The term Fisher-Bickerstaff syndrome (FBS) has been proposed to describe the clinical spectrum encompassing Miller-Fisher syndrome (MFS) and Bickerstaff brainstem encephalitis. The pathophysiology of FBS and the nature of the underlying neuropathy (demyelinating or axonal) are still subject to debate. This study describes the main findings of an early neurophysiological study on 12 patients diagnosed with FBS.
Patients and methodsRetrospective evaluation of clinical characteristics and electrophysiological findings of 12 patients with FBS seen in our neurology department within 10 days of disease onset. Follow-up electrophysiological studies were also evaluated, where available.
ResultsThe most frequent electrophysiological finding, present in 5 (42%) patients, was reduced sensory nerve action potential (SNAP) amplitude in one or more nerves. Abnormalities were rarely found in motor neurography, with no signs of demyelination. The cranial nerve exam revealed abnormalities in 3 patients (facial neurography and/or blink reflex test). Three patients showed resolution of SNAP amplitude reduction in serial neurophysiological studies, suggesting the presence of reversible sensory nerve conduction block. Results from cranial MRI scans were normal in all patients.
ConclusionAn electrophysiological pattern of sensory axonal neuropathy, with no associated signs of demyelination, is an early finding of FBS. Early neurophysiological evaluation and follow-up are essential for diagnosing patients with FBS.
El término síndrome de Fisher-Bickerstaff (SFB) ha sido propuesto para describir el espectro clínico que engloba el síndrome de Miller-Fisher y la encefalitis de Bickerstaff. Su fisiopatología y las características de la neuropatía subyacente, axonal o desmielinizante son todavía objeto de debate. En este estudio describimos los principales hallazgos del estudio neurofisiológico realizado de forma precoz en 12 pacientes diagnosticados de SFB.
Pacientes y métodosSe han evaluado de forma retrospectiva las características clínicas y los hallazgos electrofisiológicos de 12 pacientes con diagnóstico de SFB y estudio realizado en los primeros 10 días de evolución. El estudio electrofisiológico de control ha sido evaluado en los pacientes en los que estaba disponible.
ResultadosEl hallazgo electrofisiológico más frecuente fue una reducción de la amplitud del potencial de acción nervioso sensitivo (PANS) en uno o más nervios, presente en 5 (42%) pacientes. La neurografía motora presentó hallazgos aislados y poco frecuentes, ninguno de ellos dentro de un rango desmielinizante. Tres pacientes presentaron alteración en el estudio de pares craneales (neurografía facial y/o blink reflex). En el estudio neurofisiológico seriado, 3 pacientes presentaron resolución de la reducción de la amplitud del PANS, indicando la presencia de bloque de conducción reversible sensitivo. La resonancia magnética craneal fue normal en todos los pacientes.
ConclusiónUn patrón electrofisiológico de neuropatía axonal sensitiva es un hallazgo precoz en pacientes afectados de FBS, sin hallazgos indicativos de desmielinización. La realización de estudio neurofisiológico precoz y de seguimiento es fundamental en el diagnóstico de pacientes afectos de FBS.
Miller-Fisher syndrome (MFS) is considered a clinical variant of Guillain-Barré syndrome (GBS) characterised by a triad of ataxia, arreflexia, and ophthalmoplegia; when present, muscle weakness is minimal.1 Bickerstaff brainstem encephalitis (BBE) is characterised by altered level of consciousness or hyperreflexia associated with the classic triad; both MFS and BBE are associated with anti-GQ1b antibodies.2,3 Because of this shared autoimmune mechanism, both entities have recently been referred to within the clinical spectrum of the anti-GQ1b Ig antibody syndrome.4 Furthermore, from a nosological perspective, both entities constitute a continuous clinical spectrum with variable involvement of the peripheral and the central nervous system (CNS); the proposed term Fisher-Bickerstaff syndrome (FBS) covers the whole disease spectrum.5,6
The pathogenic mechanism responsible for the associated ataxia is reported to be related to altered conduction in the afferent proprioceptive system (Ia fibres in muscle spindles).7,8 Some studies suggest that anti-GQ1b antibodies play a direct physiopathological role,9 binding to paranodal regions of the oculomotor nerves, dorsal ganglia, and muscle spindles where there is no blood–brain barrier (BBB).10 The presence of CNS dysfunction in these patients has been linked to a metalloproteinase-mediated alteration in the BBB.11
Historically, several studies have discussed the axonal or demyelinating nature of the neuropathy underlying FBS. Most studies reported peripheral nervous system involvement manifesting as generally axonal neuropathy, although electrophysiological findings of demyelination have been observed in some cases.6,12–17 More recent studies support the presence of axonal or not primarily demyelinating neuropathy with reversible conduction block in sensory and motor nerves, as in acute motor axonal neuropathy (AMAN). This would explain the improved amplitude of potentials in serial studies and place FBS on the spectrum of paranodopathies.18,19
Several studies report neurophysiological testing of MFS in its early stages.12,17–19 Although they can be nonconclusive for definitive diagnosis, these early tests provide important pathophysiological data that should be corroborated by serial neurophysiological studies to establish the type of underlying neuropathy.18–22
This study presents clinical and electrophysiological findings from patients diagnosed with FBS and assessed during the first 10 days after symptom onset. We assess the hypothetical usefulness of early neurophysiological studies in the characterisation and diagnosis of FBS.
Patients and methodsWe conducted a retrospective analysis of patients admitted in the last 10 years to the neurology department at Hospital Universitario de Bellvitge (L’Hospitalet de Llobregat, Spain), with a discharge diagnosis of classic MFS or BBE. We selected patients who underwent an electrophysiological study within the first 10 days after symptom onset. We excluded patients with clinical variants of classic MFS, overlapping GBS, history of polyneuropathy, and patients with predisposing factors for neuropathy.4
Data were collected on previous and initial symptoms, neurological examination, presence of albuminocytologic dissociation (cerebrospinal fluid [CSF] protein level ≥ 45mg/dL; with a cell count ≤ 5cells/μL) and brain magnetic resonance imaging (MRI) findings. The GM1, GM2, GD1a, GD1b, GT1b, and GQ1b antiganglioside antibodies were assessed by ELISA; titres ≥ 500 were considered pathological. All neurophysiological studies were performed with the same electromyography equipment (Synergy, CareFusion; San Diego, CA, USA). Recordings were made using the standard method.23 Nerve conduction studies were performed with surface electrodes. Motor neurography (MN) was performed with stimulation of at least one unilateral median, cubital, or peroneal nerve. We assessed the motor evoked potential (MEP) amplitude, distal motor latency, motor conduction velocity, and presence of temporal dispersion. Minimal F-wave latency was assessed in at least one nerve. The F-wave was considered absent or blocked when response persistence was below 20%. Sensory neurography (SN) was performed with antidromic stimulation in at least one unilateral median, cubital, or sural nerve, with sensory nerve action potential (SNAP) amplitude being the main variable assessed. The neurophysiological study of cranial nerves included the blink reflex (BR) and the neurography of the facial nerve (FN). The BR study was performed by stimulation of the supraorbital nerve, with surface electrode recordings made in the orbicularis oculi muscles. The early and late components (R1, R2, and R2c) were assessed. Neurography of the FN recorded activity in the nasalis muscles, with stimulation anterior to the tragus. Values were considered abnormal when they were outside the age-corrected normal values used by our laboratory. Demyelination was defined according to the 2010 EFNS/PNS criteria.24
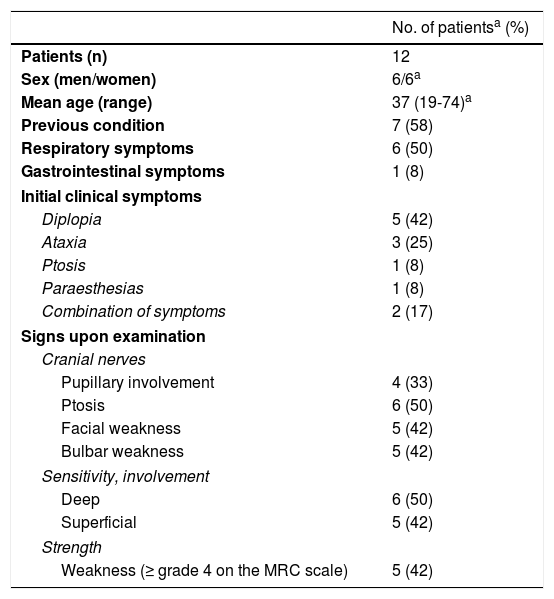
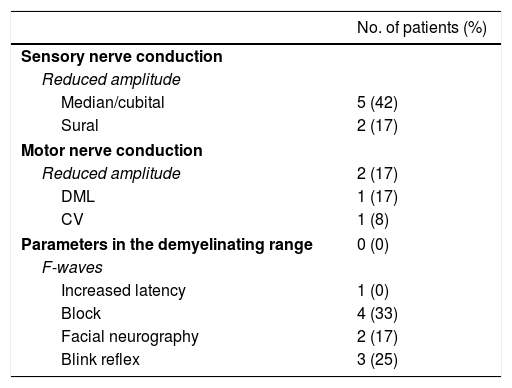
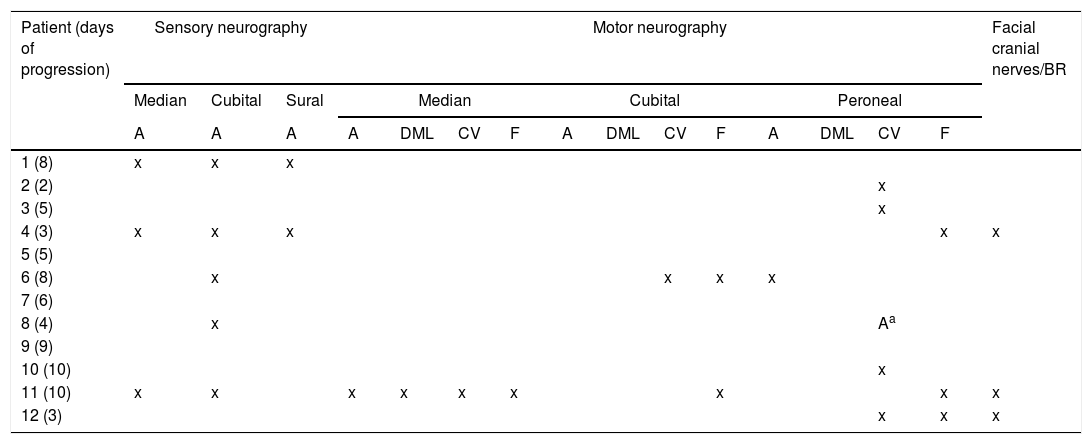
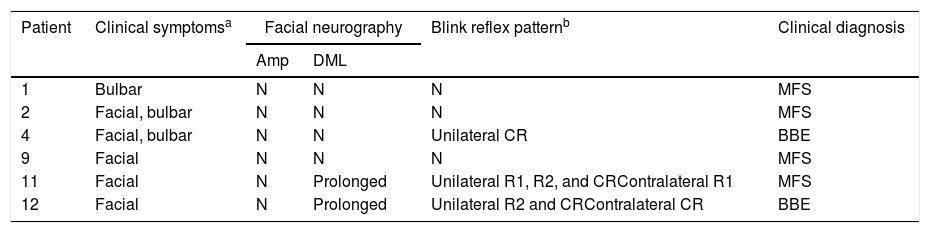
ResultsWe included 12 patients diagnosed with FBS (10 with MFS and 2 with BBE) and undergoing an electrophysiological study within 10 days of symptom onset. Table 1 lists clinical findings. All patients presented clinical symptoms of ataxia and ophthalmoparesis. Two patients presented a pattern suggestive of internal ophthalmoplegia (one patient with Parinaud syndrome and the other with internuclear ophthalmoplegia). One patient was admitted to the intensive care unit due to severe bulbar involvement. Four patients (33%) presented albuminocytological dissociation in the CSF. Antiganglioside antibodies were determined in 9 patients, with 5 (55%) presenting anti-GQ1b IgG antibodies. Eight patients underwent a brain MRI, all obtaining normal results. All electrophysiological studies were conducted within 2-10 days of symptom onset. Table 2 summarises electrophysiological findings; individual findings from each patient are displayed in Table 3. The early neurophysiological study detected no significant alterations in 3 patients (patients 5, 7, and 9). The pathological findings described in the remaining patients were mainly observed in the SN, with all the nerves assessed being affected in 2 patients. Pathological findings were less frequent in the MN, mostly affecting the F-waves (in 3 patients, F-wave block was the only remarkable finding). No patient presented MEP amplitude below 20% of the normal value and no parameters were within the demyelinating range. One patient presented altered SNAP amplitude in the upper limbs with no sural nerve abnormalities; these findings are compatible with a sural sparing pattern. Five patients presented clinical symptoms involving the FN. In 2 of these patients, FN neurography yielded pathological results, with both presenting abnormal BR consisting of homolateral absent or long latency of R1 and R2 with normal contralateral responses. One patient presented clinical symptoms in the FN, with normal neurography results and abnormal BR consisting of R2c lengthening and normal R1 (pattern suggestive of central involvement). Table 4 shows the pattern of cranial nerve involvement. No patient met electrophysiological criteria for GBS.
Clinical characteristics of our sample of patients with FBS.
| No. of patientsa (%) | |
|---|---|
| Patients (n) | 12 |
| Sex (men/women) | 6/6a |
| Mean age (range) | 37 (19-74)a |
| Previous condition | 7 (58) |
| Respiratory symptoms | 6 (50) |
| Gastrointestinal symptoms | 1 (8) |
| Initial clinical symptoms | |
| Diplopia | 5 (42) |
| Ataxia | 3 (25) |
| Ptosis | 1 (8) |
| Paraesthesias | 1 (8) |
| Combination of symptoms | 2 (17) |
| Signs upon examination | |
| Cranial nerves | |
| Pupillary involvement | 4 (33) |
| Ptosis | 6 (50) |
| Facial weakness | 5 (42) |
| Bulbar weakness | 5 (42) |
| Sensitivity, involvement | |
| Deep | 6 (50) |
| Superficial | 5 (42) |
| Strength | |
| Weakness (≥ grade 4 on the MRC scale) | 5 (42) |
MRC: Medical Research Council Scale for Muscle Strength.
Electrophysiological findings.
| No. of patients (%) | |
|---|---|
| Sensory nerve conduction | |
| Reduced amplitude | |
| Median/cubital | 5 (42) |
| Sural | 2 (17) |
| Motor nerve conduction | |
| Reduced amplitude | 2 (17) |
| DML | 1 (17) |
| CV | 1 (8) |
| Parameters in the demyelinating range | 0 (0) |
| F-waves | |
| Increased latency | 1 (0) |
| Block | 4 (33) |
| Facial neurography | 2 (17) |
| Blink reflex | 3 (25) |
A total of 12 patients were assessed; however, neurography of the facial nerve was conducted in 11.
CV: conduction velocity; DML: distal motor latency.
Pattern of electrophysiological findings.
| Patient (days of progression) | Sensory neurography | Motor neurography | Facial cranial nerves/BR | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Cubital | Sural | Median | Cubital | Peroneal | |||||||||||
| A | A | A | A | DML | CV | F | A | DML | CV | F | A | DML | CV | F | ||
| 1 (8) | x | x | x | |||||||||||||
| 2 (2) | x | |||||||||||||||
| 3 (5) | x | |||||||||||||||
| 4 (3) | x | x | x | x | x | |||||||||||
| 5 (5) | ||||||||||||||||
| 6 (8) | x | x | x | x | ||||||||||||
| 7 (6) | ||||||||||||||||
| 8 (4) | x | Aa | ||||||||||||||
| 9 (9) | ||||||||||||||||
| 10 (10) | x | |||||||||||||||
| 11 (10) | x | x | x | x | x | x | x | x | x | |||||||
| 12 (3) | x | x | x | |||||||||||||
Only pathological findings are reported.
A: amplitude; BR: blink reflex; CV: conduction velocity; DML: distal motor latency; F: F-waves.
Clinical and electrophysiological pattern of cranial nerve involvementa.
| Patient | Clinical symptomsa | Facial neurography | Blink reflex patternb | Clinical diagnosis | |
|---|---|---|---|---|---|
| Amp | DML | ||||
| 1 | Bulbar | N | N | N | MFS |
| 2 | Facial, bulbar | N | N | N | MFS |
| 4 | Facial, bulbar | N | N | Unilateral CR | BBE |
| 9 | Facial | N | N | N | MFS |
| 11 | Facial | N | Prolonged | Unilateral R1, R2, and CRContralateral R1 | MFS |
| 12 | Facial | N | Prolonged | Unilateral R2 and CRContralateral CR | BBE |
Amp: amplitude of motor evoked potential; BBE: Bickerstaff brainstem encephalitis; CR: contralateral response; DML: distal motor latency; MFS: Miller Fisher syndrome; N: normal; R1: response 1; R2: response 2.
Follow-up neurophysiological studies were performed within 14-62 days of symptom onset in 6 patients. One of these (patient 2) presented reduced MEP amplitude in the FN at 27 days after symptom onset, with SN and MN remaining within normal ranges, with the exception of F-waves, which remained blocked. At that time, the patient still presented symptoms of bulbar involvement and ataxia. Patients 4 and 11, who underwent neurophysiological testing at 15 and 18 days, respectively, showed increased sensory potential amplitudes in the upper limbs, with no changes in potential duration; patient 4 continued to present alterations in the neurography of the sural nerve. Though improved, ataxia persisted in both patients, as did arreflexia and reduced vibratory sensitivity in the lower limbs in patient 4. In patient 8, sensory potential amplitude of the sural nerve normalised at 62 days of progression, with the disappearance of the A-waves previously detected in multiple nerves. The neurological examination was normal. Finally, for patient 12, the only follow-up study was a BR study performed at 14 days of progression; the patient presented persisting increased R1 latency and severe symptoms of ophthalmoparesis and facial weakness. Patient 1 presented no significant changes in a study performed at 23 days.
DiscussionThe clinical findings in our patients are compatible with those reported in previous studies.4,25 However, anti-GQ1b antibodies were detected in a lower frequency than expected. A possible explanation is that the samples were collected more than 2 weeks after symptom onset, which may have increased the possibility of false negatives.26
Regarding the study of peripheral nervous system involvement in our patients, the fact that the first neurophysiological study was performed early increases the controversy regarding the interpretation of electrophysiological findings, as occurs in GBS.20–22
In our study, 9 of the patients assessed (75%) showed some alteration in the electrophysiological study; the most frequent finding was reduced SNAP amplitude in one or more nerves, and motor conduction study findings being mild or isolated, or in the axonal range. These findings suggest the presence of a predominantly sensory axonal neuropathy, suggesting involvement of cutaneous sensory afferents in FBS pathophysiology. The pattern detected seems to differ from that described in the early neurophysiological studies of GBS, with a predominance of MN alterations with parameters in the demyelinating range even in an early stage and with a seldom strictly normal electrodiagnostic study.20–22
A significant limitation of our study is the absence of information of the H-reflex or postural studies providing information on the involvement of the afferent proprioceptive system (Ia fibres), which may be responsible for ataxia in these patients.6,7
The resolution or improvement observed in SNAP amplitude in follow-up neurophysiological studies at 15 and 18 days in 2 of our patients, with no significant increase in potential duration, is consistent with reversible sensory conduction block, as reported in the study by Umapathi et al.19 These authors report reversible conduction failure in 6 of the 15 patients with MFS, in line with previously described findings in axonal GBS (AMAN).19 These findings also suggest nodal or paranodal dysfunction, which would place FBS on the spectrum of axonal GBS and nodo-paranodopathies.27
Evidence of CNS involvement is limited in our patients. Despite the presence of clinical symptoms suggesting CNS involvement, such as somnolence or a pattern of internal ophthalmoplegia in 2 patients, MRI results were normal in all the patients assessed. It is interesting that the BR study revealed CNS involvement in a patient whose BBE diagnosis was based on somnolence. The BR study and/or facial neurography may, therefore, provide evidence on peripheral or CNS involvement in the study of patients with FBS; we therefore recommend that this study be performed routinely.
In conclusion, electrophysiological findings in our patients support the involvement of axonal non-demyelinating neuropathy in the pathophysiology of FBS. The presence of reversible sensory conduction failure explains the fast resolution of sensory nerve conduction alterations in 3 of our patients. Performing serial neurophysiological studies is crucial for the assessment of patients with FBS or GBS.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Alberti MA, Povedano M, Montero J, Casasnovas C. Hallazgos electrofisiológicos precoces en síndrome Fisher-Bickerstaff. Neurología. Neurología. 2019. https://doi.org/10.1016/j.nrl.2017.05.012
Part of this study was presented at the 63rd Annual Meeting of the Spanish Society of Neurology, 2020;35:40–45.