Unilateral resistance training has been shown to improve muscle strength in both the trained and the untrained limb. One of the most widely accepted theories is that this improved performance is due to nervous system adaptations, specifically in the primary motor cortex. According to this hypothesis, increased corticospinal excitability (CSE), measured with transcranial magnetic stimulation, is one of the main adaptations observed following prolonged periods of training. The principal aim of this review is to determine the degree of adaptation of CSE and its possible functional association with increased strength in the untrained limb.
DevelopmentWe performed a systematic literature review of studies published between January 1970 and December 2016, extracted from Medline (via PubMed), Ovid, Web of Science, and Science Direct online databases. The search terms were as follows: (transcranial magnetic stimulation OR excitability) AND (strength training OR resistance training OR force) AND (cross transfer OR contralateral limb OR cross education). A total of 10 articles were found.
ConclusionResults regarding increased CSE were inconsistent. Although the possibility that the methodology had a role in this inconsistency cannot be ruled out, the results appear to suggest that there may not be a functional association between increases in muscle strength and in CSE.
El entrenamiento de fuerza unilateral ha demostrado provocar aumentos de fuerza tanto en la extremidad entrenada como en la no entrenada. Una de las teorías actuales más aceptadas defiende que el origen de dicho aumento de rendimiento se encuentra en adaptaciones en el sistema nervioso, concretamente en la corteza motora primaria, siendo los aumentos en la excitabilidad corticoespinal (EC) medida con estimulación magnética transcraneal una de las principales adaptaciones observadas tras periodos crónicos de entrenamiento. Por ello, el principal objetivo es hacer un análisis de la literatura actual para determinar el grado de adaptación que se da en la EC y su posible relación funcional con el aumento de fuerza de la extremidad no entrenada.
DesarrolloSe llevó a cabo una búsqueda sistemática en la literatura existente entre enero de 1970 hasta diciembre de 2016 en las bases de datos online Medline (vía PubMed), Ovid, Web of Science y Science Direct con la siguiente estrategia de búsqueda: (Transcranial magnetic stimulation OR excitability) Y (strength training OR resistance training or force) Y (cross transfer OR contralateral limb OR cross education). Finalmente se incluyeron un total de 10 artículos.
ConclusionesExiste cierta inconsistencia en los resultados referentes al aumento de la EC. Aunque no se puede descartar que dicha inconsistencia se deba a aspectos metodológicos, los resultados parecen indicar que el aumento de fuerza y el incremento en la EC podrían no estar funcionalmente relacionados.
Unilateral resistance training (training of one limb with the contralateral limb remaining at rest) has been shown to increase strength not only in the trained muscle but also in the contralateral homologous muscle.1,2 This increase in the strength of the untrained limb is known as cross-education of strength in unilateral resistance training.3 Strength changes without training provide clear evidence of the great plasticity of the nervous system. Furthermore, the peculiarity of this effect has attracted much attention from researchers working in the field of rehabilitation due to its potential use for the treatment of some unilateral lesions (hemiparesis secondary to cerebrovascular accident, unilateral bone and joint lesions, etc.).
The cross-education effect may be attributed at least in part to neural adaptation.3–6 This is mainly due to the apparent lack of peripheral adaptations in the skeletal muscle of the untrained limb.7–10 In addition to this lack of peripheral adaptations, functional magnetic resonance imaging and transcranial magnetic stimulation (TMS) have revealed bilateral activation of the corticospinal tract during unilateral contractions.11–14 Repetitive ipsilateral activation during long-term resistance training is thought to cause adaptations in these structures. There is controversy, however, around which central nervous system structures are responsible for the adaptations increasing strength in the untrained limb. Our study therefore aimed to determine which cortical, subcortical, and spinal structures change with unilateral resistance training.
The studies conducted to date rule out spinal adaptations in the untrained limb: in some studies, long-term resistance training was not found to cause changes in such parameters as the Hoffmann reflex (the H-reflex test is widely used to measure possible changes in spinal motor neuron excitability).15,16 Current evidence suggests that these adaptations affect cortical (primary motor cortex, supplementary motor area, etc.) and subcortical structures (corpus callosum, cerebellum, etc.).
According to other recent studies, however, increased strength in the untrained limb may be due to adaptations in the ipsilateral primary motor cortex (M1).17–21 Most studies supporting this hypothesis have used TMS. This painless, non-invasive technique stimulates the cerebral cortex.22 Combined with electromyography (EMG), TMS is used to record motor evoked potentials (MEP), the electrical signals recorded in muscles after a magnetic pulse is applied to the primary motor cortex. MEP amplitude reflects the excitability of the corticospinal tract and the excitation/inhibition balance of interneurons initially activated with TMS.22 Therefore, a change in the response induced by TMS reveals adaptations in the corticospinal tract. Recording MEPs in the untrained limb after long-term unilateral resistance training helps determine the impact of this type of training on the corticospinal tract controlling that limb and the potential association with the cross-education effect.
As mentioned previously, the most widely accepted hypothesis postulates that the most important changes occur in the contralateral motor cortex.6 According to this hypothesis, the excitability of the untrained corticospinal tract increases, probably due to repeated ipsilateral activation during unilateral resistance training, which improves the effectiveness of efferent discharges, increasing strength. Several studies have aimed to test this hypothesis, reporting diverging results in terms of both response (increased/unchanged MEP amplitude) and the magnitude of the change and the potential correlation between changes and increased strength.17–21,23–27 This variability may be due to differences in the muscles studied, the methodology used, or the situation in which corticospinal excitability (CE) was measured.
Our main objective in this study was to perform a systematic literature review to better understand the impact of unilateral resistance training on excitability of the corticospinal tract controlling the untrained limb. We also aimed to shed light on the association between CE changes and increased strength after training. Furthermore, we analyse the different methodologies used in the literature and their potential impact on TMS results.
DevelopmentWe followed the recommendations of the 2015 “Preferred reporting items for systematic review and meta-analysis protocols” (PRISMA-P) statement.28 As our study did not involve human participants, we did not require the approval of any research ethics committee.
Search strategyWe performed a systematic review of studies published between January 1970 and December 2016 and included in the following online databases: Medline (PubMed), Ovid, Web of Science, and Science Direct. We used the following search terms: (transcranial magnetic stimulation OR excitability) AND (strength training OR resistance training OR force) AND (cross transfer OR contralateral limb OR cross education). The same search strategy was used by 2 independent researchers (DCP and SRA). We contacted the authors of the studies included whenever additional information was needed.
Study selection criteriaWe excluded duplicated articles. We read the titles and abstracts of the remaining articles to confirm they met the inclusion criteria. Whenever the title and abstract were not sufficient to determine whether an article should be included in our review, we read the full text of the article. Randomised clinical trials were included in our review only if they measured CE in the untrained hemisphere before and after a period of unilateral resistance training of any limb (upper/lower, dominant/non-dominant). The minimum period for unilateral resistance training was set at 2 weeks; no limitations were set regarding the intensity of the training. We excluded those articles including individuals under the age of 18 years or over 65 years, or individuals with neurological diseases, disorders affecting their limbs, orthopaedic injuries, or immobilisations. To ensure all articles met the inclusion criteria, inclusion in the study was subject to agreement between 2 authors (DCP and GM).
Data collectionOne of the authors (DCP) gathered the following data on the studies included in our review: authors, date of publication, sample size, sample characteristics (age and laterality), muscle group trained, training characteristics (duration, total number of sessions, volume, intensity, and exercises performed), key outcome (methodology used to measure CE), and key outcome results.
Assessment of methodological qualityThe methodological quality of the studies included was evaluated with the PEDro scale (http://www.pedro.org.au); this tool has been shown to be sufficiently reliable for use in systematic reviews.29 The scale includes 11 items, the first of which was not considered for the total score. Each item is rated either “yes” or “no”; a “yes” response is given only when the study clearly meets the criterion. The maximum PEDro score is 10. Studies with a PEDro score≥6 were considered to be of high methodological quality, whereas those with a score≤5 were considered to be of moderate to poor methodological quality. Two authors (DCP and SRA) independently evaluated the methodological quality of the articles.
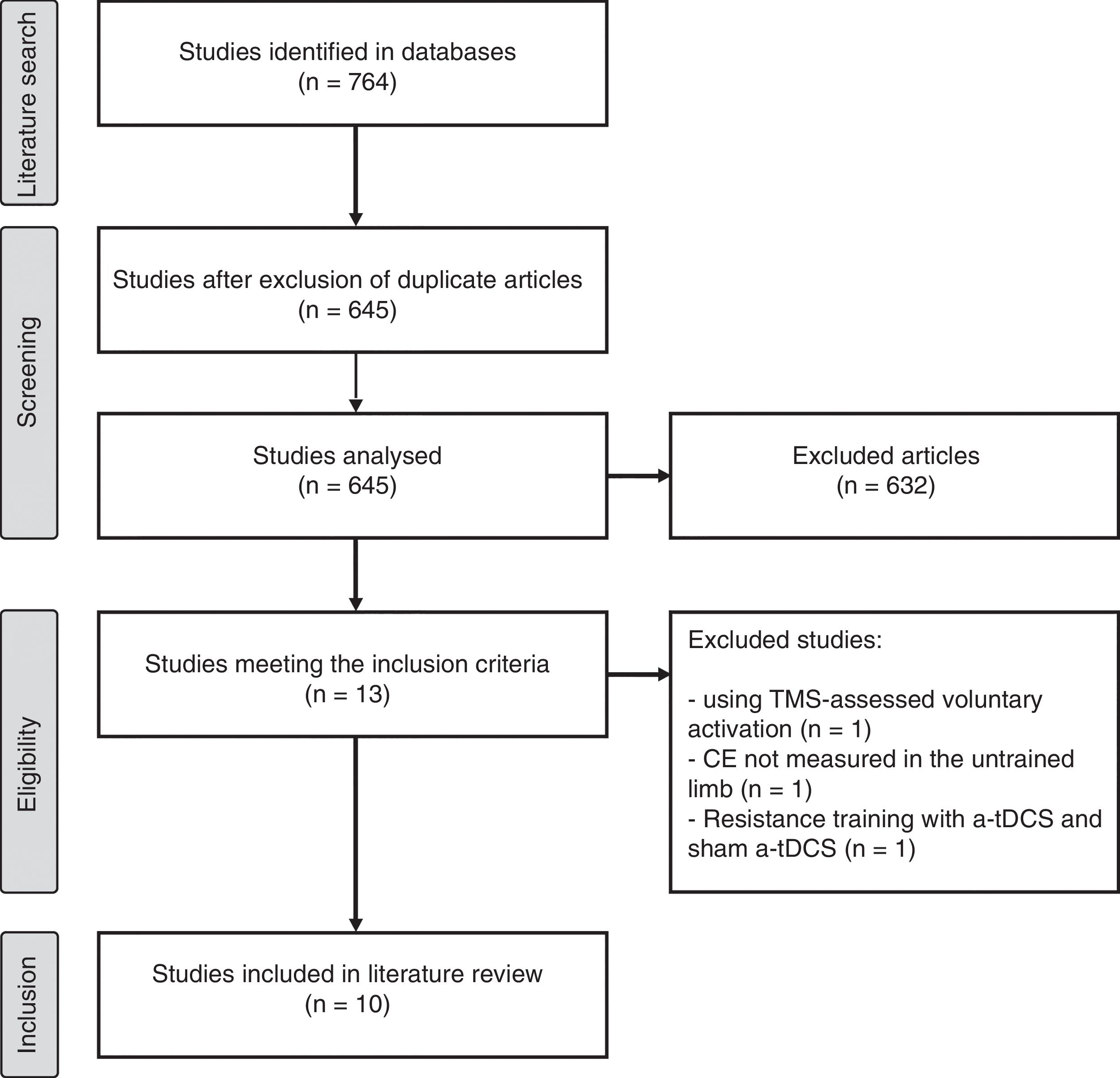
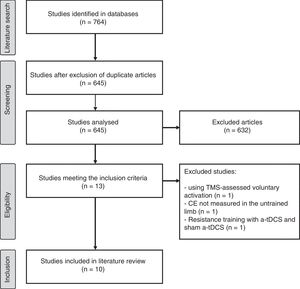
ResultsSearch resultsOur literature search yielded 764 studies, 10 of which were finally included in our review. The article selection process is summarised in Fig. 1.
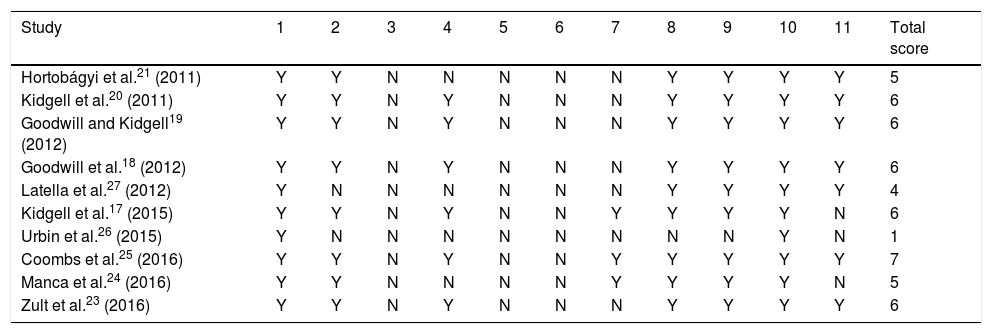
Methodological quality assessmentTable 1 summarises methodological quality scores for each study. Six studies were of high quality whereas the remaining 4 were of poor to moderate quality.
Methodological quality of studies included in the literature review.
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | Total score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hortobágyi et al.21 (2011) | Y | Y | N | N | N | N | N | Y | Y | Y | Y | 5 |
| Kidgell et al.20 (2011) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Goodwill and Kidgell19 (2012) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Goodwill et al.18 (2012) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
| Latella et al.27 (2012) | Y | N | N | N | N | N | N | Y | Y | Y | Y | 4 |
| Kidgell et al.17 (2015) | Y | Y | N | Y | N | N | Y | Y | Y | Y | N | 6 |
| Urbin et al.26 (2015) | Y | N | N | N | N | N | N | N | N | Y | N | 1 |
| Coombs et al.25 (2016) | Y | Y | N | Y | N | N | Y | Y | Y | Y | Y | 7 |
| Manca et al.24 (2016) | Y | Y | N | N | N | N | Y | Y | Y | Y | N | 5 |
| Zult et al.23 (2016) | Y | Y | N | Y | N | N | N | Y | Y | Y | Y | 6 |
1: random allocation; 2: concealment of allocation; 3: comparability of groups at baseline; 4: blinding of patients; 5: blinding of therapists; 6: blinding of assessors; 7: measures of at least one key outcome were obtained from less than 15% dropouts; 8: intention-to-treat analysis; 9: reporting between-group statistical comparisons; 10: providing point measures and measures of variability; N: does not meet the criterion; Y: meets the criterion.
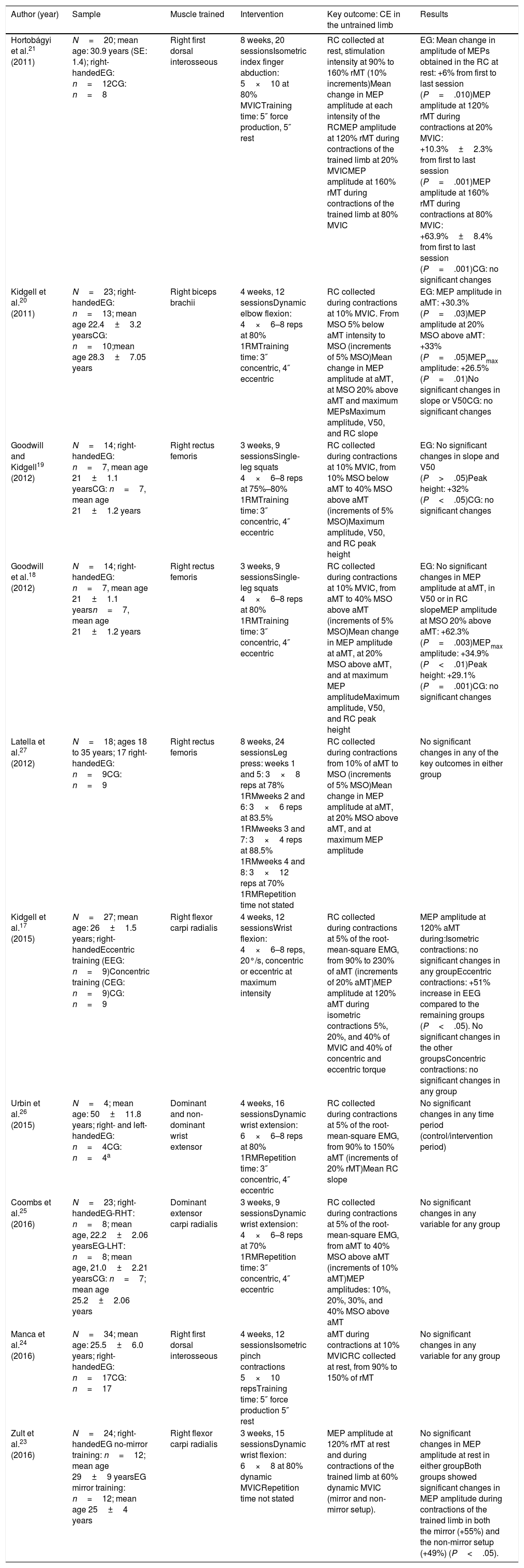
Table 2 summarises the most relevant information from each study. The studies included in our review were published between 2011 and 2016; all had a pre-post design, and all but one23 included a control group or a control period for comparisons. Sample sizes ranged from 4 to 34 individuals24,26 (mean, 20.1; total number of participants in all studies: 201), and all studies included both men and women. Six studies17,19–21,25,26 classified individuals as “untrained,” whereas the remaining 4 studies do not specify the degree of training.18,23,24,27 All but 2 participants (one in the study by Latella et al.23 and the other in the study by Urbin et al.26) were right-handed. Age ranged from 18 to 35 years, except for participants in the study by Urbin et al.,26 who had a mean age of 50 years (standard deviation: 11.8).
Characteristics of the studies included in our literature review.
| Author (year) | Sample | Muscle trained | Intervention | Key outcome: CE in the untrained limb | Results |
|---|---|---|---|---|---|
| Hortobágyi et al.21 (2011) | N=20; mean age: 30.9 years (SE: 1.4); right-handedEG: n=12CG: n=8 | Right first dorsal interosseous | 8 weeks, 20 sessionsIsometric index finger abduction: 5×10 at 80% MVICTraining time: 5″ force production, 5″ rest | RC collected at rest, stimulation intensity at 90% to 160% rMT (10% increments)Mean change in MEP amplitude at each intensity of the RCMEP amplitude at 120% rMT during contractions of the trained limb at 20% MVICMEP amplitude at 160% rMT during contractions of the trained limb at 80% MVIC | EG: Mean change in amplitude of MEPs obtained in the RC at rest: +6% from first to last session (P=.010)MEP amplitude at 120% rMT during contractions at 20% MVIC: +10.3%±2.3% from first to last session (P=.001)MEP amplitude at 160% rMT during contractions at 80% MVIC: +63.9%±8.4% from first to last session (P=.001)CG: no significant changes |
| Kidgell et al.20 (2011) | N=23; right-handedEG: n=13; mean age 22.4±3.2 yearsCG: n=10;mean age 28.3±7.05 years | Right biceps brachii | 4 weeks, 12 sessionsDynamic elbow flexion: 4×6–8 reps at 80% 1RMTraining time: 3″ concentric, 4″ eccentric | RC collected during contractions at 10% MVIC. From MSO 5% below aMT intensity to MSO (increments of 5% MSO)Mean change in MEP amplitude at aMT, at MSO 20% above aMT and maximum MEPsMaximum amplitude, V50, and RC slope | EG: MEP amplitude in aMT: +30.3% (P=.03)MEP amplitude at 20% MSO above aMT: +33% (P=.05)MEPmax amplitude: +26.5% (P=.01)No significant changes in slope or V50CG: no significant changes |
| Goodwill and Kidgell19 (2012) | N=14; right-handedEG: n=7, mean age 21±1.1 yearsCG: n=7, mean age 21±1.2 years | Right rectus femoris | 3 weeks, 9 sessionsSingle-leg squats 4×6–8 reps at 75%–80% 1RMTraining time: 3″ concentric, 4″ eccentric | RC collected during contractions at 10% MVIC, from 10% MSO below aMT to 40% MSO above aMT (increments of 5% MSO)Maximum amplitude, V50, and RC peak height | EG: No significant changes in slope and V50 (P>.05)Peak height: +32% (P<.05)CG: no significant changes |
| Goodwill et al.18 (2012) | N=14; right-handedEG: n=7, mean age 21±1.1 yearsn=7, mean age 21±1.2 years | Right rectus femoris | 3 weeks, 9 sessionsSingle-leg squats 4×6–8 reps at 80% 1RMTraining time: 3″ concentric, 4″ eccentric | RC collected during contractions at 10% MVIC, from aMT to 40% MSO above aMT (increments of 5% MSO)Mean change in MEP amplitude at aMT, at 20% MSO above aMT, and at maximum MEP amplitudeMaximum amplitude, V50, and RC peak height | EG: No significant changes in MEP amplitude at aMT, in V50 or in RC slopeMEP amplitude at MSO 20% above aMT: +62.3% (P=.003)MEPmax amplitude: +34.9% (P<.01)Peak height: +29.1% (P=.001)CG: no significant changes |
| Latella et al.27 (2012) | N=18; ages 18 to 35 years; 17 right-handedEG: n=9CG: n=9 | Right rectus femoris | 8 weeks, 24 sessionsLeg press: weeks 1 and 5: 3×8 reps at 78% 1RMweeks 2 and 6: 3×6 reps at 83.5% 1RMweeks 3 and 7: 3×4 reps at 88.5% 1RMweeks 4 and 8: 3×12 reps at 70% 1RMRepetition time not stated | RC collected during contractions from 10% of aMT to MSO (increments of 5% MSO)Mean change in MEP amplitude at aMT, at 20% MSO above aMT, and at maximum MEP amplitude | No significant changes in any of the key outcomes in either group |
| Kidgell et al.17 (2015) | N=27; mean age: 26±1.5 years; right-handedEccentric training (EEG: n=9)Concentric training (CEG: n=9)CG: n=9 | Right flexor carpi radialis | 4 weeks, 12 sessionsWrist flexion: 4×6–8 reps, 20°/s, concentric or eccentric at maximum intensity | RC collected during contractions at 5% of the root-mean-square EMG, from 90% to 230% of aMT (increments of 20% aMT)MEP amplitude at 120% aMT during isometric contractions 5%, 20%, and 40% of MVIC and 40% of concentric and eccentric torque | MEP amplitude at 120% aMT during:Isometric contractions: no significant changes in any groupEccentric contractions: +51% increase in EEG compared to the remaining groups (P<.05). No significant changes in the other groupsConcentric contractions: no significant changes in any group |
| Urbin et al.26 (2015) | N=4; mean age: 50±11.8 years; right- and left-handedEG: n=4CG: n=4a | Dominant and non-dominant wrist extensor | 4 weeks, 16 sessionsDynamic wrist extension: 6×6–8 reps at 80% 1RMRepetition time: 3″ concentric, 4″ eccentric | RC collected during contractions at 5% of the root-mean-square EMG, from 90% to 150% aMT (increments of 20% rMT)Mean RC slope | No significant changes in any time period (control/intervention period) |
| Coombs et al.25 (2016) | N=23; right-handedEG-RHT: n=8; mean age, 22.2±2.06 yearsEG-LHT: n=8; mean age, 21.0±2.21 yearsCG: n=7; mean age 25.2±2.06 years | Dominant extensor carpi radialis | 3 weeks, 9 sessionsDynamic wrist extension: 4×6–8 reps at 70% 1RMRepetition time: 3″ concentric, 4″ eccentric | RC collected during contractions at 5% of the root-mean-square EMG, from aMT to 40% MSO above aMT (increments of 10% aMT)MEP amplitudes: 10%, 20%, 30%, and 40% MSO above aMT | No significant changes in any variable for any group |
| Manca et al.24 (2016) | N=34; mean age: 25.5±6.0 years; right-handedEG: n=17CG: n=17 | Right first dorsal interosseous | 4 weeks, 12 sessionsIsometric pinch contractions 5×10 repsTraining time: 5″ force production 5″ rest | aMT during contractions at 10% MVICRC collected at rest, from 90% to 150% of rMT | No significant changes in any variable for any group |
| Zult et al.23 (2016) | N=24; right-handedEG no-mirror training: n=12; mean age 29±9 yearsEG mirror training: n=12; mean age 25±4 years | Right flexor carpi radialis | 3 weeks, 15 sessionsDynamic wrist flexion: 6×8 at 80% dynamic MVICRepetition time not stated | MEP amplitude at 120% rMT at rest and during contractions of the trained limb at 60% dynamic MVIC (mirror and non-mirror setup). | No significant changes in MEP amplitude at rest in either groupBoth groups showed significant changes in MEP amplitude during contractions of the trained limb in both the mirror (+55%) and the non-mirror setup (+49%) (P<.05). |
1RM: one-repetition maximum; aMT: active motor threshold; CEG: concentric experimental group; CG: control group; EEG: eccentric experimental group; EG: experimental group; LHT: left hand training; MEP: motor evoked potential; MSO: maximal stimulator output; MVIC: maximum voluntary isometric contraction; RC: recruitment curve; reps: repetitions; RHT: right hand training; rMT: resting motor threshold; V50: stimulus intensity at which the MEP amplitude is halfway between top and bottom.
Unilateral resistance training involved the upper limb in 7 studies17,20,21,23–26 and the lower limb in the remaining 3.18,19,27 The duration of the intervention period ranged from 3 to 8 weeks (9 to 24 sessions). Eight studies used dynamic muscle contractions17–20,23,25–27 whereas the other 2 used isometric contractions.21,24 All studies used high-intensity resistance training, with intensities ranging from 70% of one-repetition maximum (1RM)25 to maximal contractions (isometric or dynamic).17,24 The most frequent intensity was approximately 80% of maximum strength (1RM or maximal voluntary isometric contraction [MVIC]).18–21,23,26,27
Key outcomeChanges in the strength of the untrained limb as a result of unilateral resistance training were not regarded as the key outcome in our literature review, since previously published systematic reviews with meta-analyses have confirmed the effectiveness of this type of training for improving strength in the untrained limb.1 However, the studies included in our literature review do confirm the existence of the cross-education effect. Though not displayed in Table 2, all interventions achieved significant strength increases in the untrained limb as compared to baseline strength and to controls. Strength in the untrained limb increased by 6.4%24 to 61%23; the upper limit may have been due to the use of a mirror, which creates a visual illusion of movement in the untrained limb.
Regardless of the methodology used to measure CE, 6 studies show increased CE17–21,23 (from 6%, with both limbs at rest, to 63% and 62.3% during trained and untrained contraction, respectively), whereas the other 4 studies report no changes in CE.24–27 The methodologies used to measure the key outcome varied greatly. One of the main differences between studies is when CE was measured: with the untrained limb at rest in 3 studies,21,23,24 during low-intensity isometric contraction of the untrained limb in 8 studies,17–20,24–27 and during contraction of the trained limb in 2 studies.21,23 The degree of contraction for CE measurement was also heterogeneous, with contractions ranging from 10% MVIC18–20,24,27 or 5% of the pre-stimulus root-mean-square on EMG during MVIC of the untrained limb17,25 to 20%, 60%, and 80% MVIC of the trained limb.21,23 Other methodological differences include the way in which CE was measured. Most studies collected recruitment curves for MEP amplitudes with different stimulus intensities, from intensities corresponding to the motor threshold or slightly below it to different percentages of stimulator output. Two exceptions are the studies by Zult et al.23 and Kidgell et al.17 The former only obtained MEP amplitudes at a stimulus intensity of 120% of the active and resting motor thresholds. The latter collected recruitment curves but only reported data for changes in MEP amplitude for a stimulus intensity of 120% of the active motor threshold during different degrees of isometric and dynamic contraction. However, although most studies collected recruitment curves, different stimulus intensities were used. Five studies used intensities ranging from 5% to 10% of maximum stimulator output,18–20,25,27 whereas other studies used intensities ranging from 10%21,24 to 20%17,26 of the motor threshold. Furthermore, not all studies analysed recruitment curve data in the same way. One study measured the change in the mean amplitude of all MEPs recorded during all intensities used in the recruitment curve.21 Other studies measured changes in MEP amplitude at certain stimulus intensities: motor threshold, 120% of the motor threshold, 20% of the maximum stimulator output above the motor threshold, or changes in maximum MEP amplitude for the most frequently used values.17,18,20,23–25,27 Some studies used the nonlinear Boltzmann sigmoid equation to measure changes in maximum MEP amplitude, V50 (stimulus intensity at which the MEP amplitude is halfway between top and bottom), and slope.18–20
DiscussionOur study aimed to determine the impact of unilateral resistance training on CE in the untrained hemisphere. Although the studies included in our review report varying results, most show increased excitability of the corticospinal tract controlling the untrained limb after a training period of at least 3 weeks.17–21,23
According to several studies, unilateral contractions facilitate MEPs in the ipsilateral hemisphere; this phenomenon is known as cross-facilitation.11–14,30 Cross-facilitation seems to be cortical in origin, and is probably due to transcallosal or interhemispheric connections between the right and the left M1: facilitation has been observed only when MEPs are induced with TMS, but not with direct stimulation of corticospinal axons via the cervicomedullary junction.14,30 The phenomenon is thought to be responsible for chronic increases in ipsilateral CE following long-term unilateral resistance training. Long-term increases in CE may be due to changes in the properties of corticospinal neuronal membranes or increases in the efficacy of excitatory synapses towards those neurons, strengthening corticomotor projections.19,21 However, none of the studies included allow us to rule out the hypothesis that modulation of TMS-induced MEPs following ipsilateral resistance training is not due to changes in the spinal cord. Therefore, although other studies examining the H-reflex in the untrained limb after unilateral resistance training report no changes in motor neuron excitability,15,16 we cannot state that increased CE is solely due to cortical adaptations.
A number of studies suggest a causal relationship between increased CE and increased strength in the untrained limb.17–21 It should be stressed, however, that several of the studies included report no changes in CE,24–27 although all interventions increased strength in the untrained limb; this suggests that these 2 variables may not be related. Several factors may have been responsible for the lack of changes in CE in these studies. For example, the specificity of the task used to evaluate neural changes related to the training task seems to be decisive when measuring adaptations.31,32 In the study by Latella et al.,27 the absence of changes in CE may have been due to lack of specificity of the training task (leg press, multiple joint exercises) to the TMS testing task (leg extension, single joint exercises). Manca et al.24 report a 6.4% increase in the strength of the untrained limb, a modest increase compared to those reported by other studies included in our review.17–21,23 This small increase in the strength of the untrained limb may have limited CE changes in the muscle studied: as the authors themselves suggest, increased strength may also be due to improved activation of other muscles that stabilise the wrist. The study by Urbin et al.26 is among those that report no changes in CE after unilateral resistance training. Only 4 individuals in the study received TMS; changes in the corticospinal tract may have gone undetected due to the small sample size. In the study by Coombs et al.,25 the only possible explanation for the lack of CE changes is the limited number of sessions; however, neural adaptations have traditionally been thought to be responsible for increased strength after the first training sessions. Furthermore, the researchers report strength increases of 10%-15% after only 9 sessions.
However, while these factors may have had an impact on the lack of CE changes, the lack of changes itself may point to the lack of any functional relationship between increased strength in the untrained limb and increased excitability of the corticospinal tract controlling that limb. In fact, 2 of the 6 studies reporting improvements in CE results suggest that CE may not be linked to increased strength.17,23 The study by Kidgell et al.17 provides a clear example of this; the study included a control group, a group receiving concentric training, and another group receiving eccentric training. Eccentric training was found to improve performance to a greater extent than concentric training, both in MVIC (43% vs 11%) and in concentric (49% vs 28%) and eccentric torque (47% vs 14%); this is consistent with the findings of previous studies reporting a more marked cross-education effect in eccentric resistance training.33 CE was measured during isometric, concentric, and eccentric contractions, but significant increases in all CE measures were only observed during eccentric contractions. Although this points to a certain degree of specificity of the situation in which CE is measured to the trained movement, it shows that the group performing eccentric contractions, who also displayed improvements in isometric and concentric strength of the untrained limb, experienced no CE changes in those situations. Furthermore, the group receiving concentric training showed improvements in maximal concentric strength of the untrained limb during concentric contractions, with no changes in CE.
The study by Zult et al.23 provides another example of the lack of correlation between CE changes and increased strength in the untrained limb. The authors used a strength training programme based on dynamic wrist flexion contractions at 60% 1RM. In one group, a mirror was placed between both wrists during training so that participants could see the reflection of the trained limb, creating the illusion of the untrained limb performing the training; the other group received no visual feedback. Both groups displayed similar increases in M1 excitability on the untrained side during contractions of the trained limb. However, the group receiving mirror training showed more marked strength increases in the untrained limb than the no-mirror training group (61% vs 34%), which shows that greater cross-education of strength in the untrained limb was not accompanied by greater excitability in the untrained M1.
Only 2 studies analysed the correlation between strength and CE, with conflicting results.20,21 Kidgell et al.20 reported a moderate correlation (r=0.57; P=.04) between increased strength in the untrained limb and CE during contraction of the untrained limb. Hortobágyi et al.21 found no correlation (r=0.20; P=.293) between these variables when CE was measured at rest. The remaining studies found no correlation between strength and CE. Hortobágyi et al.21 did observe a correlation between increased strength in the untrained limb and increased MEP amplitude after stimulation of the untrained M1 during contractions of the trained limb. Given that measuring CE during contractions of the trained limb is not specific to a situation where strength changes occur, this correlation probably suggests that the greater the activity in M1 at rest (as a result of contraction of the ipsilateral limb) and the greater the MEP amplitude, the greater the stimulus received by that hemisphere (not only M1 or the structures tested with single-pulse TMS); a higher degree of improvement in the untrained limb may therefore be expected.
The cross-education effect may therefore not be attributed exclusively to adaptations specifically in the ipsilateral M1; rather, changes may also occur in other areas of the ipsilateral cerebral cortex, directly or indirectly connected with M1,34 or even at the spinal level.35 In fact, such areas as the supplementary motor cortex; sensory regions; the prefrontal, premotor, cingulate, and parietal cortices; and the cerebellum have shown bilateral activation during unilateral contractions36; repeated crossed activation may therefore result in long-term adaptations. Farthing et al.9 observed that unilateral resistance training of the flexor carpi ulnaris muscle increased strength in the untrained limb by 47.1%, with functional magnetic resonance imaging revealing changes in the cortical activation pattern during training. More specifically, participants showed increased activation of the contralateral sensorimotor cortex and the ipsilateral temporal lobe after training; these areas are associated with motor learning.37 These findings support the hypothesis that adaptations underlying increased strength of the untrained limb may be due to changes in non-cortical areas linked to motor planning.34,38 Further research is needed to locate other possible adaptive brain regions.
Adaptations may also occur in M1 structures other than those associated with MEP generation after single-pulse TMS. For example, changes in the excitation/inhibition balance of M1 intracortical circuits due to decreased excitability of GABAergic inhibitory interneurons may also play a role in the cross-education effect of unilateral resistance training. In fact, there is evidence of a slight decrease in intracortical inhibition following unilateral resistance training,17,18 in both the trained and the untrained hemispheres. This suggests that changes in synaptic efficacy between inhibitory interneurons and corticospinal neurons may play a role in the strength increases observed following unilateral resistance training.
Our findings reveal great variability in the methodologies used to measure CE changes; the fact that some studies observed no changes in CE may therefore be due to methodological reasons. One of the main factors affecting CE results is the situation in which CE is measured. As previously suggested, in order to detect any neurophysiological adaptations after training, the task performed during testing should be similar to that performed during training.31,32
It therefore seems logical to test CE during contractions of the muscle where changes are expected to occur: this way, the M1 structures evaluated will be in a similar situation to that in which increased strength was observed. Furthermore, evaluating CE in the untrained hemisphere during contraction of the trained limb may show the level of cross-activation of the apparently inactive hemisphere, reflecting the training stimulus received by that hemisphere (either M1 or other areas with an impact on the ipsilateral M1). However, although it may be useful for measuring potential changes in the level of cross-activation, and therefore in the training stimulus received by the untrained hemisphere, this situation is far from similar to the situation in which strength changes are measured. It would be interesting to determine whether specificity only applies to the task and to the type of contraction, or whether the intensity of contractions performed while measuring EC may also play a role, since all the studies included collected recruitment curves or even measurements at a fixed stimulus intensity during low intensity contractions (10% MVIC or 5% of the root-mean-square EMG during MVIC).17–20,24–27 Comparisons between studies can be difficult if CE is evaluated with different variables: some studies report changes in MEP amplitude at different stimulus intensities or even at a fixed stimulus intensity, whereas other studies evaluate peak slope, V50, or the maximum value of the nonlinear Boltzmann sigmoid curve.18–20
ConclusionIncreased CE in the untrained hemisphere after unilateral resistance training is a controversial issue. Sixty percent of studies report increased CE in the untrained M1 region, whereas 40% observe no significant changes. Furthermore, our results suggest that strength and CE changes may not be related. Therefore, although the lack of change in CE may be due to methodological limitations in some studies, it seems reasonable to hypothesise that the adaptations underlying the cross-education effect of unilateral resistance training may be due to changes to other areas of the untrained hemisphere linked to motor planning, or even to changes to M1 structures other than those determining or affecting MEP amplitude after single-pulse TMS. In conclusion, the effect of long-term unilateral resistance training on CE in the untrained hemisphere continues to be subject to debate. Future studies should adopt methodologies achieving greater specificity of testing conditions and greater homogeneity and detail in the measures obtained, and analyse the correlation between increased strength and changes in CE in order to determine the potential functional association between them.
FundingThis study was financed by the Spanish Ministry of Economy, Industry, and Competitiveness (project reference: PSI2015-71061-P).
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Colomer-Poveda D, Romero-Arenas S, Hortobagyi T, Márquez G. ¿Desempeña un papel decisivo la excitabilidad corticoespinal ipsilateral en el efecto cruzado provocado por el entrenamiento de fuerza unilateral? Una revisión sistemática. Neurología. 2021;36:285–297.