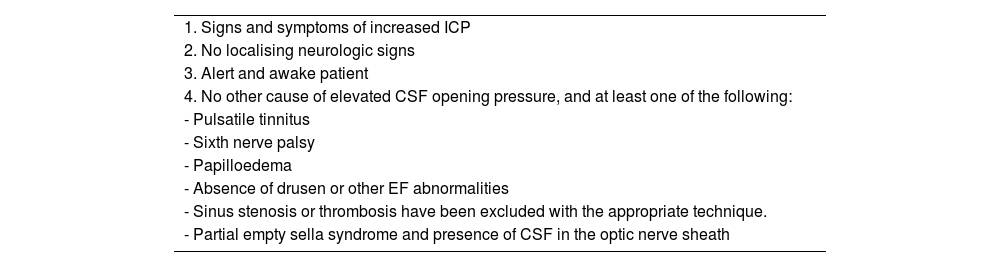
Modified Dandy criteria.23
| 1. Signs and symptoms of increased ICP |
| 2. No localising neurologic signs |
| 3. Alert and awake patient |
| 4. No other cause of elevated CSF opening pressure, and at least one of the following: |
| - Pulsatile tinnitus |
| - Sixth nerve palsy |
| - Papilloedema |
| - Absence of drusen or other EF abnormalities |
| - Sinus stenosis or thrombosis have been excluded with the appropriate technique. |
| - Partial empty sella syndrome and presence of CSF in the optic nerve sheath |
CSF: cerebrospinal fluid; EF: eye fundus; ICP: intracranial pressure.
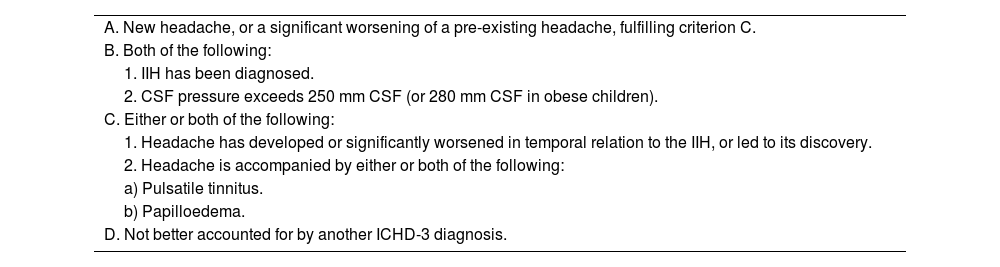
Diagnostic criteria for headache attributed to idiopathic intracranial hypertension. 7.1.1 (ICHD-3).
| A. New headache, or a significant worsening of a pre-existing headache, fulfilling criterion C. |
| B. Both of the following: |
| 1. IIH has been diagnosed. |
| 2. CSF pressure exceeds 250 mm CSF (or 280 mm CSF in obese children). |
| C. Either or both of the following: |
| 1. Headache has developed or significantly worsened in temporal relation to the IIH, or led to its discovery. |
| 2. Headache is accompanied by either or both of the following: |
| a) Pulsatile tinnitus. |
| b) Papilloedema. |
| D. Not better accounted for by another ICHD-3 diagnosis. |
CSF: cerebrospinal fluid; ICHD-3: International Classification of Headache Disorders (third edition); IIH: idiopathic intracranial hypertension.
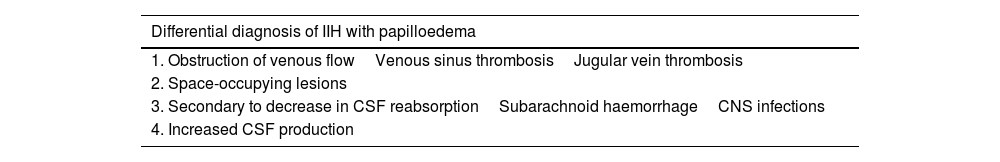
Differential diagnosis may follow different approaches, depending on whether papilloedema is present. If papilloedema is detected, differential diagnosis should seek to rule out secondary causes of intracranial hypertension (Table 4): obstructed venous blood flow (cerebral venous sinus thrombosis is the main entity to rule out, although obstructions in the jugular vein are also a possibility), space-occupying lesions, secondary causes of decreased CSF reabsorption (central nervous system infections, subarachnoid haemorrhage), and increased CSF production. If papilloedema is absent or has already resolved at the time of diagnosis, differential diagnosis should consider primary headaches, and especially migraine, in which pain can be phenotypically indistinguishable from IIH.
Secondary causes of idiopathic intracranial hypertension.
| Differential diagnosis of IIH with papilloedema |
|---|
| 1. Obstruction of venous flowVenous sinus thrombosisJugular vein thrombosis |
| 2. Space-occupying lesions |
| 3. Secondary to decrease in CSF reabsorptionSubarachnoid haemorrhageCNS infections |
| 4. Increased CSF production |
CNS: central nervous system; CSF: cerebrospinal fluid; IIH: idiopathic intracranial hypertension.
We should also consider other conditions or drugs that may be associated with increased ICP (Table 5).
Neuro-ophthalmological examinationNeuro-ophthalmological examination of patients with IIH should include direct ophthalmoscopy; examination of visual acuity, the pupils, and visual field; and optical coherence tomography (OCT), wherever possible.
Ophthalmoscopy may reveal papilloedema, the most specific finding in the diagnosis of IIH. It is usually bilateral and symmetrical, although up to 7% of patients present asymmetrical papilloedema. The underlying pathophysiology is oedema of retinal fibres from the optic disc, secondary to axoplasmic transport alterations. Severity is classified according to the modified Frisen scale (Table 6), and ranges from disappearance of venous pulsation, in earlier stages, to elevation of the disc, associated with retinal nerve fibre layer displaying splinter and flame haemorrhages and cotton-wool exudates.24 Optic disc atrophy may be observed at later stages.21,24
Modified Frisen scale.
| Grade 1 (minimal papilloedema) | Subtle C-shaped halo of disc oedema at the nasal border, with a normal temporal margin |
| Grade 2 (low-degree papilloedema) | Halo covers the entire circumference of optic disc, with elevated nasal border. |
| Grade 3 (moderate papilloedema) | Obscuration of one or more segments of a major blood vessel leaving the disc, with elevation of the entire border |
| Grade 4 (marked papilloedema) | Total obscuration of a major blood vessel leaving the disc, with elevation of the entire optic nerve head and obscuration of the border |
| Grade 5 (severe papilloedema) | Total obscuration of all blood vessels of the optic disc and leaving the disc |
The most widely used visual field test is the Humphrey test; most patients present enlargement of the physiological blind spot. More severe cases present with concentric reduction of the visual field, or appearance of scotoma, with inferior nasal or central arcuate scotoma being most frequent.
In recent years, OCT has become an increasingly important tool in diagnosis and follow-up; the technique enables measurement of retinal nerve fibre layer thickness, which is correlated with the severity of papilloedema, particularly in milder cases, and facilitates follow-up. The technique is not useful in cases of established optic atrophy, which may display thinning of the retinal ganglion cell complex and inner plexiform layer.21
NeuroimagingBrain MRI is fundamental in diagnosis, particularly in ruling out other causes of intracranial hypertension, and should always include a venography sequence in order to rule out venous sinus thrombosis, among other disorders. MRI findings can be highly suggestive, although they are neither pathognomonic nor a defining feature of IIH. The most typical findings are total or partial empty sella syndrome, posterior globe flattening or even optic disc protrusion into the vitreous humour, and tortuous optic nerves with thickening of the optic nerve sheath, although none of these findings are specific, as they may also be observed in healthy individuals; nonetheless, sensitivity is increased if a combination of signs is observed.24,25
MRI or CT venographyStenosis of one or both transverse sinuses, particularly at the transverse-sigmoid sinus junction, is highly characteristic, appearing in 10% to 90% of cases, depending on the series, a higher prevalence rate than in the general population. The cause is thought to be mechanical compression of the sinus due to increased pressure, although other studies suggest that this stenosis may play an aetiopathogenic role or even exacerbate the process.26
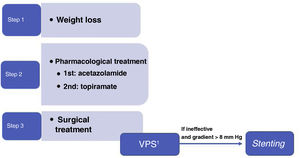
TreatmentWeight lossIIH is strongly associated with weight gain. Obesity is observed in 57% to 100% of patients,15 and the risk of IIH is heightened in patients presenting weight gain of 5% to 15% in the 2-12 months prior to diagnosis.15,20
Weight loss is the only established disease-modifying treatment15,20,27,28 and, in the absence of fulminant vision loss, is considered the recommended first line of treatment (LE I, GR A).
The degree of weight loss required for symptom remission and the optimal method for losing weight have not yet been established. Dietary strategies are recommended, and should ideally be followed through a community or hospital programme.29 Bariatric surgery has been proposed as a lasting treatment to induce remission of IIH, as it achieves a greater magnitude of weight loss, which persists in the long term, with a low mortality rate.15,18
A systematic meta-analysis of cases of IIH treated with bariatric surgery describes resolution of papilloedema in 100% of cases, compared to 66.7% of those following dietary strategies, and improvement or resolution of headache in 90.2% of cases, compared to 23.2% of those following dietary strategies.30 The results of the Idiopathic Intracranial Hypertension Weight Trial (IIH-WT), a multicentre, open-label, randomised controlled clinical trial, were published in 2021.31 The trial compared bariatric surgery against a community weight loss programme in patients with active IIH and BMI > 35 kg/m2, reporting favourable results for bariatric surgery, with treatment response persisting over 2 years. Comparison of the 2 arms revealed a difference of –8.2 cm CSF, –26.6 kg weight, and 7.3 points for quality of life on the 36-Item Short-Form Health Survey at 24 months.
Medical treatmentAcetazolamideAcetazolamide inhibits carbonic anhydrase and, consequently, the production of CSF by the choroid plexi. A randomised controlled clinical trial, the Idiopathic Intracranial Hypertension Treatment Trial (acetazolamide and diet), found a modest improvement in visual field in patients with mild vision loss, as well as a quality of life improvement at 6 months.32 However, the latest Cochrane review of the management of IIH concluded that “there is insufficient evidence to recommend or reject the efficacy of this intervention, or any other treatments currently available, for treating people with IIH” (LE II, GR C).33
The optimal dose of acetazolamide is not well established, although the most frequently used is 250 mg 2-3 times daily, increased progressively according to tolerability. The drug has not been shown to improve headache, and adverse effects are frequent (diarrhoea, dysgeusia, fatigue, nausea, paraesthesia).34
TopiramateTopiramate inhibits carbonic anhydrase to an extent, and may also suppress appetite. It has been compared against acetazolamide in a non-controlled open-label study of IIH treatment,35 and showed similar results for visual field, but greater weight loss. It may play a relevant role in cases of IIH with headache phenotype resembling migraine, or with concomitant migraine. The initial recommended dose is 25-50 mg per day, progressively increasing to a maximum of 100 mg twice daily.21
FurosemideLow or moderate doses of furosemide may be added to acetazolamide to potentiate its diuretic effect, with periodic monitoring of blood electrolyte levels and potassium supplementation. Furosemide is not recommended in monotherapy.21
New treatmentsGLP-1 agonists have been suggested as a treatment alternative. Studies have found that the GLP-1 receptor is expressed in the human choroid plexus. Treatment with the GLP-1 receptor agonist exendin-4 modulates the receptor in the rat choroid plexus via agonist-induced internalisation of the receptor, increasing AMPc levels and reducing Na+/K+-ATPase activity. In a clinical trial with rats, exendin-4 significantly reduced ICP to 60% at 30 minutes after administration.36 A recently published phase 2 double-blind clinical trial, with exenatide as placebo, found a reduction in ICP in 15 of 16 patients who completed the study, with no severe adverse reactions.37
The 11-beta hydroxysteroid dehydrogenase type 1 (11ß-HSD1) inhibitors were developed to treat obesity and metabolic syndrome. A phase 2 trial is currently underway to evaluate the capacity of this drug to reduce CSF secretion and, therefore, ICP. 11ß-HSD1 inhibitors do not affect the metabolism of systemic glucocorticoids, but have been shown to reduce CSF secretion by decreasing the local availability of cortisol in the choroid plexus; this decreases the osmotic gradient, mediated by the glucocorticoid receptor, and ultimately reduces ICP.38
Management of headache attributed to idiopathic intracranial hypertensionPatients should be informed at all stages about the potential effects of analgesic medication overuse. Non-steroidal anti-inflammatory drugs (NSAID), and particularly indometacin, can be used for symptomatic treatment due to their ability to reduce ICP. Opioids are not recommended; occipital nerve block may be useful in some cases. Preventive treatments that may favour weight gain, such as beta-blockers, tricyclic antidepressants, and flunarizine, should be avoided.34
Surgical treatmentSurgical treatment should be considered in patients with IIH who present no improvement or even worsening of symptoms despite receiving the maximally tolerated medical therapy, and earlier in patients with fulminant progression.
Evacuation by lumbar punctureRepeated lumbar puncture is not recommended as a long-term treatment strategy for IIH (LE II, GR B). Small improvements in headache (one point on the Numerical Pain Rating Scale) have been reported in 71% of patients; the procedure is also estimated to have a 64% likelihood of exacerbating headache in the first week following lumbar puncture. Furthermore, the procedure may result in acute or chronic lumbar pain in some patients. In the light of the above, therapeutic use of the procedure should be restricted to patients with fulminant IIH awaiting CSF shunt, and in pregnant women in whom surgery cannot be performed.20,34
Peritoneal shunts and valvesCSF shunting techniques are effective in decreasing papilloedema, visual field defects, and headache in 78.9%, 66.8%, and 69.8% of cases, respectively (LE II, GR B).39 Ventriculoperitoneal shunting is preferred over lumboperitoneal shunting as it is better tolerated (LE II, GR B). In any case, this technique has a relatively high rate of complications (9.4%), including infection, obstruction, and migration of the drainage tube; shunts therefore require frequent revisions. The use of this type of technique in patients with headache as the sole symptom of IIH is controversial, as these patients frequently also present migraine, and there is a risk of headache due to low CSF pressure (LE II, GR B). Furthermore, this technique presents a failure rate greater than 40%.40
StentingTransverse sinus stenting can be considered in patients presenting stenosis of the transverse sinus with a pressure gradient > 8 mm Hg and increased venous blood pressure at the superior sagittal sinus and venous sinuses near the stenosis (LE III, GR B). Larger trans-stenotic pressure gradients are known to benefit more from the procedure,40 whereas the degree of stenosis seems not to be related to ICP or to predict the risk of vision loss.18 It has been suggested that stenting reduces cerebral venous hypertension, facilitating an increase in CSF absorption, a reduction in intracranial hypertension, and improvement of signs and symptoms.39
This technique may improve papilloedema, visual field defects, and headache in 87.1%, 72.7%, and 72.1% of cases, respectively.39 However, these results are subject to selection bias, and no long-term data are available.20 Complications may occur in 2.3% of patients, and include intrastent thrombosis, subdural haemorrhage, and recurrent stenosis immediately proximal to the stent.25 Furthermore, it should be noted that stent placement requires antiplatelet therapy for at least 6 months, which may represent a contraindication against the procedure in some patients with high haemorrhagic risk.34 The failure rate is approximately 11%.25
Optic nerve sheath fenestrationOptic nerve sheath fenestration is an effective treatment to be considered, especially in patients with severe visual involvement (LE II, GR B). The treatment consists in creating a window in the retrolaminar portion of the optic nerve sheath, resulting in a fistula between the subarachnoid space and the orbital cavity. This results in a decrease in pressure on the optic nerve, reducing papilloedema and improving visual function. It is probably the most effective technique for improving papilloedema (90.5%), although success rates are more modest for visual field defects (65.2%) and headache (49.3%).41 Complications (which are severe in approximately 2% of patients) include transient or permanent vision loss (mainly due to trauma to the optic nerve), tonic pupil, and diplopia. The failure rate of the technique is approximately 10%, but it is not unusual for patients’ vision to worsen after an initial period of stabilisation (up to one-third of cases in the first year).42
In our setting, indication is currently limited to pregnant women and patients with fulminant IIH in whom rapid access for shunting cannot be established.
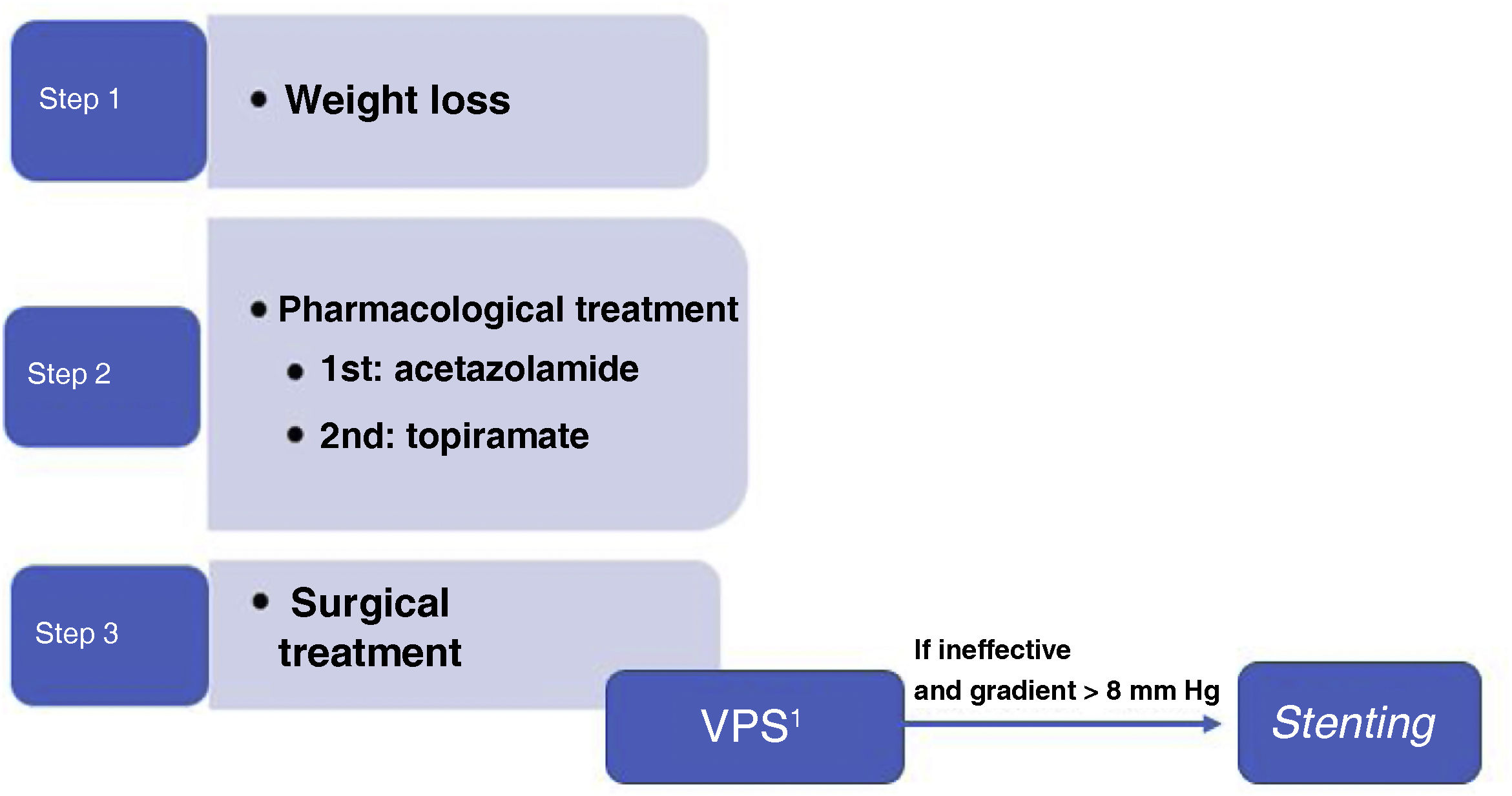
Conclusions and proposed surgical treatment algorithmWe currently lack clear scientific evidence on the different surgical options for the treatment of IIH. Therefore, selection of the type of surgical treatment remains controversial, and often depends on availability and experience at each centre. The GECSEN proposals are shown in Fig. 1, and should be adapted according to the characteristics of each centre.
PrognosisNo large patient series have been published in the literature that may characterise the natural history of this disease. The most frequent course is slow progression over a period of months or even years. If treatment is effective, prognosis is usually favourable, with symptoms improving and/or stabilising. Some patients continue to present papilloedema, high ICP, and even mild residual visual deficits.15,43
Disabling vision loss is the most significant comorbidity of IIH; fortunately, it is rare, ranging from 6% to 14% of cases according to different series, although higher rates (up to 24%) are also reported.43 The main risk factors are moderate-severe papilloedema (grade 3−5 on the modified Frisen scale) and vision loss, with or without other visual symptoms.43 Transient visual obscurations and marked vision loss as the initial manifestation are moderate and severe risk factors, respectively.
Other factors of poor prognosis include male sex, black race, younger age of onset or onset during puberty, elevated opening pressure, more severe obesity and/or significant weight gain, and elevated arterial blood pressure. Interestingly, neuroimaging findings do not predict progression.44
Lack of symptom remission is associated with certain ophthalmological parameters, such as reduced ganglion cell and retinal nerve fibre layer thickness, as measured with OCT.43
Recurrence, understood as detection of papilloedema and/or symptoms suggestive of IIH with elevated opening pressure, is described in 8% to 38% of patients, years after prolonged remission or stabilisation of symptoms. The main predisposing factor is weight gain.44 Finally, headache may persist in up to 67% of cases; headache is heterogeneous and may also be present in patients with symptom remission.45
Spontaneous intracranial hypotensionEpidemiologySIH can appear at any age, including during childhood and adolescence, but most frequently affects women older than 35 years. Very few epidemiological data are available, with only 2 studies having been performed, both at the same centre. The first calculated the incidence rate according to the number of patients attended at the emergency department over a 4-year period, finding a rate of 5 cases per 100 000 person-years.46 A prospective study by the same research group reported an annual incidence rate in the total population of 3.7 cases per 100 000 person-years, with a clear female predominance (4.3, vs. 2.9 in men).47 Incidence may be higher; the limitation of the study cited is that it included a very specific population with specific demographic characteristics. Predisposing factors are shown in Table 7.48
Causes of intracranial hypotension.
Spontaneous intracranial hypotension
|
CSF: cerebrospinal fluid.
The term spontaneous intracranial hypotension is not entirely accurate, as hypotension is not always spontaneous; furthermore, there are occasions when, despite presence of CSF leaks, pressure is not low (< 60 mm). As a result, the term “CSF hypovolaemia” has been proposed.49,50 The mechanism underlying headache and neurological manifestations is thought to be caudal displacement of cerebral structures and traction or distortion of pain-sensitive nerve endings in the cranial dura mater and its blood vessels.51 Despite a certain controversy about the mechanisms, 3 hypotheses are currently considered plausible52,53:
- 1)
Meningeal diverticula (42%): areas of dural dehiscence allowing protrusion of the leptomeninges through the dura mater, resulting in a fragile, rupture-prone outpouching. Diverticula are most frequent in the thoracic and lumbar spine, involving the nerve root or its meeting point with the dural sac. Some diverticula involve large meningeal tears that result in rapid outward flow of CSF, whereas in other cases the CSF leak is slower and more marked with Valsalva manoeuvres.
- 2)
Dural tears (27%): these are usually caused by calcified disc protrusions or sharp bone spurs, which cause longitudinal tears by impacting against the dura mater. They most frequently affect the thoracic or lower cervical spine, as calcified discs are generally more common in this region. CSF leakage tends to be rapid, leading to extensive epidural CSF collections.
- 3)
CSF-venous fistulae (3%): prevalence may increase with the development of more reliable techniques for their detection. In this entity, the spinal subarachnoid space is directly connected to a draining paraspinal vein, allowing rapid loss of CSF into the venous circulation. CSF is normally reabsorbed at the spinal nerve roots via transport across the walls of the arachnoid granulations, mediated by vacuoles. Loss of CSF volume due to CSF-venous fistulae is unregulated, resulting in decreased CSF volume and intracranial hypotension. These fistulae are most frequent in the thoracic spine, and less common in the lumbar and cervical regions. They are associated with perineural diverticula in approximately 80% of cases.
A not-insignificant percentage of cases (28%) are of undetermined cause.53
Several predisposing factors for SIH have been identified, including joint hypermobility disorders, dolichostenomelia (abnormally long limbs), Ehlers-Danlos syndrome, and Marfan syndrome. However, most patients do not present clear signs of connective tissue laxity, and genetic studies screening for associated mutations have returned negative results; therefore, further studies are needed to demonstrate the role of these conditions in predisposing to SIH.54
Clinical manifestationsThe signs and symptoms of SIH vary according to severity and progression time. The most frequent clinical manifestation is orthostatic headache, resulting from a greater reduction in ICP when standing, with pain usually appearing 15 minutes after standing up.55 Pain may be diffuse or located in the frontal, temporal, or more frequently the occipital/suboccipital regions. Pain intensity varies, and may be exacerbated by coughing or Valsalva manoeuvres. The orthostatic nature of headache is less marked, or may disappear entirely, at later stages of progression. Less typical manifestations include non-orthostatic headache, thunderclap headache (15% of cases), and headache triggered by effort.56,57
Some patients also present cranial nerve involvement, which may result in diplopia. Ophthalmoplegia is most frequently caused by involvement of the external oculomotor nerve, due to its long intracranial trajectory, which makes it susceptible to traction. Third or fourth nerve paresis in isolation is less common. Patients may present signs or symptoms related to traction of any cranial nerve, including facial palsy, dysgeusia, or hiccuping.56
Cochleovestibular manifestations (e.g., unilateral hypoacusia, dizziness, tinnitus, or vertigo) may be associated with traction of the eighth cranial nerve. However, these symptoms may also be attributed to alterations in the pressure of the perilymph/endolymph of the inner ear. These symptoms are more common among patients older than 45 years.49
Cases of cervical pain (in up to 71% of patients) and lumbar back pain have been reported.54 These symptoms may appear at the same time as orthostatic headache, or days or weeks before the other classical manifestations. Likewise, radiculopathy affecting the upper limbs (generally with cervical nerve root involvement) is reported in up to 6% of patients. It occurs at the site of the CSF leak, and is secondary to the mass effect of the extradural CSF collection.56
An unusual form of presentation is nonconvulsive status epilepticus and isolated seizures in advanced stages of SIH. This diagnosis should be considered in patients with seizures of unclear aetiology, presenting epileptiform EEG anomalies and lack of response to antiepileptic drugs.54
Drowsiness and impaired level of consciousness may appear in advanced stages of SIH. Displacement of cranial structures can cause brain herniation with cephalocaudal progression of signs and symptoms including paralysis of the lower cranial nerves, respiratory failure, and coma.
Less frequent clinical manifestations of SIH include galactorrhoea, diabetes insipidus, superficial siderosis, or such movement disorders as parkinsonism, ataxia, postural tremor, and chorea.58
In patients with SIH presenting a change in headache characteristics, we should rule out possible complications including cerebral venous thrombosis, cerebellar haemorrhage, and subdural haematoma. It is important to be aware of the potential clinical manifestations of these complications, as they are similar to those resulting from SIH.
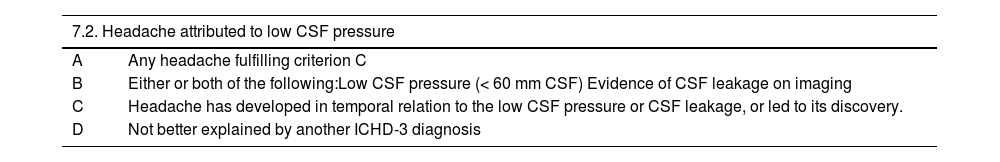
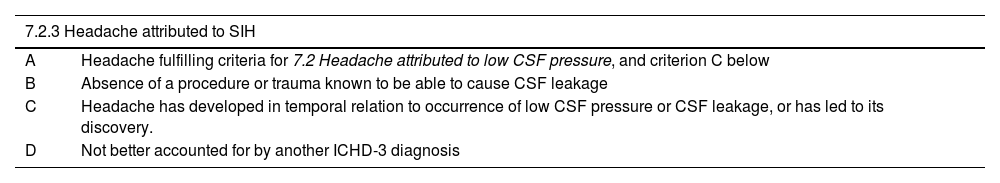
DiagnosisDiagnostic criteria for headache attributed to spontaneous intracranial hypotensionAs noted above, the most characteristic aspect of headache attributed to SIH is its orthostatic nature. However, this is not a necessary condition in the ICHD-3 diagnostic criteria,1 as the orthostatic nature of pain is not always evident, particularly in more chronic cases or in patients with low-flow fistulae. Lumbar puncture is also not a requirement for diagnosis, as some patients may present non-pathological values at the lower limit of normal; lumbar puncture may also exacerbate the patient’s clinical condition. The ICHD-3 criteria are shown in Tables 8 and 9.1
Diagnostic criteria for headache attributed to low cerebrospinal fluid pressure.1
| 7.2. Headache attributed to low CSF pressure | |
|---|---|
| A | Any headache fulfilling criterion C |
| B | Either or both of the following:Low CSF pressure (< 60 mm CSF) Evidence of CSF leakage on imaging |
| C | Headache has developed in temporal relation to the low CSF pressure or CSF leakage, or led to its discovery. |
| D | Not better explained by another ICHD-3 diagnosis |
CSF: cerebrospinal fluid; ICHD-3: International Classification of Headache Disorders (third edition).
Diagnostic criteria for headache attributed to spontaneous intracranial hypotension.
| 7.2.3 Headache attributed to SIH | |
|---|---|
| A | Headache fulfilling criteria for 7.2 Headache attributed to low CSF pressure, and criterion C below |
| B | Absence of a procedure or trauma known to be able to cause CSF leakage |
| C | Headache has developed in temporal relation to occurrence of low CSF pressure or CSF leakage, or has led to its discovery. |
| D | Not better accounted for by another ICHD-3 diagnosis |
CSF: cerebrospinal fluid; ICHD-3: International Classification of Headache Disorders (third edition); SIH: spontaneous intracranial hypotension.
Differential diagnosis of headache attributed to SIH should consider headaches that worsen with standing or improve with decubitus positions, such as migraine, headache attributed to Chiari malformation, arterial hypertension, or postural orthostatic tachycardia syndrome.
Some headaches are exacerbated by head movement, regardless of the direction of movement, such as headaches in the context of sinusitis, meningitis, primary exercise headache, alcohol-induced headache, and migraine. SIH should also be included in the differential diagnosis of thunderclap headache.52
Complementary testsDiagnosis is based on the presence of indirect radiological signs in patients with compatible symptoms and detection of the cause of the disorder. Most cases of SIH result from spinal CSF leaks.57,59 Strictly speaking, diagnosis requires the presence of low CSF pressure or identification of the leak. Many centres, even tertiary hospitals, lack some of the diagnostic and therapeutic techniques recommended in this consensus statement, or do not have sufficient experience in performing and interpreting them. This is probably influenced by the relatively low prevalence of patients on whom these techniques are performed, and the low level of certainty due to the lack of controlled trials, as a result of which their application is fundamentally empirical.
- -
CSF study: lumbar puncture is not essential to the diagnosis of SIH, and in fact may worsen the patient’s condition. If lumbar puncture is performed, measurement of opening pressure is essential. CSF biochemistry, cytology, and culture results should be normal.
- -
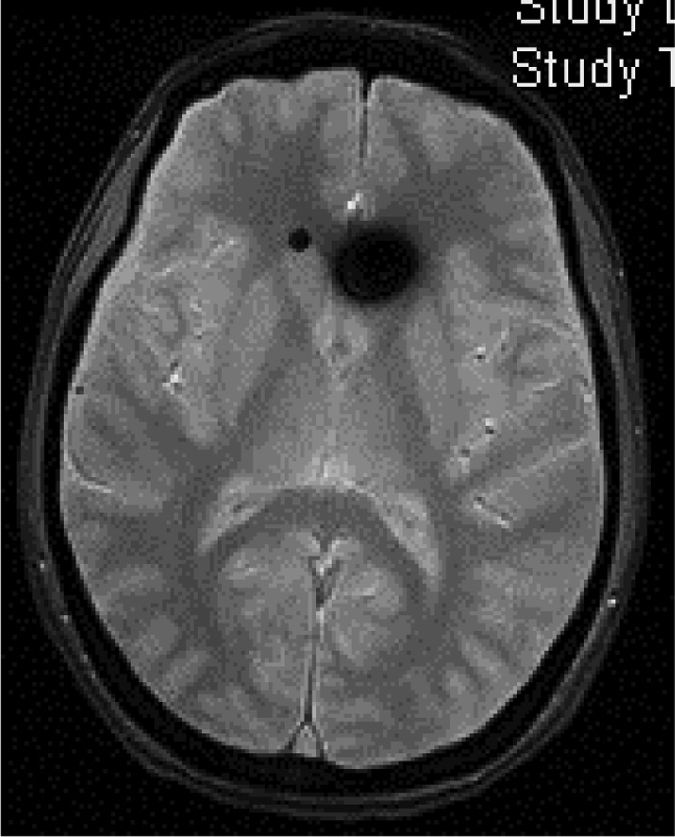
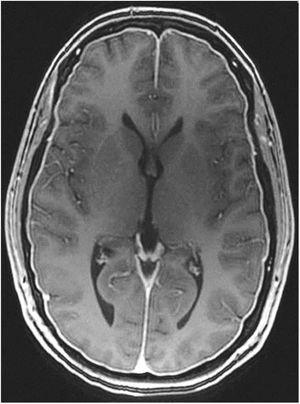
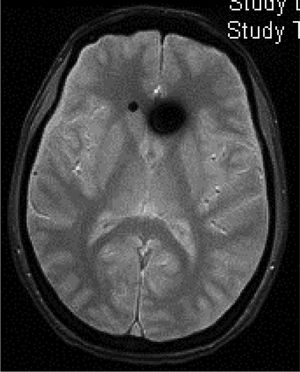
Head CT: findings are usually normal, although some studies have reported engorged venous sinuses, small ventricles, subdural fluid collections (Fig. 4), obliteration of the prepontine cistern, and images of pseudosubarachnoid haemorrhage (hyperdensity of the tentorium and Sylvian fissure).49
- -
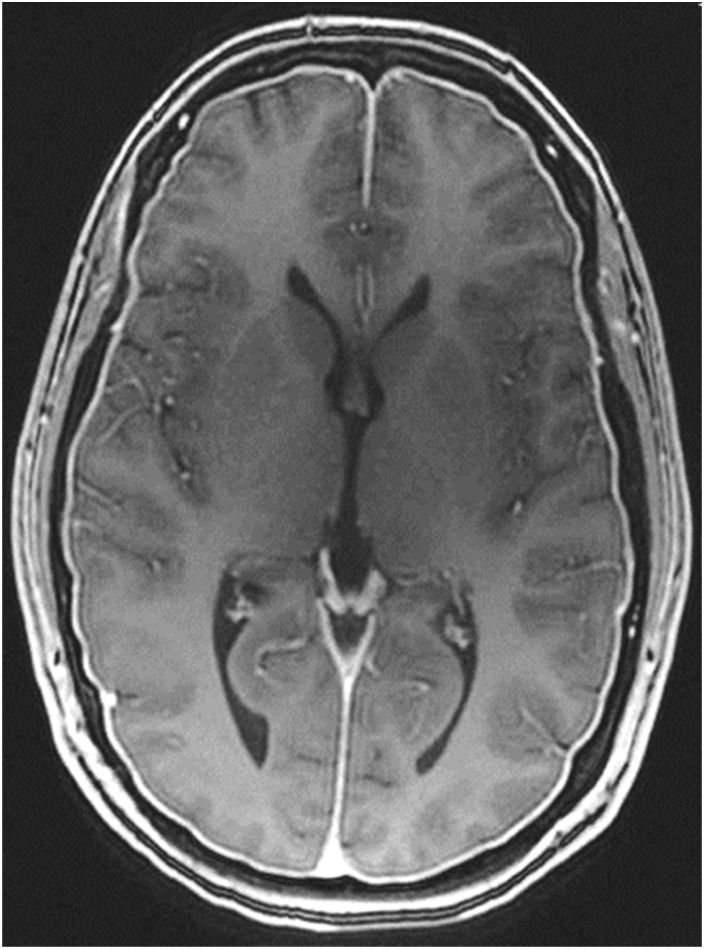
Gadolinium-enhanced brain MRI: this is the neuroimaging study of choice to confirm clinical suspicion of SIH; results are diagnostic in 80% of cases.49 MRI results from more than 2000 patients with SIH have been reported; the signs described are summarised in Table 10.48,49 Diffuse, homogeneous pachymeningeal enhancement is the most frequent radiological sign (Fig. 2). However, evaluation of these signs requires training, to prevent conclusions from being drawn arbitrarily. This has led to the development of such instruments as the Bern score (Table 11).59,60 MRI findings vary according to disease progression time.61
Table 10.Magnetic resonance imaging signs of spontaneous intracranial hypotension.48,49
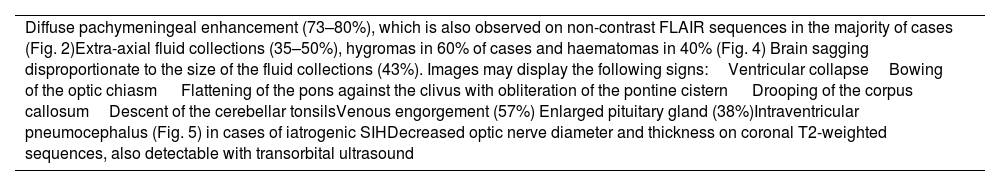
Diffuse pachymeningeal enhancement (73–80%), which is also observed on non-contrast FLAIR sequences in the majority of cases (Fig. 2)Extra-axial fluid collections (35–50%), hygromas in 60% of cases and haematomas in 40% (Fig. 4) Brain sagging disproportionate to the size of the fluid collections (43%). Images may display the following signs:Ventricular collapseBowing of the optic chiasm Flattening of the pons against the clivus with obliteration of the pontine cistern Drooping of the corpus callosumDescent of the cerebellar tonsilsVenous engorgement (57%) Enlarged pituitary gland (38%)Intraventricular pneumocephalus (Fig. 5) in cases of iatrogenic SIHDecreased optic nerve diameter and thickness on coronal T2-weighted sequences, also detectable with transorbital ultrasound SIH: spontaneous intracranial hypotension.
Figure 2.Diffuse, homogeneous pachymeningeal enhancement in the brain MRI study of a 42-year-old woman with spontaneous intracranial hypotension confirmed with cerebrospinal fluid manometry in a lumbar puncture procedure. Courtesy of Dr. Belvís. Neurology department, Hospital de la Santa Creu i Sant Pau. Barcelona.
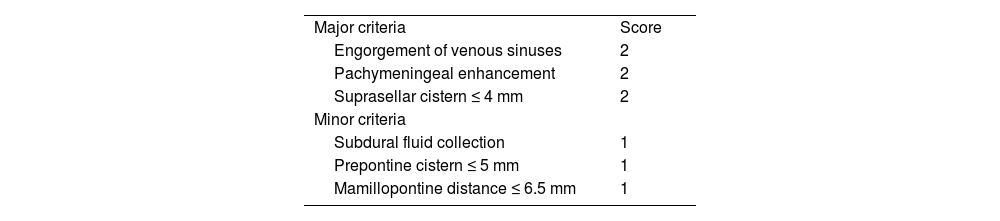
(0.09MB).Table 11.Bern score. Signs of spontaneous intracranial hypotension on brain MRI.
Major criteria Score Engorgement of venous sinuses 2 Pachymeningeal enhancement 2 Suprasellar cistern ≤ 4 mm 2 Minor criteria Subdural fluid collection 1 Prepontine cistern ≤ 5 mm 1 Mamillopontine distance ≤ 6.5 mm 1 Low risk: ≤ 2 points.
Intermediate risk: 3−4 points.
High risk: ≥ 5 points
- -
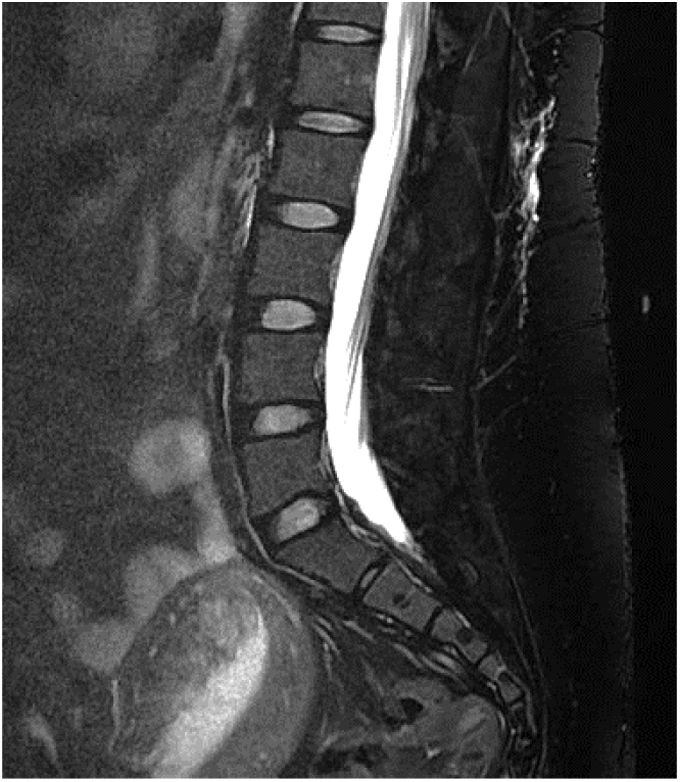
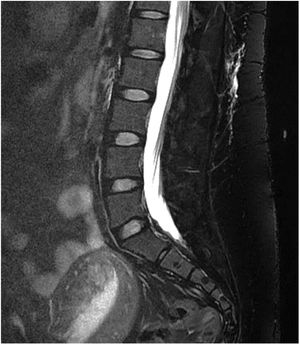
Gadolinium-enhanced spinal MRI: a fat-suppressed T2 sequence should be included. Images may show pachymeningeal enhancement, dilated nerve root sheaths, engorged epidural venous sinuses, or meningeal diverticula.50,62 In addition to indirect signs of SIH, spinal MRI may also be useful in locating the CSF leak, with the observation of fluid collections in the epidural soft tissues (Fig. 3), which are generally non-compressive (60% of cases).59 Therefore, spinal MRI should always be performed simultaneously with brain MRI if SIH is suspected.59
- -
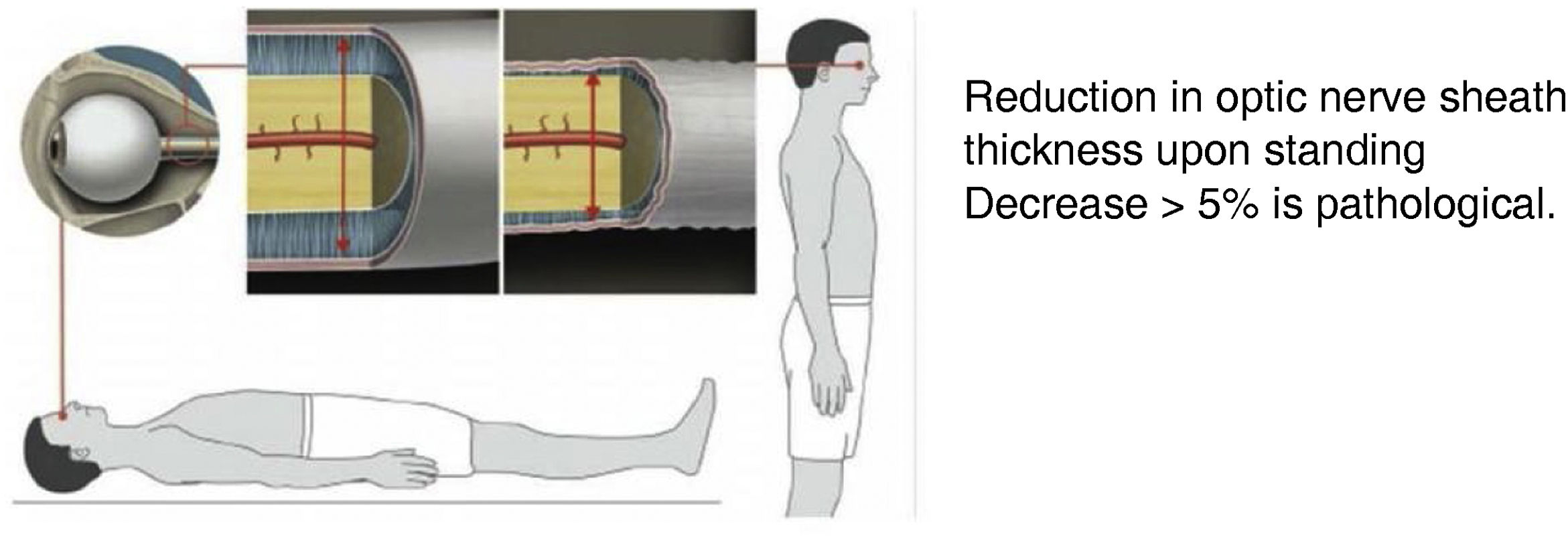
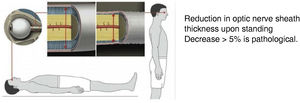
Transorbital ultrasound (Fig. 4): this test is used to measure optic nerve sheath thickness with the patient in a decubitus or seated position. Findings are classed as pathological if sheath thickness decreases by over 5% when the patient stands up.61
Figure 4.Subdural fluid collection (subdural haematoma) in the parieto-occipital convexity of a 46-year-old man with spontaneous intracranial hypotension following spinal anaesthesia administered for knee surgery. Courtesy of Dr. Belvís. Neurology department, Hospital de la Santa Creu i Sant Pau. Barcelona.
(0.07MB).Figure 5.Transorbital ultrasound study. Adapted from Fichtner et al.63
(0.13MB). - -
Other complementary studies aim to locate the CSF leak by detecting extra-arachnoid fluid collections in the spine. In iatrogenic cases (accidental dural puncture during spinal anaesthesia or following spinal surgery), these studies are not initially indicated, as the location of the leak is already known. All these techniques require a lumbar puncture, which should be performed with as little trauma as possible in order not to exacerbate SIH. This disadvantage is countered by the fact that CSF pressure can also be determined during the study. According to data from meta-analyses, these neuroimaging techniques present a sensitivity of 48% to 76% for detecting the CSF leak.50,59 CSF leaks have been classified into 4 categories (Table 12)59,60; they most frequently affect the thoracic spine (41%), followed by the cervico-thoracic (25%), cervical (14%), and lumbar regions (12%). Twenty-four percent of patients present multiple leaks.49
Table 12.Farb radiological classification of cerebrospinal fluid leaks.
Type 1 (48–60%). Ventral dural tear associated with degenerative disc disease and, occasionally, bone spurs. Accompanied by EFC Type 2 (13–20%). Lateral proximal nerve root sleeve dural tear. Not associated with degenerative disc disease but typically presents with EFC Type 3 (20%). CSF-venous fistula. Associated with meningeal diverticulum (actually, an embryonic pseudomeningocele). Not associated with EFC Type 4. Distal nerve root sheath leak. Not associated with EFC. This is the rarest type. CSF: cerebrospinal fluid; EFC: extradural fluid collection.
- -
Isotope cisternography. This study involves intrathecal injection of a radioisotope, typically indium-111. Serial CT scans are performed after injection, at 24 hours, and even at 48 hours.50 In addition to CSF leaks, the detection of isotope uptake in the kidneys/bladder before 6 hours is a very specific indicator of SIH, as is the absence of uptake in the cerebral convexities at 24 hours. This study presents the advantage that it can also detect cranial CSF leaks, with cotton swabs placed in the nasal choana displaying isotope uptake in patients with CSF rhinorrhoea. Due to its limited spatial resolution, the technique is gradually being replaced by myelography techniques.
- -
Myelography.
- o
Conventional digital subtraction myelography. This technique presents near-perfect sensitivity for detecting CSF leaks.49 The published studies only include a total of 133 patients. The technique can detect extra-arachnoid collections, meningeal diverticula, and venous-CSF fistulae. The study can be performed with the patient in the lateral decubitus position, enabling better detection of type 2 CSF leaks.
- o
CT myelography with intrathecal contrast. This technique presents the same indications and resolution as conventional myelography and is simpler to perform, and as a result is the study of first choice for CSF leaks when contrast MRI myelography findings are normal. A novel technique, dynamic CT myelography, seems to be the best option for studying high-flow leaks, which often go undetected with other studies.50,51
- o
MRI myelography with intrathecal gadolinium. This technique presents 75% sensitivity for detecting CSF leaks.49 The literature only includes reports of its performance in 87 patients. It should be noted that gadolinium contrast is not indicated for intrathecal administration, and may be neurotoxic at high doses. Furthermore, a recent meta-analysis62 including 643 patients found no differences in diagnostic performance between MRI myelography with intravenous (86%) and with intrathecal administration of gadolinium (83%).
- o
No data are available from randomised clinical trials on the treatment of SIH; therefore, the available evidence is mainly from observational studies (mostly including patients with postdural puncture headache) and expert opinions.
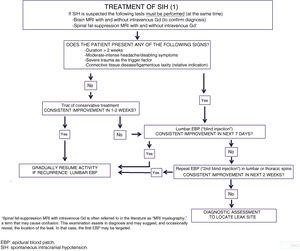
Both in spontaneous and in postdural puncture intracranial hypotension, we recommend initial conservative treatment including rest, hydration, and abdominal binder use (LE IV, GR C).
From a pharmacological perspective, most guidelines recommend the use of analgesics, corticosteroids, or caffeine. Multiple cases have been reported of clinical response to NSAIDs and corticosteroids, although their efficacy has not been demonstrated in randomised clinical trials (LE IV, GR C). The use of caffeine and other xanthines (theophylline, aminophylline) is correlated with a reduction in postdural puncture headache intensity, which tends to be transient (LE IV, GR C).64,65 Although it is widely used, the available evidence on the efficacy of caffeine is poor, and it should be noted that regular, prolonged use of the substance is a risk factor for headache chronification; therefore, short schedules are recommended. Bilateral occipital nerve block may be useful (LE IV, GR C),66 although its effect is probably also transient.
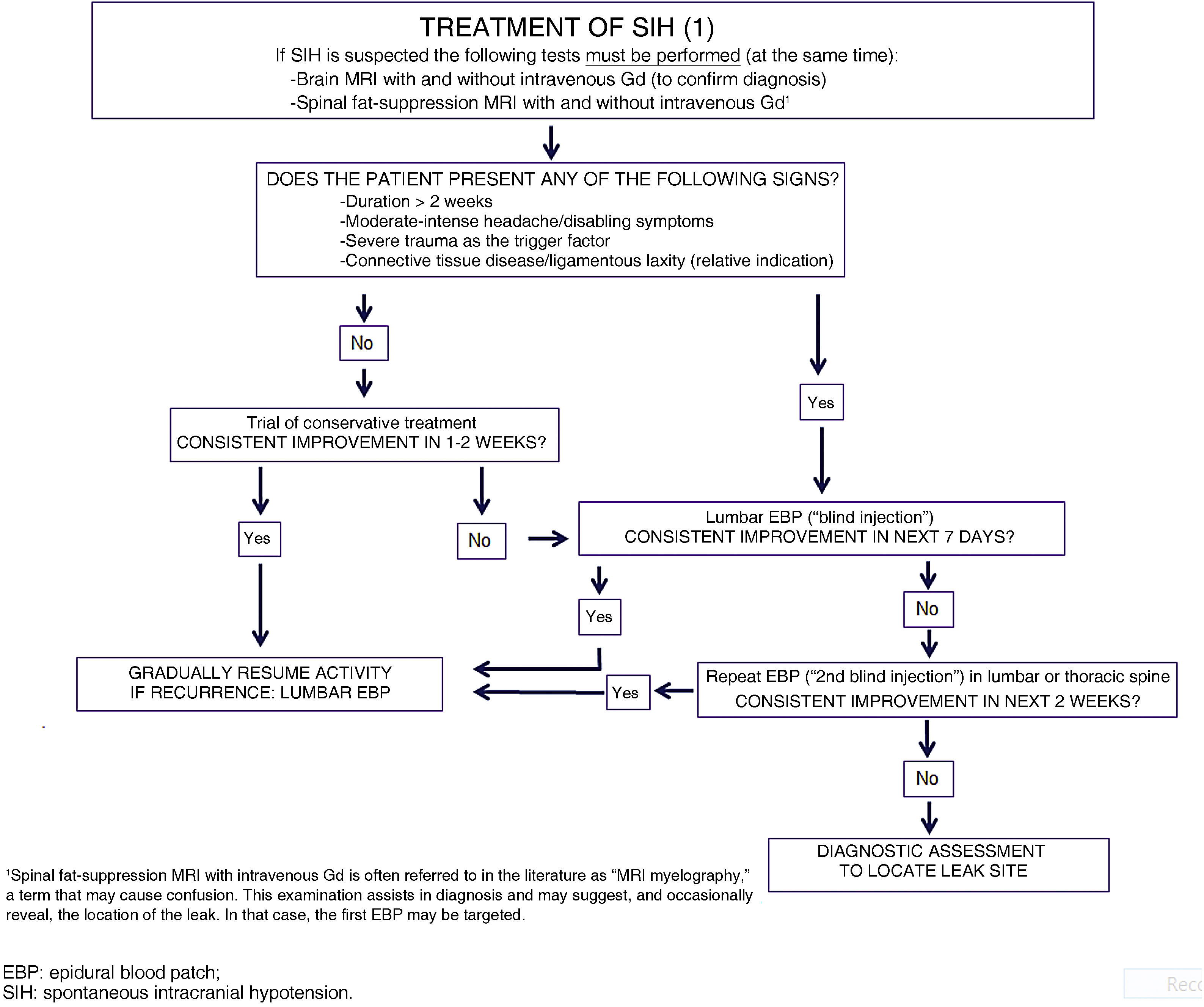
Only 15% to 30% of cases of SIH are resolved with conservative treatment.49 Given the shortage of evidence and the severity of symptoms and the associated disability, as well as the potential for severe complications, experts recommend avoiding delay in administering blood patch treatment, following neuroimaging confirmation of the diagnosis,54 which should be performed on an emergent or priority basis (LE IV, GR GECSEN).
Epidural blood patchThis treatment for SIH is essentially aetiological, aiming to close off the CSF leak. Empirical data clearly show its efficacy (LE III, GR B). Furthermore, numerous reviews and a recent meta-analysis sought to provide the most objective perspective possible for clinical practice48,67–71 and for future research, and served as the basis for this consensus statement.
The initial epidural blood patch (EBP) is usually performed at the level of the lumbar spine, with the location of the leak being unknown (“blind injection”). Indications for this treatment are summarised in Table 13. Contraindications are similar to those for any puncture technique in general, and lumbar puncture in particular. EBP has a dual effect: immediately, the injected blood is distributed throughout the epidural space, both caudally and, above all, cephalically (nearly twice as much). This increases the volume of the epidural space, compressing the sac, and counteracting the flow of CSF from the intradural space. This mechanism usually brings about very rapid (usually immediate) relief of symptoms. In the following 24-48 hours, during which time most of the blood is reabsorbed, the formation of fibrin clots and a certain degree of dural inflammation facilitate the repair and closure of the tear; this may take up to 3 weeks.
Indications for epidural blood patch treatment of spontaneous intracranial hypotension.
Patient with symptom duration > 2 weeks at time of consultation (especially if they have been resting at home).Persistence of symptoms after 1−2 weeks of conservative treatment* in:
|
The blood to be injected is collected in situ from the median cubital vein, with the patient already in position, and generally is not processed in any way; the volume injected should be sufficient to fill the epidural space, according to the constitution of the patient. Larger volumes are associated with greater likelihood of success (77% for volumes > 20 mL; 66% for smaller amounts), but also greater risk of adverse events. Injection should be slow, at a rate of 5 mL/minute. The most widely accepted approach is to inject 10-25 mL,72 although authors with extensive experience have injected up to 50 mL, in some cases, taking into account the height and weight of the patient and the length of the spine,49 even though the impact of these factors is unclear.68 In practice, the final volume administered usually depends mainly on the patient’s tolerance, with the potential appearance of back pain that may irradiate to and be associated with paraesthesia in the neck, back, or limbs. If the patient presents pain or intense paraesthesia, administration should be stopped for 30 seconds. If pain remits, administration can be continued. If it reappears, the procedure should be stopped definitively.
After the procedure, the patient should remain at rest in a decubitus position for between 2 and 24 hours, according to different sources. In this indication (SIH), longer periods (8−24 hours) are probably needed, and the patient should be in the Trendelenburg position for at least one-third of the time.49,70–72 At discharge, patients should be instructed to take certain measures to reduce the risk of recurrence (Table 14).70–72 Success rates after an initial EBP (complete response without recurrence in the next 6 months, with gradual resolution of radiological signs) range from 43% to 64%,70–73 although the latter figure may be an overestimation.48 It should be noted that EBP presents very poor efficacy in SIH due to CSF-venous fistula, and is not indicated for these patients.74–77 EBP is considered ineffective if symptoms do not remit in the 24-48 hours following administration. If this is the case, it is reasonable at this time (not before) to search more actively for the location of the CSF leak, while scheduling a second injection. Some authors only request further studies to locate the CSF leak after the second EBP attempt,49 which should in any case not be delayed, even when aetiology is unknown.
Recommendations at discharge following epidural blood patch.
| For 7 days, avoid: Physical exerciseValsalva manoeuvresBending over and crouchingLong trips in vehicles or other means of transport (rapid increase in ICP, movements, or vibrations while sitting may dislodge a stable clot) Sitting with a straight back Constipation (laxatives should be used if needed, avoiding stimulants or irritants, such as sennosides) |
ICP: intracranial pressure.
Apart from the risk of infection (for which reason the procedure should be performed under total asepsis), EBP is generally safe, although complications are possible, and can be severe in exceptional cases. The most frequent complication is lumbar back pain, which may last several weeks. Pain sometimes irradiates to the lower limbs, which may present paraesthesia or dysaesthesia, attributed to the irritative effect of blood. If these symptoms last longer than 2 weeks, other possibilities should be considered. Accidental transdural puncture may also occur, exacerbating SIH. Injection of blood into the subarachnoid space also entails a risk, albeit small, of arachnoiditis, meningitis, or ventriculitis. Another relatively frequent effect is rebound intracranial hypertension after the procedure (7%–27% of cases, considering both EBP and other percutaneous or surgical treatments), which usually responds to acetazolamide or topiramate.46Table 15 summarises all the complications described to date.74–77
Potential complications of epidural blood patch.
| Frequent (usually mild and transient) Back painLess frequent (usually mild and transient): During procedure (monitoring is recommended): 1. Bradycardia and/or hypotension 2. Palpitations 3. DizzinessNeck painRadicular painParaesthesia, hypoaesthesiaMild pyretic reactionTinnitusDural puncture Intracranial hypertension Rare (< 1%):Sixth nerve palsy, facial palsyCauda equina syndromeSpinal cord compressionArachnoiditis, meningitis, ventriculitis (aseptic, chemical, infectious)Chronic adhesive arachnoiditisSubdural haematoma, subarachnoid haemorrhage, spinal haematomaPneumocephalus Venous sinus thrombosisSuperficial siderosis |
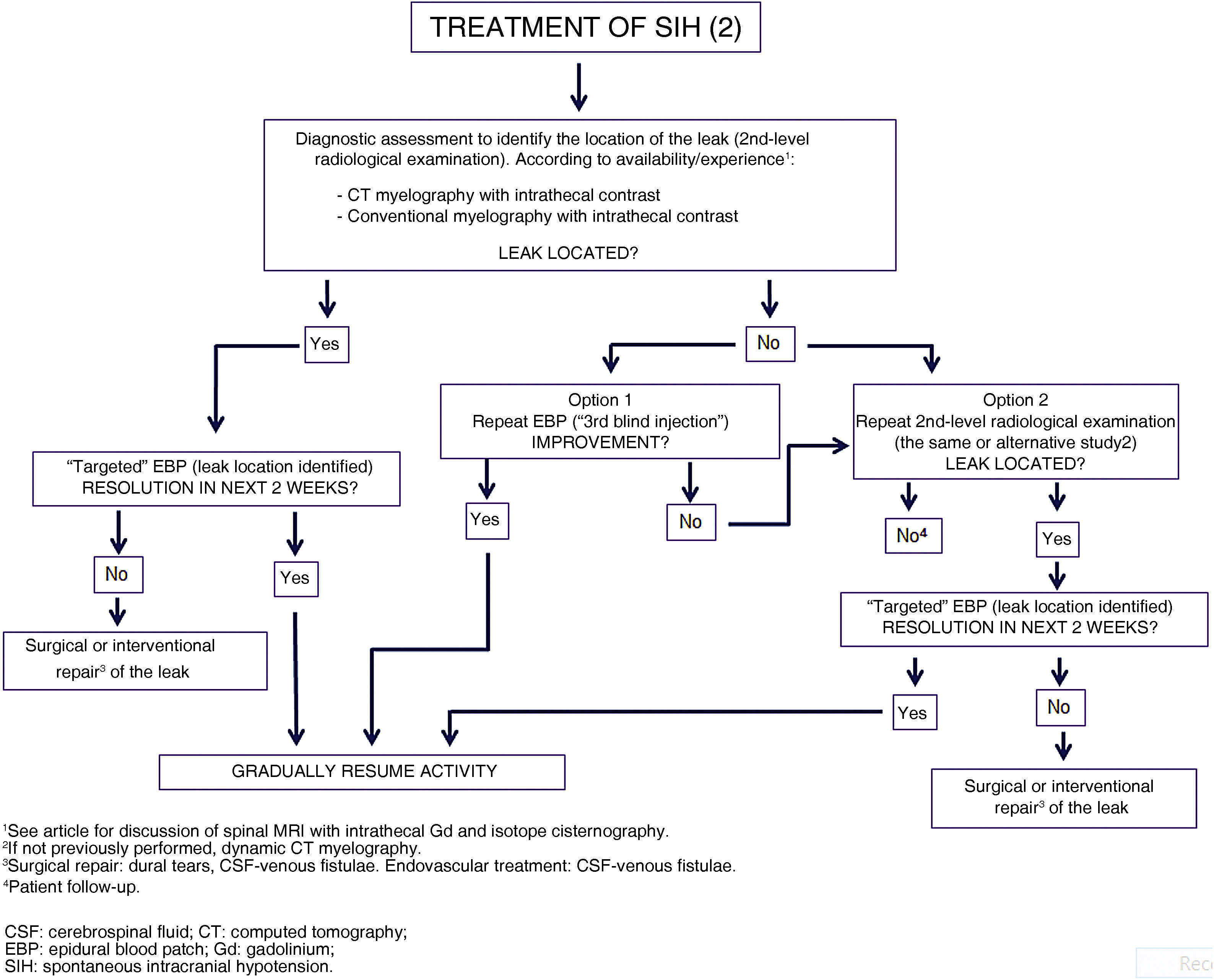
If the first injection is not effective (40%–50%), a second attempt can be made after an interval of at least 7 days. If the leak has been located by that time, the second injection can be targeted, in which case the procedure should be guided by fluoroscopy, with iodinated contrast (1−5 mL) mixed into the blood; some centres subsequently use helical CT (at 15−20 min) to confirm correct administration. Targeted injections require an interlaminal or transforaminal approach. The maximum recommended volume of blood is 20 mL for interlaminal and 5 mL for transforaminal injections. Success rates as high as 88% have been reported for these targeted injections.49
If the location of the leak is still unknown, this second injection will also be “blind,” and may also be performed at the lumbar level. However, given the fact that the majority of leaks occur at the cervical/thoracic level, this blind injection may be performed at a higher (normally thoracic) level, with radiological monitoring to minimise the risk of spinal cord damage. In any case, if results are not favourable, a third injection may be considered, taking the same factors into account.
A factor with a great impact on the success rate is the position of the patient. Placing the patient in the Trendelenburg position before, during, and after the procedure has been shown to improve the success rate in more than half of cases.71Table 16 shows other potential reasons for treatment failure.67,68 As noted above, randomised clinical trials are needed to compare differences between positions, between blind and targeted injections, and between large- and small-volume injections.
Potential causes of epidural blood patch failure in the treatment of spontaneous intracranial hypotension.
| Patient not positioned correctlyInsufficient blood volume (< 22.5 mL) Corticosteroid use (may delay dural scarring)Large CSF collection in the anterior epidural spaceSignificant diencephalic/midbrain deformity due to brain sagging |
CSF: cerebrospinal fluid.
The use of allogeneic blood has been proposed in patients with haematologic neoplasms, fever, or HIV or COVID-19 infection. In these cases, blood must be cross-matched and analysed.69
Epidural injection of saline solution or dextran is not currently recommended as this does not facilitate scarring of the dural tear.67 Fibrin glues may be an alternative, although they are not widely used76; they may be considered for targeted closure of leaks identified after failure of at least one targeted EBP. They were recently suggested as an effective alternative for closure of CSF-venous fistulae.78 These adhesives present some risk of anaphylaxis, particularly if they contain bovine aprotinin, or in patients with prior exposure, given their use for haemostasis or repair in some surgical procedures.69
Early treatment with EBP should be attempted in patients with severe SIH with altered level of consciousness and neurological deficits secondary to brain sagging; in patients presenting venous sinus thrombosis as a complication, EBP should be performed before anticoagulant treatment is started.67 An emergency temporary measure to increase ICP is intrathecal or epidural infusion of physiological saline, although few cases have been reported.69,79 Surgery may also be considered if the leak location is known. Evacuation of large subdural haematomas is not recommended without prior management of the CSF leak, as the risk outweighs the (unclear) benefit; this procedure should only be considered a posteriori in patients with ICP inversion as a complication.67
Surgical treatment. Endovascular treatmentSurgery is indicated if the location of the CSF leak is clearly identified and after failure of at least 3 EBPs, at least one of which was targeted (GR GECSEN). Table 17 presents the different available techniques, some of which can be combined.67,69 If surgery is considered to be indicated, excessive delay should be avoided.
Surgical procedures in the treatment of spontaneous intracranial hypotension.
Intraoperative neurophysiological monitoring is required.Procedures:
|
| NOTES: With current microsurgical techniques, both ventral and lateral tears (e.g., in diverticula) and CSF-venous fistulae (generally also lateral) may be treated with a minimally invasive procedure, generally following a dorsal approach via hemilaminectomy or interlaminar fenestration. Ventral dural tears require a transdural approach with separation of the denticulate ligaments, enabling mobilisation of the spinal cord outside the surgical field. If surgical and endovascular options are not possible or are ineffective, targeted EBP may be repeated. |
CSF: cerebrospinal fluid; EBP: epidural blood patch.
In patients with SIH secondary to CSF-venous fistula, it is possible to perform embolisation of paraspinal veins through which the fistula drains, resulting in its closure. The first series of patients treated with this novel technique was published recently.79
Other treatmentsBilateral greater occipital nerve block may be useful in postdural puncture headache. The benefit of this treatment is due to its effect on a caudal trigeminal nucleus receiving abnormal stimulation due to traction, contributing to pain; the only evidence available in SIH is from case reports.80 Some cases have been reported of headache attributed to SIH responding to botulinum toxin injected according to the PREEMPT protocol.81 This benefit is due to modulation of the nociceptive system, similar to that described in migraine and other primary headaches.
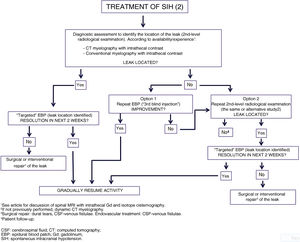
Figs. 6 and 7 propose a treatment algorithm. Given the great variability in spontaneous CSF leaks and their differing characteristics, treatment should be based at least on prospective observational studies and trials comparing different radiological and therapeutic techniques, which are not currently available.
PrognosisThere is evidence of spontaneous resolution of SIH within a period of 2 weeks82; at the other end of the spectrum, some patients may continue to present symptoms even after resolution of the CSF leak.83 Symptoms may last months or even years. If the leak is intermittent, with intervals of weeks or months, patients may present episodic headache without other symptoms between episodes. Nearly 10% of patients with spontaneous CSF leaks will present recurrence despite adequate treatment.84
Complications of CSF leaksIn addition to the most frequent symptoms of SIH, a latent or severe course may present with various complications secondary to brain sagging and traction of brain structures. The literature includes reports of visual field impairment or blurred vision due to congestion or compression of the optic nerves, upper limb symptoms due to traction or irritation of cervical nerve roots, cognitive alterations due to brain sagging or compression of the frontal or temporal lobes, hyperprolactinaemia due to distortion of the pituitary stalk, gait alterations due to spinal cord compression, chorea due to pressure on the basal ganglia or their connections, bulbar signs, and parkinsonism or ataxia due to compression of structures of the posterior fossa; more severe cases may present with symptoms of encephalopathy, reduced level of consciousness, or coma due to diencephalic compression.85
Therefore, it is essential to ensure correct diagnosis and appropriate treatment.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: García-Ull J, González-García N, Torres-Ferrús M, García-Azorín D, Molina-Martínez IFJ, Beltrán-Blasco Iet al., Diagnóstico y tratamiento de los trastornos de la presión intracraneal: documento de consenso del Grupo de Estudio de Cefaleas de la Sociedad Española de Neurología, Neurología. 2023. https://doi.org/10.1016/j.nrl.2023.06.003