Phantom limb pain (PLP) is a type of neuropathic pain that affects the territory of an amputated limb or other surgically removed body parts. Between 60% and 90% of amputees suffer from PLP during follow-up. There are a range of therapeutic options for PLP, both pharmacological (gabapentin, amitriptyline, tricyclic antidepressants, etc) and non-pharmacological (transcutaneous electrical nerve stimulation, hypnosis, acupuncture, etc). A widely accepted hypothesis considers PLP to be the consequence of postamputation cortical reorganisation. New treatment approaches, such as mirror therapy (MT), have been developed as a result of Ramachandran's groundbreaking research in the 1990s. This review analyses the current evidence on the efficacy of MT for treating PLP.
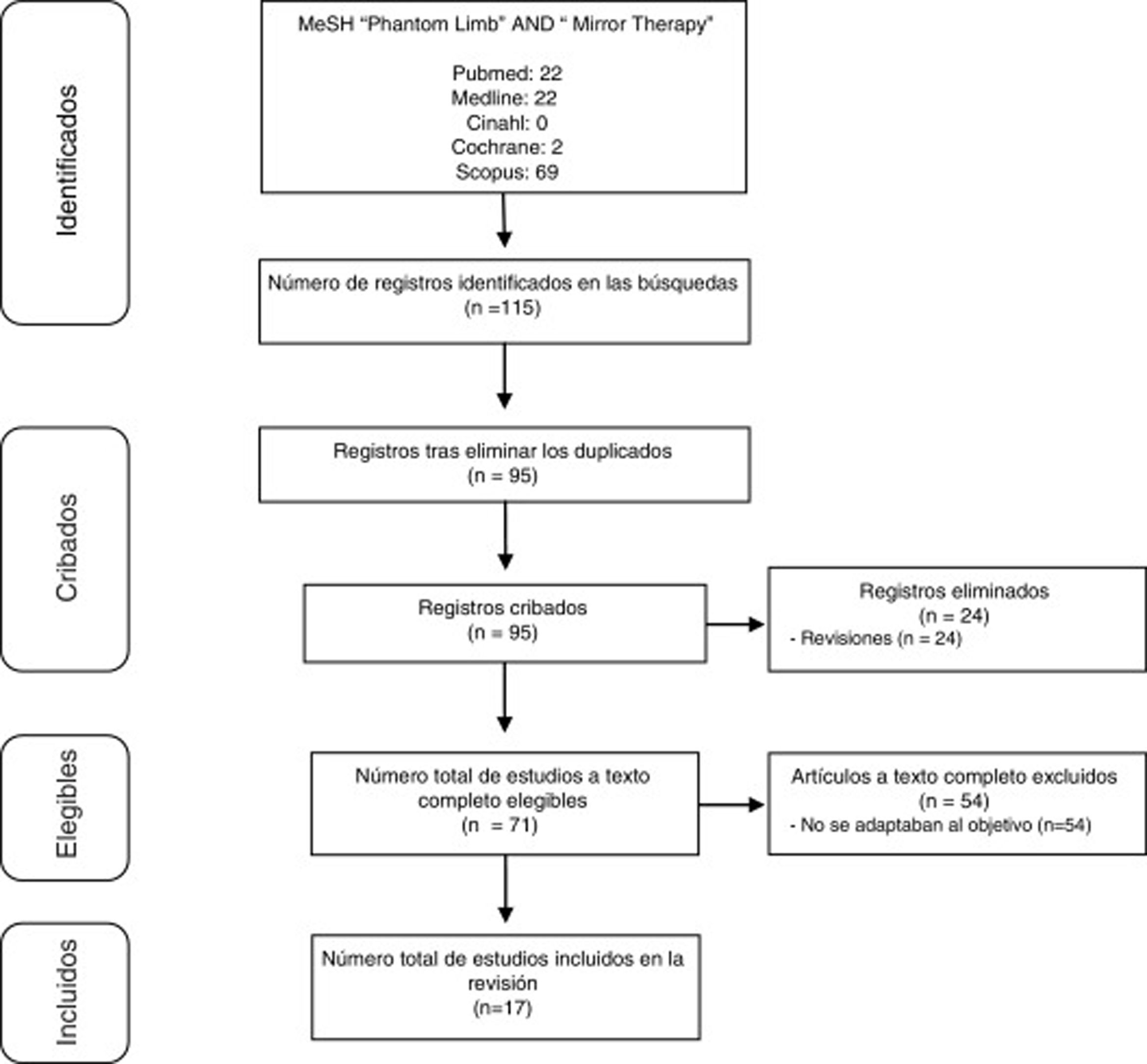
DevelopmentWe performed a literature review of publications registered from 2012 to 2017 on the CINAHL, Cochrane, Scopus, and PubMed (including Medline) databases using the descriptors “phantom limb” and “mirror therapy.” We identified 115 publications addressing MT in PLP. Of these, 17 (15%) contributed useful information for pooled analysis.
ConclusionsMT seems to be effective in relieving PLP, reducing the intensity and duration of daily pain episodes. It is a valid, simple, and inexpensive treatment for PLP. The methodological quality of most publications in this field is very limited, highlighting the need for additional, high-quality studies to develop clinical protocols that could maximise the benefits of MT for patients with PLP.
El dolor del miembro fantasma (DMF) es un dolor de tipo neuropático que afecta al territorio de una extremidad amputada o a otras partes del cuerpo extirpadas quirúrgicamente. El 60-90% de los amputados sufren DMF durante el seguimiento. Se han descrito opciones terapéuticas para DMF, farmacológicas (gabapentina, amitriptilina, antidepresivos tricíclicos…) y no farmacológicas (TENS, hipnosis, acupuntura…). Una hipótesis predominante considera este fenómeno consecuencia de la reorganización cortical postamputación, y, tras investigaciones innovadoras de Ramachandran en los 90, se han desarrollado nuevos enfoques de tratamiento como la terapia de espejo (TE). En la presente revisión se analiza la evidencia actual publicada sobre la eficacia de la TE para el tratamiento del DMF.
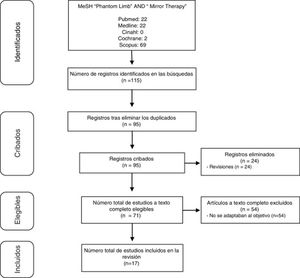
DesarrolloSe realizó una revisión bibliográfica, abarcando desde 2012 a 2017, de publicaciones registradas en las bases de datos Cinahl, Cochrane, Scopus y Pubmed (incluyendo Medline). Los descriptores utilizados para la búsqueda fueron los términos Phantom Limb y Mirror Therapy. Se identificaron 115 publicaciones que abordaban TE en DMF. De estas, 17 (15%) aportaban información útil para el análisis conjunto.
ConclusionesLa TE parece ser efectiva en el alivio del DMF, reduciendo la intensidad y duración de los episodios de dolor diarios. Por otra parte, constituye un método válido, sencillo y muy económico. La calidad metodológica de la mayoría de las publicaciones en este campo es muy limitada, destacando la necesidad de estudios adicionales de alta calidad para desarrollar protocolos clínicos que puedan maximizar los beneficios de TE en pacientes con DMF.
The phantom limb was first described as a painful condition by the military surgeon Ambrose Paré in the 16th century. Three centuries later, the neurologist Silas Weir Mitchell coined the term “phantom limb pain” (PLP) and described the syndrome in detail.1
PLP is a type of neuropathic pain that generally manifests in amputated limbs, although it can also occur after the surgical removal of other body parts.2 The phantom limb phenomenon includes 3 different elements: PLP, phantom sensations, and stump pain. These elements frequently coexist, and may be difficult to distinguish.3 These sensations may range from a pleasant warm sensation to an uncomfortable itching,4 which may be described as “burning, cramping, crushing, or lancinating” and may be felt intermittently or permanently.5 A peculiar phenomenon known as “telescoping” occurs when the distal part of the phantom limb seems gradually to move closer to the residual limb, sometimes to the point where patients have the sensation that it is “implanted.”6 PLP frequently affects patients’ quality of life and increases the limitations caused by amputation, potentially compromising social and/or professional reintegration.7
According to the literature, 60%–90% of amputees experience PLP at some point of their lives, and incidence is generally higher after traumatic limb loss or when pain was already present prior to amputation.8 Incidence is much lower in children than in adults,8 with cases rarely reported in children younger than 4 years.6 Various pharmacological treatment options for PLP have been described, including gabapentin, amitriptyline, tricyclic antidepressants, morphine, or ketamine. Non-pharmacological options include transcutaneous electrical nerve stimulation, transcranial magnetic stimulation, spinal cord stimulation, use of prostheses, hypnosis, acupuncture, etc.7
PLP remains an enigma, and better understanding of its mechanisms is needed. A widely accepted hypothesis suggests that following limb amputation, the loss of afferent input allows for invasion of adjacent cortical regions into the former representation zone of the limb in the primary sensorimotor cortex. It has been suggested that these maladaptive changes constitute the neural basis of PLP, and this view has generated new treatment approaches, such as sensory discrimination or mirror therapy (MT).9 In MT, a mirror is placed parasagittally between the arms or legs and the patient views the reflected movements of their intact limb while simultaneously attempting to move the phantom limb in a similar manner. The aim of the technique is for the patient to perceive the reflection in the mirror as the amputated limb, which may reflect the incongruence between proprioceptive and visual inputs caused by cortical reorganisation.10 The hypothesis that cortical reorganisation occurring after amputation contributes to PLP has been proposed in several functional magnetic resonance imaging studies in which reversion to a normal/pre-amputation state coincides with alleviation of PLP.11
In the 1990s, Ramachandran and Rogers-Ramachandran12 became the first researchers to successfully use this technique to alleviate phantom limb paralysis and spasms. Later, Chan et al.13 conducted a study in which all participants showed improvements.
Considering the above, the aim of the present study is to assess the effectiveness of MT in patients with PLP in order to consolidate its status as the conservative non-pharmacological treatment of choice.
DevelopmentTo collect data on the effectiveness of MT in PLP, we conducted a literature review on the CINAHL, Cochrane, Scopus, and PubMed (including MEDLINE) databases. In order to include only the most recent data, we limited our search to studies published since 2012.
Both authors searched for, selected, and analysed the results independently, and consensus was reached in the event of discrepancy.
The search terms used were “phantom limb” and “mirror therapy,” combined with the Boolean operator AND. We included studies with human patients published since 2012 and excluded review studies, duplicates, and studies not focusing on the established subject matter.
Study selectionStudies were preselected based on their abstracts, and full texts were accessed to establish the final selection. Fig. 1 summarises the selection process.
ResultsOf the 115 studies found, we finally selected 17 that met the inclusion criteria. Studies are summarised in Tables 1–3. Table 1 includes case reports14–18 and case series.1,3,19Table 2 includes the remaining studies.8,20–27 Finally, Table 3 includes the treatment protocols used.
Main data from the case reports included in the review.
| Study (year) | Objective | Sample (sex)Type of amputationAge | Assessment (time points) | Results |
|---|---|---|---|---|
| González García et al.1(2013) | To analyse MT in the functional rehabilitation of amputees in a geriatrics department | 3 patients (1♀, 2♂)LLA65 (♂), 80 (♂), and 67 (♀) years | VASBarthel index(at baseline, at discharge after 68, 95, and 98 sessions respectively, and after follow-up of 16–22 months) | - Decrease in pain intensity (VAS 6/1, 7/4, and 7/2 respectively) and mean reduction in analgesic use/patient from 4.33 to 0.66- Decrease in the number and duration of episodes- Functional improvement: mean increase of 36.6 points in the Barthel Index- Beneficial effects persist in the long-term, both in pain control and in emotional and functional condition |
| Kim and Kim14(2012) | To report the successful reduction of PLP by MT after failure of other therapies | 1 patient (1♂)ULA30 years | VAS(at baseline, one week, one month, and 3 months) | - PLP decreased from 8/10 to 7/10 during the first week, to 5/10 at one month, and to 4/10 after 3 months of therapy (previous, pharmacological and non-pharmacological therapies were unsuccessful in controlling PLP)- The electrical sensation persisted- Lower doses of pharmacological treatment were needed with MT |
| Clerici et al.15(2012) | To describe the advantages of MT in an oncological patient with PLP | 1 patient (1♂)LLA17 years | VASZung test(at baseline and every week until week 26) | - Gradual reduction of PLP until pain was stable and tolerable- Mean pain reduction between the first 6 weeks and weeks 20 to 25 (P<0.05)- No evidence of significant depression was found with the Zung test- Benefits remained at 6 months |
| Wosnitzka et al.16(2014) | To report on the use of MT in a patient with bilateral amputation of the lower limbs | 1 patient (1♂)LLDA66 years | NRS(at baseline and daily until week 3) | - The use of prostheses may also facilitate the use of MT- Alleviation of PLP for several weeks (8-9/10 to 2-4/10) after few MT sessions- 90% decrease in pain episodes per day- Benefits remained after several months |
| Datta and Dhar3(2015) | To confirm whether MT may support conventional pharmacological treatment in PLP | 2 patients (2♂)LLA21 and 65 years | VAS(at baseline, one week, and 3 months) | - Decrease vs baseline PLP after one week (6 to 4/10 and 5 to 2/10, respectively)- Decrease vs baseline PLP after 3 months (2-3/10 and 1-2/10, respectively)- The younger patient responded poorly to the initial pharmacological treatment |
| Thomas17(2015) | To assess the effectiveness of MT, electromyographic biofeedback, and tactile stimulation in decreasing PLP | 1 patient (1♂)ULA48 years | NPRDASH(at baseline, and at 4 and 8 weeks) | - Significant improvement in control of the pectoralis major and infraspinatus muscles at 4 and 8 weeks- Significant improvement in PLP (9/10 to 6/10 at 4 weeks and 3/10 at 8 weeks)- Significant improvement in DASH score (from 80% to 65% disability at 4 weeks and 50% at 8 weeks)- Reduced oedema (from 115 to 108cm at 4 weeks and 105cm at 8 weeks) |
| Ramsey et al.18(2017) | To describe the beneficial effects of MT in combination with drug therapy | 1 patient (1♂)ULA7 years | Interview with the patient and his family on symptoms(at baseline and at 2 weeks) | - Follow-up at 2 weeks showed significant improvement in psychomotor agitation, mood, and sleep quality- No PLP after 2 weeks except when jumping off steps- Previously, pharmacological treatment alone did not obtain good results |
| Toita et al.19(2017) | To report on the results obtained after the application of MT or sensory discrimination techniques at the stump, according to PLP characteristics | 2 patients (2♂)LLA and ULA50 years (both) | NRS(at baseline, at one week, and at 2 weeks) | - MT was only applied to the patient with LLA- Improvement in PLP in both patients |
♀: woman; ♂: man; DASH: disability of the arm, shoulder, and hand; LLA: lower limb amputation; LLDA: lower limb double amputation; MT: mirror therapy; NPR: Numeric Pain Rating; NRS: Numerical Rating Scale; PLP: phantom limb pain; ULA: upper limb amputation; VAS: visual analogue scale.
Main data from the remaining studies included in the review.
| Study (year) | ObjectiveStudy design | SampleType of amputationAge (range) | Assessment (time points) | Results |
|---|---|---|---|---|
| Darnall and Li20(2012) | To assess the variability and effectiveness of self-delivered MT for PLP- PS | 31 patients (13♀, 18♂)LLA and ULA61 (32-74) | CES-DNRSMT diary(at baseline, at one month, and at 2 months) | - Reduced mean intensity of initial PLP at the first month (P<.001)- Reduced mean intensity of initial PLP at the second month (P<.001)- Reduced mean intensity of PLP in patients with university education vs basic studies (P=.01)- PLP was exacerbated in 4 patients (2 reported increased pain and 2 phantom sensation, although the latter 2 improved quickly) and 6 reported no changes- Adverse effects: boredom (2), greater awareness of the missing limb (2), increased PLP (2), increased phantom sensation (2), feeling depressed (1), and cramps (2 cases; cramps resolved after changing to gentler movements)- The benefits cannot exclusively be explained by MT, but drug therapy alone did not achieve them |
| Foell et al.21(2014) | To assess the effects of MT on PLP, to compare pre- and post-therapy brain changes, and to identify success predictors- PS | 13 patients (4♀, 9♂)ULA50.6±15.8 years (26-74) | VAS(daily for 8 weeks: 2 weeks before starting treatment, 4 weeks of treatment, and 2 weeks after treatment)fMRIMPI(pre- and post-intervention) | - PLP reduction of 27%- Decreased mean pain at week 7 vs week 2 (P<.05), although not all patients reported pain improvement- Positive correlation between PLP reduction and reduction in the cortical shift of S1 (P<.01)- Positive correlation between PLP reduction and decrease of activity in the inferior parietal cortex (P<.001)- Negative correlation between telescoping and MT effect (P<.01) |
| Schmalzl et al.22(2013) | To report an alternative to traditional MT and analyse whether illusory touch may alleviate PLP- PS | 6 patients (4♀, 2♂)ULA55 years (39-80) | NRS(before and after each intervention)Questionnaire on the illusory perception of movement/sensitivity in the phantom limb(at the end of the eighth session) | - In group results, PLP is increased in the movement session (P=.011) and decreased in the stroking session (P=.011)- In individual results:* 3 participants presented no changes and 3 presented increased PLP (P<.007, P<.046, and P<.039) in the movement session* 4 participants presented decreased PLP (P<.038, P<.010, P<.010, and P<.016) and 2 presented no changes in the stroking session- Illusory touch created by stroking the stump with a paintbrush may be an alternative when MT increases PLP |
| Tilak et al.23(2016) | To assess and compare the effectiveness of TENS and MT in the management of PLP- RCT | 26 patients (13 EG1; 13 EG2)LLA and ULAEG1: MT, 13 (1♀, 12♂)42.62±10.69 yearsEG2: TENS, 13 (2♀, 11♂)36.38±9.55 years | VASUPS(pre- and post-intervention) | - Improved PLP with MT (VAS, P=.003; UPS, P=.001)- Improved PLP with TENS (VAS, P=.003; UPS, P=.002)- MT vs TENS showed no significant differences (VAS, P=.223; UPS, P=.956) |
| Houston and Dickerson24(2016) | To analyse the effectiveness of Farabloc™ and an MT programme to improve functionality in vascular amputees- CS | 14 patients (EG1: 9; EG2: 5)LLAEG1 (acute): 9 (4♀, 5♂)58.2±11.2 years (48-78)EG2 (subacute): 5 (2♀, 3♂)61.6±7.9 years (51-73) | PEQBPIDaily record(pre- and post-intervention and at 4 weeks) | - Significant improvements in EG1 in the post-intervention and maintenance periods for self-care, walking, car transfer, sleep, mood, and quality of life with regard to the pre-intervention period- Improvements in EG2 in the post-intervention and maintenance period for sleep and satisfaction with regard to the pre-intervention period- The complete group of all patients showed improvements in the post-intervention and maintenance periods with regard to the pre-intervention period but no significant changes were observed between the post-intervention and maintenance periods- 7/9 participants from EG1 were ready for prosthetic fitting at 8 weeks (4 weeks earlier than in normal protocols)- All participants in EG2 showed increased wearing tolerance (from 0-2 to 8-12h) |
| Anghelescu et al.8(2016) | To describe the differences between paediatric patients with PLP who receive standard therapy with or without MT- RS | 18 patients- EG: MT (n=9) (1♀, 8♂)- CG: no MT (n=9) (2♀, 7♂)LLA and ULA13 years (8-24) | NPS(at baseline and at weeks 1, 2, 3, 4, 8, 12, 16, 20, and 24 or until the patient's discharge) | - The MT group presented PLP of shorter duration and lower frequency over a year than the CG (246±200 vs 541±363 days, P=.08)- No difference was observed between groups in pain intensity- The use of MT was not associated with a decrease in pain markers or reduced consumption of medication |
| Yildirim and Kanan25(2016) | To assess the effects of MT in alleviating PLP- QES | 15 patients (2♀, 13♂)LLA and ULA52.13±16.68 years | NPS(at baseline and weekly for 4 weeks) | - Decrease in mean PLP scores each week of the study period and 1-month total PLP score (P<.01)- 5/15 wore prostheses (cosmetic prostheses)- An increased benefit was observed in patients not wearing prostheses (P<.05), with prosthesis usage explaining 30.6% of the change in PLP scores before and after MT (P<.05) |
| Griffin et al.26(2017) | To assess the effects of MT according to the degree of PLP in order to report treatment parameters and promote a standard for clinical practice- RCS | 31 patients (2 cohorts: 21 and 10) (4♀, 27♂)LLA33.5 years (19-60) | VASSF-MPQ(at baseline and after the 7th, 14th, and 21st sessions) | - Overall, MT was effective in 87% of patients, with a decrease in PLP over time (P<.0001) and improvements for most of the pain qualities included in the SF-MPQ- Pain mainly decreased in the first 7 sessions and from the 14th session onwards- Patients with low baseline pain (VAS≤30mm) showed significant improvements after 7 sessions, those with medium baseline pain level (VAS=31-60mm) after 14 sessions, and those with high baseline pain level (VAS>61mm) after 21 sessions |
| Finn et al.27(2017) | To assess the effectiveness of MT in alleviating PLP in unilateral upper limb amputees- RCT | 15 patients (15♂)EG1 (9): MTEG2 (6): mental visualisation or covered mirror therapyULA(19-68 years) | VASPLP frequency and duration(daily and throughout the intervention) | - Improvements were observed in EG1 only- EG1: 8/9 showed improvements in PLP (P=.001)- EG1: decrease in total daily PLP time (P=.003)- EG1: the response to MT after the first 5 sessions was predictive of the response at 4 weeks in 6/9 patients; the remaining 3 reported a change after 10 weeks- EG2: 5/6 patients received MT before or after completing mental visualisation or covered mirror therapy; PLP and total daily time experiencing pain improved in these 5 patients |
♀: woman; ♂: man; BPI: Brief Pain Inventory; CES-D: Centers for Epidemiologic Studies Depression subscale; CG: control group; CS: cross-over study; EG: experimental group; fMRI: functional magnetic resonance imaging; LLA: lower limb amputee; MPI: West Haven-Yale Multidimensional Pain Inventory; MT: mirror therapy; NPS: Numerical Pain Scale; NRS: Numerical Rating Scale; PEQ: Prosthetic Evaluation Questionnaire; PLP: phantom limb pain; PS: prospective study; QES: quasi-experimental study; RCS: retrospective cohort study; RCT: randomised clinical trial; RS: retrospective study; SF-MPQ: Short-Form McGill Pain Questionnaire; TENS: transcutaneous electrical nerve stimulation; ULA: upper limb amputee; UPS: universal pain scale; VAS: visual analogue scale.
MT application protocols followed in the analysed studies.
| Study | Type of intervention | Intervention period | Session frequency | Duration of treatment session | Place of intervention |
|---|---|---|---|---|---|
| González García et al.1 | MT+DT+PT | 68/95/68 sessionsa | – | 15min | Healthcare centre |
| Kim and Kim14 | MT+DT | 3 months | 4/week | 15min | Healthcare centre+home |
| Clerici et al.15 | MT+DT | 26 weeks | Daily | 30min | Healthcare centre+home |
| Wosnitzka et al.16 | MT+DT | 3 weeks | Daily | – | Healthcare centre |
| Datta and Dhar3 | MT+DT | 1 week (hospital)+3 months (at home) | 2-3/day | – | Healthcare centre+home |
| Thomas17 | MT+DT+PT | 8 weeks+6 weeksc | 1-2/week | – | Healthcare centre+home |
| Ramsey et al.18 | MT+DT | 2 weeks | 2/day | 10min | Healthcare centre+home |
| Toita et al.19 | MT+DT | 2 weeks | Daily | 15min | Healthcare centre |
| Darnall and Li20 | MT | 1-2 months | Daily | 25min | Home |
| Foell et al.21 | MT (+DT)b | 1 month | Daily | 15min | Healthcare centre/home |
| Schmalzl et al.22 | MT+MT without visual feedback+tactile stimulation | 8 sessions | – | 5min | Healthcare centre |
| Tilak et al.23 | MT/TENS | 4 days | Daily | 20min | Healthcare centre |
| Houston and Dickerson24 | MT+Farabloc™ mesh | 4 weeks | Daily | 15min | – |
| Anghelescu et al.8 | MT+DT or only | 4 sessions (hospital)+unknown number (at home) | Several times a day | Variable | Healthcare centre+home |
| Yildirim and Kanan25 | MT (+DT)b | 4 weeks | Daily | 20-25min | Healthcare centre+home |
| Griffin et al.26 | MT | Mean of 19 sessions (range: 3-40 sessions) | 5/week (variable) | 15min (20min)d | Healthcare centre |
| Finn et al.27 | MT/mental visualisation therapy or MT without visual feedback+DT | 4 weeks | 5/week | 15min | Healthcare centre |
DT: drug therapy; MT: mirror therapy; PT: physiotherapy; TENS: transcutaneous electrical nerve stimulation.
Our main findings are:
- -
MT reduced the intensity,1,3,8,14–21,23–27 frequency,1,8,16,18,26 and duration of PLP episodes.8,27 We also found that patients are reported to respond well to the therapy, whereas no positive response to previous pharmacological3,14,18 or non-pharmacological treatment have been obtained.14,27 However, in some cases, this response cannot be attributed exclusively to MT; nor was it superior to that obtained with other therapies (sensory discrimination at the stump,19 visuotactile illusion,22 electrical transdermal nerve stimulation23).
- -
In patients undergoing follow-up, beneficial effects on PLP were maintained,1,3,15,16,18,24 especially when MT had been continued at home.3,21
- -
Improvements were observed in functional status,1,17,24 oedema,17 mood,18,24 and sleep quality18,24; patients also showed improved prosthesis wearing tolerance and reduced time from surgery to prosthetic fitting.24
- -
Furthermore, in cases of MT performed with a prosthesis,16,25 the beneficial effects on PLP were also obtained.
- -
Benefits have been observed with MT regardless of the cause leading to amputation and PLP: trauma,3,14,17,19–22,27 cancer,8,15,18,22 or peripheral vascular disease.1,3,16,21,24,25 Patient age does not seem to influence this possible benefit, unlike other factors including the patient's level of schooling, with a positive correlation between improvement with MT and higher level of studies,20 or the degree of telescoping, which shows a negative correlation with the effects of MT.19,21,25
- -
However, in some cases MT was not a better option than other therapies,19,22,23 either showing no positive effects, with persistence of such symptoms20 as electric sensation14,20 or increased PLP,20–22 or causing new symptoms, such as sadness1 or depression20 after seeing the virtual image of the amputated limb. In some cases, there was no reduction in the consumption of medication.8
- -
Finally, MT was usually applied at the patient's reference centre and then continued at home,3,8,14,15,17,18,21,25 which requires the patient to be trained in using the technique8,15,16,20,25; MT sessions usually lasted approximately 15minutes1,14,19,21,24,26,27 and were generally performed on a daily basis.3,8,15,16,18–21,23–27 MT achieved a significant reduction of PLP in 2–4 weeks3,8,16–27 and was usually accompanied by or complemented1,3,8,14–19,27 with pharmacological treatment.21,25
In the light of the above-mentioned findings, MT seems to be an effective treatment in alleviating pain in patients with PLP, since all studies selected in this review,1,3,8,14–21,23–27 with one exception,22 support the use of this therapy. Schmalzl et al.,22 who compared 2 variants of MT (movement condition vs stroking condition), obtained good results in only one of the conditions (tactile stimulus using a paintbrush) and only in certain patients (in those who initially experienced increased PLP when performing a movement). This is probably linked to the progression time of PLP or, as suggested by Griffin et al.,26 to the degree of PLP at baseline, with more and/or longer sessions needed in patients with more intense PLP.
However, despite the positive outcomes reported in the different studies, certain clinically relevant questions must be clarified. In most studies, pharmacological treatment was administered before and during the intervention1,3,8,14–19,21,25,27 and/or MT was complemented by other treatments: physiotherapy on the stump,1,17 laterality training and imagined movement,16 or use of a Farabloc™ mesh to protect the stump from electromagnetic fields.24 Therefore, we cannot unequivocally assert the effectiveness of MT. Nevertheless, we should also point out that MT also reduced medication use14 (although Anghelescu et al.8 did not achieve the same outcomes), or good results were obtained in patients who did not initially improve with pharmacological treatment only.3,18,20 However, the possible effect of pharmacological treatment should be identified in future studies, since many patients prescribed MT for PLP receive medication in accordance with the post-amputation protocol and/or due to comorbidities. Studies comparing a group with/without pharmacological treatment against a group receiving pharmacological treatment and MT are needed.
Furthermore, according to our findings, such factors as aetiology, the limb affected, and age do not affect the outcomes of MT. Nevertheless, certain relevant factors will necessarily require further study: level of schooling,20 PLP progression time,24,26 number and duration of daily PLP episodes,8,27 and the use of prostheses.25 Establishing the influence of these factors would shed light on the clinical relevance of whether patient training in MT can be performed in only one session,5 and whether sessions should be directed by a professional8,15 or if audiovisual material is sufficient20; whether training should be introduced immediately after amputation, even in the absence of PLP, or only after PLP manifests26; whether prosthetic fitting limits the therapeutic benefit of MT (results have been obtained both suggesting improvements in prosthetic fitting24 and that prosthetic use limits the benefits of MT)25; moreover, the final results may be linked to symptoms secondary to PLP, such as electric sensation14 or telescoping.19,21,25 MT generally seems to have beneficial effects on PLP; however, not all studies describe and/or analyse these variables in relation with the sample and the clinical condition; this should be addressed in future studies. Thus, for example, the variable number of episodes and the presence/absence of telescoping and its influence seem to be decisive factors in treatment success; assessing only the decrease or increase in pain intensity does not seem appropriate.
Table 3 shows some similarities between MT application protocols, as mentioned in the previous section. However, clear differences are observed in certain aspects, regardless of the fact that not all studies reported the duration of the intervention,1,8,21 the frequency1,22 and duration of treatment sessions,3,8,16,17 and the place where MT sessions were performed.24 Thus, the protocols used present numerous differences: interventions lasted from 4 days23 to 26 weeks,15 frequency of sessions ranged from once or twice a week17 to several times per day,3,8 duration was between 5minutes22 and 30minutes15, and sessions were either performed both at the healthcare centre and at home,3,14,15,17,18,21,25 or exclusively at the centre1,16,19,22,23,26,27 or at home.20 These variations suggest a lack of standardised protocols for MT, confirming the need to standardise the therapy, facilitating comparison of studies and their results; this would enable us not only to assess the effectiveness of MT but also to adapt its application to patients’ specific characteristics. Treatment protocols would therefore help to compare results and to establish the most adequate parameters for MT intervention according to PLP and other associated symptoms.
The lack of standardisation is also patent in some other areas. In terms of the scales used to assess patients, we observed that subjective scales were mostly used to assess the degree of variation of PLP. These scales include the visual analogue scale (VAS),1,3,14,15,21,23,26,27 the Numeric Rating Scale16,19,20,22 and its variant the Numerical Pain Rating scale,17 the West Haven-Yale Multidimensional Pain Inventory,21 the universal pain score (UPS),23 the Brief Pain Inventory (BPI),24 and the short-form McGill Pain Questionnaire.26 The following scales were also used: the Barthel Index1 to assess physical disability; the DASH questionnaire17 to assess shoulder, elbow, and hand function; the Zung test15 and the Centers for Epidemiologic Studies Depression subscale (CES-D)20; the Prosthesis Evaluation Questionnaire (PEQ)24 to functionally assess the patient; and functional magnetic resonance imaging.21 This shows that the assessment of PLP is essentially subjective; therefore, it is advisable to complement pain measurement with functional scales and to include objective variables in order to establish a standard assessment protocol.
No standard procedure for data collection or medium- or long-term follow-up of patients is observed. We recommend that patients with PLP be followed up in the medium and long term to assess the degree of MT use not only during the period in which they are supervised by professionals, but also when they are using MT at home, and its influence on PLP progression. In those studies including follow-up, the benefits obtained with MT persisted over time1,3,8,14–16,21; however, most of these studies are case reports,1,3,14–16 which limits their value. Furthermore, although patients undergoing MT did show improvements in the level, duration, and incidence of pain in the study by Anghelescu et al.,8 this improvement was not associated with any decrease in pain markers or in medication consumption.
Having analysed the studies published in recent years, we deem them to be of low methodological quality: we found 8 case reports14–18 or case studies,1,3,19 3 prospective studies,20–22 2 retrospective studies,8,26 one cross-sectional study,24 one quasi-experimental study,25 and only 2 randomised clinical trials.23,27 This shows that the level of evidence contributed by most studies could be improved. In the future, it would be beneficial to better control bias, enabling higher internal validity and increasing the chance of establishing a cause-effect relationship. Therefore, it would be advisable for future studies to follow a randomised clinical trial methodology.
The external validity of these future studies should also be improved. The sample sizes used in the studies selected and analysed vary greatly, from a single patient14–18 to 31,20,26 but they are generally small. Furthermore, no study calculated the sample size, which has a negative impact on the external validity of the studies and on our ability to generalise the results obtained.
According to the results of our review, MT seems to be effective in alleviating PLP as the studies analysed show its clinical benefit in controlling symptoms, regardless of aetiology; this is a safe, simple, and inexpensive treatment that enables patients to manage and treat PLP by themselves. However, the studies show a clear lack of methodological quality; several are case reports or case series, which limits the level of evidence to a grade of recommendation C (favourable, but inconclusive recommendation), demonstrating the need for further studies following an adequate methodological design to analyse the appropriateness of indicating MT to patients with PLP.
ConclusionMT seems to be efficacious in alleviating PLP and in controlling the frequency and duration of pain episodes. These clinical benefits have been obtained regardless of the aetiology of the amputation, the limb amputated, or the age of patients.
However, several outstanding issues remain. These include the influence of baseline pain level and associated symptoms on treatment outcomes, the most appropriate time to start MT, the standardisation of a protocol for application of the treatment, and establishing the actual effectiveness of MT as compared to other treatments.
Furthermore, there is a need to increase the methodological quality of studies, mainly by increasing sample sizes and improving study designs, as well as through the inclusion of medium- and long-term follow-up to verify the therapy's functionality as a self-management and self-treatment method.
Finally, in the light of the evidence presented, we can conclude that MT seems to be a valid option for managing PLP, as well as being simple and very inexpensive; therefore, with initial training, MT could be performed at the patient's home, making it accessible to more remote and/or disadvantaged populations, which would also contribute to healthcare system management.
FundingThis study received no public or private financial support.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Campo-Prieto P, Rodríguez-Fuentes G. Efectividad de la terapia de espejo en el dolor del miembro fantasma. Una revisión actual de la literatura. Neurología. 2022;37:668–681.
This study has not been presented partially or in full at any Annual Meeting of the Spanish Society of Neurology.