Spinal cord infarction, a rare cause of paraplegia, represents less than 1% of all ischaemic strokes. Presentation as transient spinal cord ischaemia is even rarer. Given the low incidence of this condition, the frequency of different aetiologies is unknown. We present the case of a patient with intermittent spinal cord symptoms which were finally diagnosed as a dissecting aortic aneurysm presenting as spinal cord ischaemia.
Clinical caseOur patient is a 64-year-old active smoker who consumed 60g of alcohol per day and had no known allergies. He had a diagnosis of emphysema-type chronic obstructive pulmonary disease and was under treatment with tiotropium bromide, budesonide, and formoterol.
Six months prior to admission to our department, he attended another hospital due to sudden lower back pain, together with loss of strength in the lower limbs, preventing him from walking. He also reported lower limb paraesthesia with no previous overexertion. The patient spontaneously recovered strength, and symptoms disappeared several hours after his admission; he was then discharged with no identified aetiological diagnosis of the symptoms.
Six months later, he attended our hospital due to sudden lower back pain irradiating to the genital area and lower limbs, again with no previous overexertion. He reported bilateral lower limb paraesthesia (predominantly distal) and progressive loss of strength, which impeded the ability to walk.
The patient was alert and correctly oriented upon arrival, maintaining his general and eupneic condition at rest; he was afebrile, with arterial pressure of 160/99 and oxygen saturation of 97% breathing room air. Cardiorespiratory auscultation and examination of the abdomen revealed no abnormalities. The lower limbs showed bilateral lesions in the anterior tibial region, consisting of dark, scaly patches which had progressed for several months. Although no pain was observed on palpation of the spinous processes, the patient did experience pain when pressure was applied to the bilateral lumbar paraspinal muscles.
Speech, cranial nerves, strength, and lower limb sensitivity were normal. We identified paraparesis (1/5) with abolished patellar reflexes and present but weak Achilles reflexes. Plantar reflexes were flexor bilaterally. We also identified tactile, painful hypaesthesia from the L1 level. Position and vibration sense were preserved. No dysmetria or meningeal signs were observed. The patient experienced difficulty urinating and required urinary catheterisation.
A blood test performed by the emergency department revealed haemoglobin levels of 19.3g/dL and a mean corpuscular volume of 106fL. No leukocytosis or neutrophilia was detected; kidney and liver function presented normal values. We did not observe alterations in sodium, potassium, or calcium levels. Erythrocyte sedimentation rate was 18mm/h; the patient had a C-reactive protein level of 22mg/L.
We requested an emergency magnetic resonance imaging (MRI) scan of the lumbar spine. This revealed widening and increased central signal intensity in the proximal segment of the conus medullaris (at T12 level) on the T2-weighted and STIR sequences, ruling out inflammatory/infectious demyelinating disease (such as transverse myelitis), spinal cord ischaemia, and toxic-metabolic disorder. There were no signs of compressive myelopathy.
The patient was admitted due to diagnosis of myelopathy at the level of the conus medullaris. We started treatment with a daily dose of 300mg acetylsalicylic acid, prophylactic treatment with low molecular weight heparin, and step 1 analgesics. No corticosteroids were administered since no signs of spinal cord compression were detected in the MRI. At 72hours, he displayed slightly improved sensitivity and discreetly moved both lower limbs when lying down.
The blood count performed at the admission ward revealed GGT of 155U/L, LDH of 567U/L, ferritin of 360ng/mL, and LDL-cholesterol of 119mg/dL. Neuron-specific enolase level was 46.3ng/dL (normal range, 0 to 17ng/mL). Positive antinuclear antibodies were present at a low titre (1:160), displaying a fine, speckled pattern. Results were normal for the remaining analytical parameters requested: HDL-cholesterol, triglycerides, direct and indirect bilirubin, GOT, GPT, AF, serum ferritin levels, transferrin, transferrin saturation rate, total protein levels, thyroid hormones, folic acid, and vitamin B12. Anti-neutrophil cytoplasmic antibodies, anticardiolipin antibodies, and lupus anticoagulant antibodies tested negative; the serology test for Brucella, Borrelia, Treponema pallidum, and HIV also returned negative results.
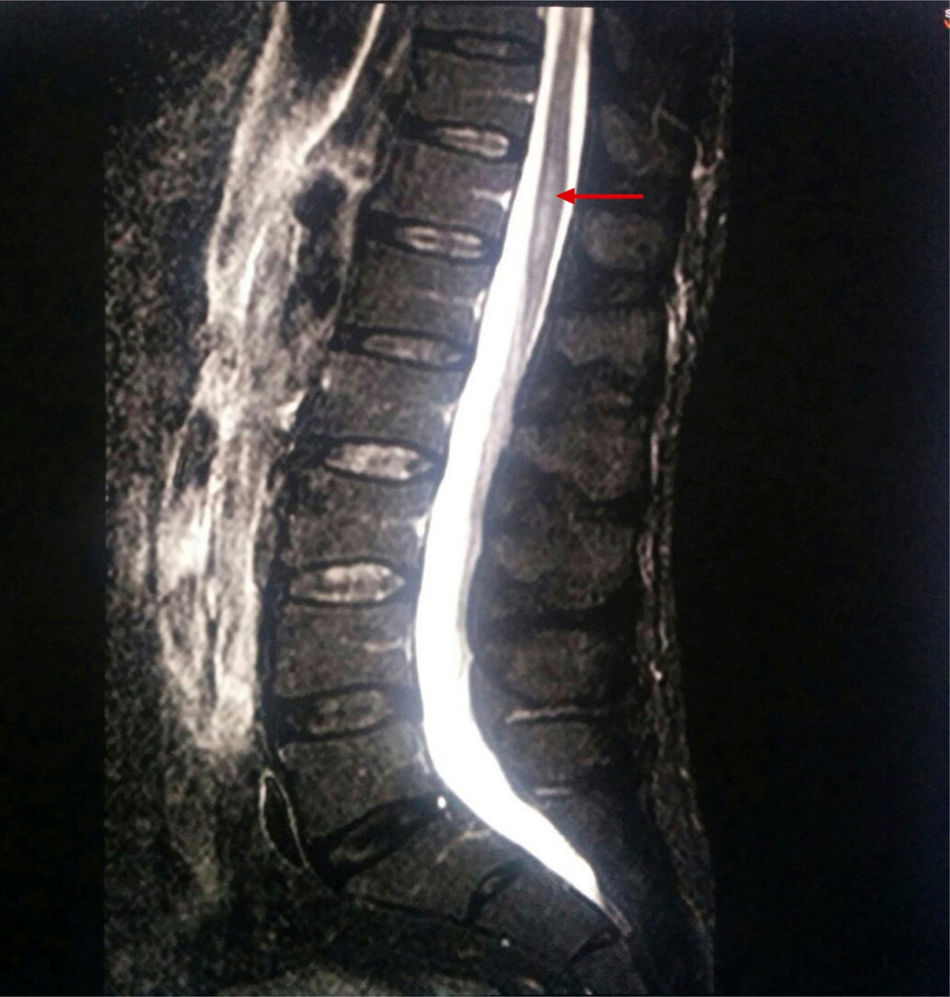
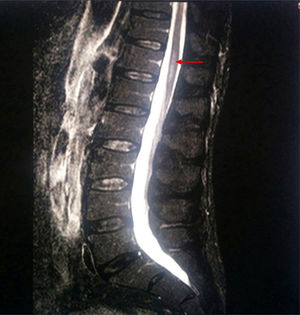
A new MRI scan including the thoracolumbar spine (Fig. 1) revealed the following findings: widening of the spinal canal and spinal cord with hyperintensity in the T2-weighted and FLAIR sequences, between T9 and T10 and the end of the conus medullaris, and abnormal diffusion restriction. Again, there were no signs of compressive myelopathy. No contrast was used in the MRI scan due to the patient's intolerance to the contrast agent and refusal to be administered contrast intravenously.
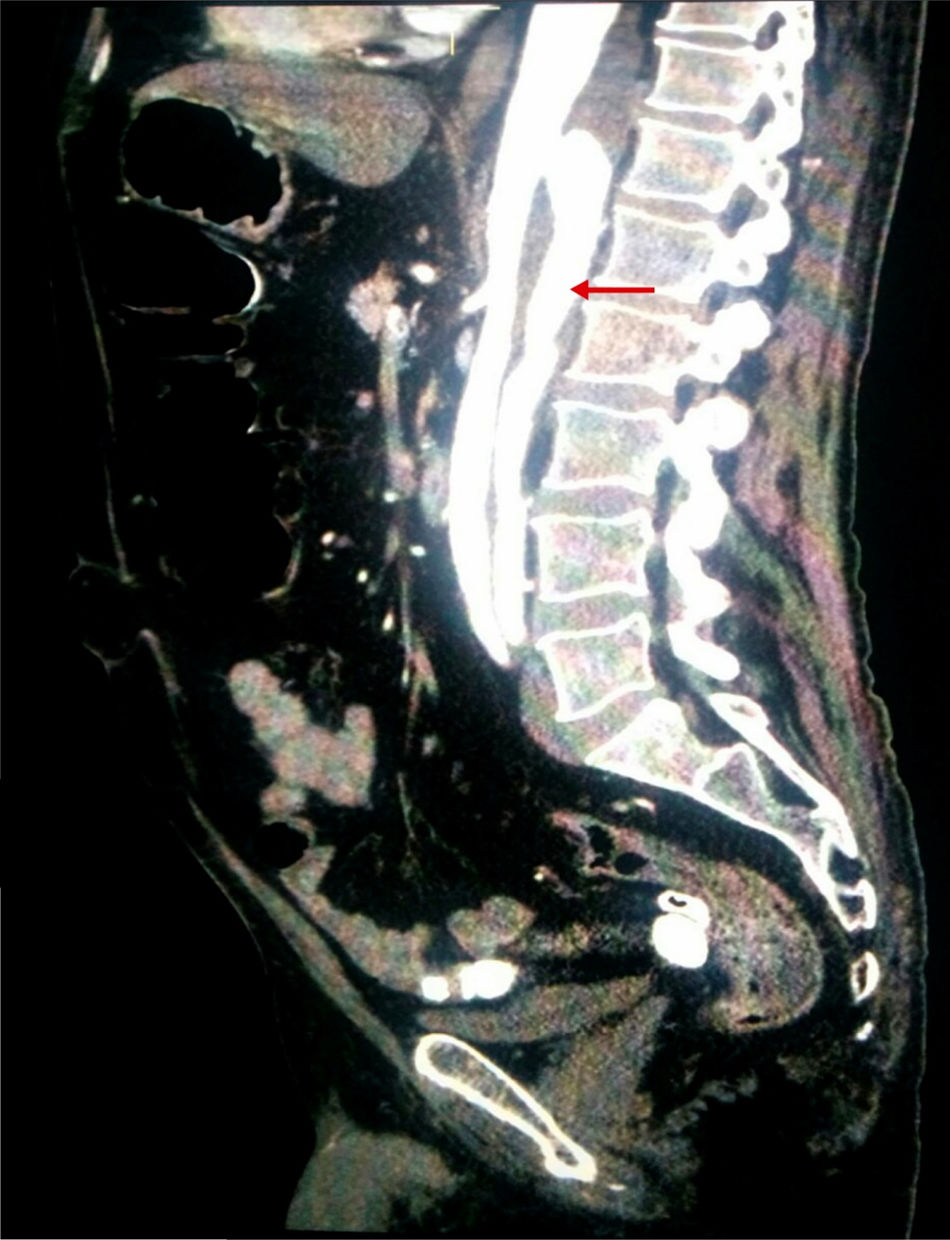
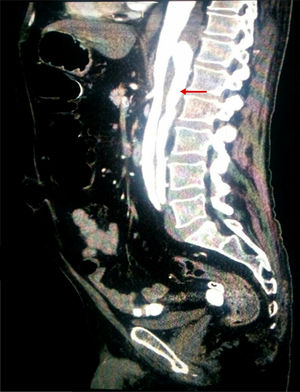
Considering the patient's vascular risk factors, we performed an abdominal CT-angiography scan (Fig. 2) to rule out acute vascular alterations. This study revealed a distal thoracic aortic dissection extending to the left renal and external iliac arteries, with false lumen in the posterior section.
He underwent emergency implantation of a thoracic endoprosthesis. The post-operative period was uneventful and the patient started rehabilitation. He was transferred to a long-stay hospital to continue treatment and rehabilitation.
DiscussionSpinal cord ischaemia is a rare disease accounting for less than 1% of all ischaemic strokes.1 Aetiology varies,2 and includes vertebral artery dissection, aortic pathology, atherosclerosis or embolism, hypotension, adverse drug reactions, and autoimmune syndromes.3 Cases have also been described in divers and in patients undergoing spinal surgery and lumbar punctures. Fibrocartilaginous embolism, caused by material from the intervertebral discs, is mentioned in the literature as a cause of spinal cord infarctions in young patients.4
Frequency of the different aetiologies is unknown, as the low incidence of this condition only provides data on personal series and isolated cases.5 No aetiological diagnosis is available for a high percentage of patients with spinal cord infarction.
Very few cases with acute symptoms and spontaneous resolution, compatible with transient spinal cord ischaemia (transient spinal cord ischaemic attack), have been reported. The literature refers to these cases as “intermittent spinal claudication”. One Spanish study6 reports the case of a patient who presented 3 transient episodes before symptom onset. In another study, a review of the post-mortem findings of 2 patients showed that the patient reporting these episodes presented advanced atheromatous lesions in the aorta.7
The spinal cord is irrigated by 3 main vessels that run along its length: the anterior spinal artery (located on the anterior median fissure) and 2 posterior spinal arteries descending medial to the dorsal roots. These arteries are supplied by different arterial branches in each spinal segment. The cervical spinal cord is supplied by the vertebral and deep cervical arteries. From the dorsal segment, the various radicular arteries (which are in turn branches of the intercostal and lumbar arteries of the aorta) are responsible for blood supply. The arteria radicularis magna or the artery of Adamkiewicz, in most cases originating at the level of the 9th to 11th intercostal arteries, supplies the lumbar enlargement of the spinal cord. Anastomosis of the anterior and posterior spinal arteries creates an arterial network in the conus medullaris. There are also transversal connections between the 3 main arterial trunks, which makes ischaemic spinal pathologies far less frequent than brain ischaemia. However, the watershed territory located in the upper thoracic region may present deficient vascularisation and be affected in a situation of global hypoperfusion.4
In young patients, dissection of the vertebral artery is a predisposing factor to spinal cord ischaemia.8 In elderly patients, aortic pathology is the main trigger factor, and has been widely reported as a complication of aortic aneurysm repair surgery9 and stenting.10 Cases reported in relation to aortic dissection account for no more than 3% of cases of this condition.1,11,12
The most frequent clinical presentation is the syndrome caused by ischaemia of the anterior spinal artery territory; symptoms are usually acute and may progress for 30 to 45minutes, with lower back or neck pain being a frequent initial manifestation.5 If the cause of the spinal cord infarction is an aortic pathology, it usually affects the blood supply of the anterior two-thirds of the spinal cord. This vascular involvement, denominated a “syndrome of the artery of Adamkiewicz”,13,4 is described as a complete or incomplete spinal syndrome with paralysis, which is initially flaccid and hyporeflexive at the level of the lesion. Signs of upper motor neuron involvement (spasticity, hyperreflexia, clonus, and Babinski sign) then manifest, as the inhibitory control exerted by the corticospinal tract on the motor neurons of the anterior horn is lost due to involvement of the tract. It is accompanied by sensory deficit caused by lesion to the spinothalamic tracts, whereas proprioceptive and vibratory sensitivity are preserved (since the dorsal columns, supplied by the posterior spinal arteries, are unaffected). Loss of sphincter control and intestinal disorders are frequent manifestations of autonomic dysfunction.
Posterior spinal syndromes are exceptional and are difficult to diagnose clinically.4
Emergency MRI is the diagnostic method of choice in patients with symptoms of spinal involvement. This imaging technique detects both extraspinal pathologies (space-occupying lesions, haematomas, infectious collections, and disc herniation) and intraspinal lesions (tumours, vascular alterations, haemorrhages, or myelitis), which should be treated as a matter of urgency.
In most cases, management is aimed at identifying and treating the underlying causes to prevent symptom worsening or relapse, and accelerating the patient's recovery. This includes surgical repair of the vascular pathology, if one is identified. Thrombolytic therapy, with only a few published cases, is complex due to the uncertainty of the initial diagnosis and the possibility of aortic dissection, which contraindicates this treatment. Antiplatelet drugs are recommended as secondary prevention in patients with cardiovascular or vascular pathology risk factors.14
Once the symptoms have been identified, rehabilitative treatment should be started. Complete motor recovery is achieved in less than one-third of patients, and is usually observed in the first 2 to 4 weeks. It is associated with better results in neurological examinations and preserved strength in the abductor and extensor muscles of the knees. More extensive deficits with no initial improvement are usually associated with poorer prognosis.15
In conclusion, spinal infarction is an infrequent, acute, and incapacitating pathology, involving a considerable impact on patients’ quality of life. There are few reported cases of patients experiencing prodromal symptoms that do not fully develop, and remit spontaneously. Maintaining a high level of suspicion, identifying treatable causes, and establishing the correct treatment must be part of our everyday therapeutic actions.
FundingThis study has received no funding.
Conflicts of interestThe authors have no conflicts of interest to declare, either directly or indirectly related with the contents of the manuscript.
The authors would like to thank the radiology department at the Complejo Hospitalario Ciudad de Jaén for their collaboration to this study, and particularly Dr Ana María Carrillo Colmenero.
Please cite this article as: Almenara Escribano MD, Jódar Morente FJ, Ortega Armenteros MC. Accidente isquémico transitorio medular como presentación de aneurisma disecante de aorta. Descripción de caso y revisión de la literatura. Neurología. 2018;33:339–342.