In cirrhosis some toxic substances accumulate in brain and modify the expression of several neuronal receptors. Thus, the use of medicinal plants such as Rosmarinus officinalis L. has been proposed in several pathologies due to its hepatoprotective, antioxidant and neuroprotective activity. In this study we evaluated the expression of the subunits NR1, NR2A and NR2B of the glutamate receptor in rat prefrontal cortex in a model of hepatic damage induced with carbon tetrachloride after a treatment with R. officinalis L.
MethodsWe used a total of 24 male Wistar rats weighing 80–90g body weight. We formed three study groups: control group (C) without a treatment, carbon tetrachloride group (CC14), and CC14 group plus R. officinalis L. (CCl4+ROM; 1.5g/kg of extract orally).
ResultsThe expression of the NR1, NR2A and NR2B subunits in cirrhotic animals increased compared to the control group; however treatment with R. officinalis L. was able to reduce this expression to normal levels compared with CC14 and CCl4+ROM groups. These results could be due to an improvement in hepatic function.
ConclusionTreatment with extract of R. officinalis L. in cirrhotic animals modifies the expression of subunits of the N-methyl-d-aspartate (NMDA) receptor due to an improvement in hepatocellular function in the presence of antioxidant compounds and flavonoids.
En la cirrosis, algunas sustancias tóxicas se acumulan en el cerebro y alteran la expresión de diversos receptores neuronales. En este sentido, se ha propuesto el uso de plantas medicinales como el Rosmarinus officinalis L. en diversas patologías debido su actividad hepatoprotectora, antioxidante y neuroprotectora. En el presente trabajo se evaluó la expresión de las subunidades NR1, NR2A y NR2B del receptor a Glutamato en la corteza prefrontal de la rata en un modelo de daño hepático inducido con tetracloruro de carbono después del tratamiento con Rosmarinus officinalis L.
MétodosSe utilizaron un total de 24 ratas macho Wistar de 80–90g. de peso corporal. Se formaron 3 grupos de trabajo: grupo testigo (T) sin ningún tratamiento, grupo tetracloruro de carbono (CCl4) y grupo CCl4 más Rosmarinus officinalis L. (CCl4+ROM; 1.5g/kg del extracto por vía oral).
ResultadosLa expresión de las subunidades NR1, NR2A y NR2B incrementaron en los animales cirróticos con respecto al grupo T, sin embargo el tratamiento con Rosmarinus officinalis L. fue capaz de disminuir la expresión a niveles normales comparados con los grupos de CCl4 y T. Estos resultados podrían deberse a una mejora en la función hepática.
ConclusiónEl tratamiento con el extracto de Rosmarinus officinalis L. en los animales cirróticos modifica la expresión de las subunidades del receptor NMDA debido a la mejora en la función hepatocelular dada la presencia de compuestos antioxidantes y flavonoides.
Liver cirrhosis is a pathological state that occurs when chronic hepatic damage causes diffuse scarring of the tissue, thereby preventing blood from circulating freely through the scar tissue.1 The mechanisms leading to cirrhosis are alcohol abuse and chronic liver damage secondary to infection with hepatitis B or C. We also know that tissue damage resulting from oxidative stress is one of the important factors in liver failure.2 The use of experimental models of hepatic damage has suggested some mechanisms that may be active in the development of this disease. Moreover, these models have shown the effect of these mechanisms on other organs, such as the brain, where they may trigger the onset of hepatic encephalopathy (HE).3 In HE, disorders in brain function are the consequence of a prior failure in normal liver function.4 Liver function failure impedes the detoxification process for ammonia and other toxic substances that may reach the brain and modify its function.5 Hyperammonaemia is one of the factors that contribute to neurological disorders caused by both acute and chronic liver failure.5
The glutamatergic system is the neurotransmission system most commonly involved in liver failure.6 The alterations of this system affect the function of NMDA receptors and some glutamate (Glu) transporters. This in turn is related to motor and cognitive impairment in subjects with HE.7 It has been reported that acute liver failure decreases expression of astrocytic glutamate transporters (GLT-1) and increases the extracellular concentration of Glu in different regions of the brain.8,9 Additionally, different models of chronic hyperammonaemia and chronic liver failure have shown a decrease in NMDA receptor binding sites in the cerebral cortex, hippocampus, striatum and thalamus.10 It has been suggested that this decrease takes place as an adaptive response by these receptors so that they do not become over-activated. We should also point out that hyperammonaemia and acute liver failure do not modify binding by these receptors.11 It has also been reported that in cases of hepatic damage, certain toxic substances may reach the brain and provoke morphological changes affecting only the astrocytes. Nevertheless, we read reports of neuronal death mediated by excitotoxicity mechanisms via activation of NMDA receptors, similar to that occurring in cerebral ischaemia, in models of carbon tetrachloride (CCI4) hepatotoxicity.12,13
Treating cirrhosis is expensive and treatment must be increased as the disease progresses. The incidence of diseases associated with liver failure, such as HE, is high. Such diseases have a considerable impact on the quality of life of a large number of people, and in advanced stages may result in coma or death.14 Moreover, despite the progress that has been made in liver diseases, there is no effective treatment. Therapeutic strategies use a palliative approach that delays the symptoms associated with cirrhosis. They mainly consist of low-fat diets and vitamin supplements.15 At present, the only effective treatment is liver transplantation. Survival rates at 1 year after the surgery are as high as 70%.15 However, it has also been suggested that medicinal plants are a viable alternative that can contribute to resolving health problems. Therefore, studies in both ethnobotany and popular medicine have reported a wide variety of medicinal plants used for gastrointestinal complaints which have a protective effect on the liver due to their high hydroxyphenolic compound content. For instance, silymarin (a compound obtained from Silybum marianum [Asteraceae]) has been shown to protect the liver from multiple types of damage.16 In addition, HD-03, a polyherbal formulation containing 7 plants, is reported to be effective in different models of hepatotoxicity. It is able to regulate ammonia metabolism and reduce pathological neuronal morphological characteristics in the case of CCI4-induced HE.17 Moreover, we know that the extract obtained from Rosmarinus officinalis L. contains flavonoids, phenols, volatile oils, and terpenes, and it has a high antioxidant activity attributed to carnosol and carnosic acid (CA).18 In fact, earlier laboratory studies have demonstrated that R. officinalis L. extract protects the liver from the onset of liver cirrhosis in a model of sustained CCI4 administration.19 This extract also exerts a protective effect on neuronal morphology and the expression of mRNA by the glutamate transporter GLT-1.20 With all of the above in mind, the purpose of our study is to evaluate the expression of subunits NR1, NR2A, and NR2B in glutamate receptors in the prefrontal cortex of rats with CCI4-induced hepatic damage after treatment with R. officinalis L.
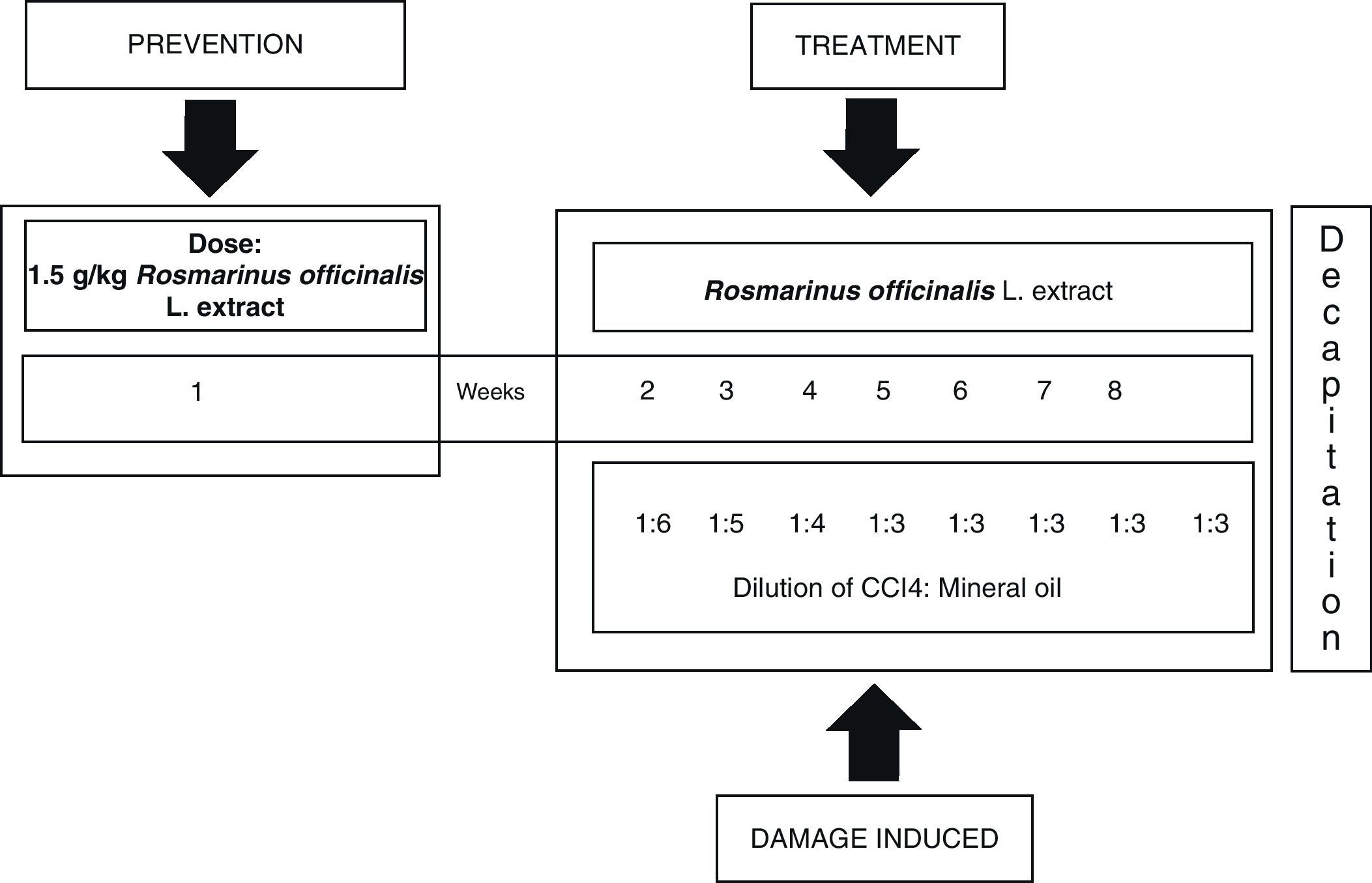
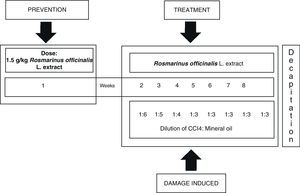
Subject and methodsAll experiments were carried out according to the Guide for the Care and Use of Laboratory Animals by the National Institute of Health (NIH Publication No. 8023, 1978). Our study included 24 male rats (Wistar strain) with body weights ranging from 80 to 90g. They lived in standard vivarium conditions, with 12:12 light–dark cycles and free access to food and water. We established three study groups: a control group without treatment (C), a CCI4 group, and a group receiving CCI4 plus extract of R. officinalis L. (CCI4+ROM; 1.5g extract/kg body weight administered orally). Treatments were administered 3 times a week during 8 weeks.
Inducing hepatic damage with CCI4 and treatment with extract of R. officinalis L.Hepatic damage was induced by intraperitoneal administration of a blend of 0.2mL CCI4 and mineral oil in a ratio of 1:1 (v/v), 3 times a week during 8 weeks, decreasing the volume of the initial blend of mineral oil and CCI4; first week 1:6, second week 1:5, third week 1:4 and fourth week, 1:3 continuing until the eighth week.21 The plant extract was administered orally prior to hepatic damage (1.5g/kg body weight) one week before beginning administration of CCI4. Treatment resumed on day 8, 1h before administration of CCI4, followed by every third day during 8 weeks (Fig. 1).
Preparation of the extractThe plant was harvested in July and August of 2009 in the San Javier communal tract of land located on the Barca Atotonilco road in Guadalajara, Jalisco, Mexico. A specimen was identified (register number IBUG-156 409) and deposited in the herbarium at the Botanical Institute of the University of Guadalajara. The leaves were dried in the shade at 22°C. We then performed a first extraction from 1kg of leaves with 5 L of n-hexane at 60°C during 1h. A second extraction was prepared with 2L of n-hexane. The resulting liquid was extracted with 6L of ethanol (60%) at 60°C during 2h. The extract was filtered and the residue washed with 2L of ethanol (60%) at 60°C. Lastly, the liquid was evaporated using a rotary evaporator (Buchi R152 rotary evaporator) at 40°C and stored at −20°C until the day it was used.
Experiments in molecular biologyAt 24h after undergoing treatment, the animals in all 3 groups were killed by decapitation. The brain was extracted under aseptic conditions, and we used previously sterilised material to dissect the prefrontal cortex. The tissue was weighed and stored at −70°C until it was used for extraction of total ribonucleic acid (RNA).
Obtaining and quantifying total ribonucleic acidTotal RNA was extracted using the guanidinium isothiocyanate–phenol–chloroform method.22 The resulting RNA precipitate was suspended in diethylpyrocarbonate-treated water (1%). The quantity and quality of the extracted RNA were evaluated by determining the 260/280nm absorbance ratio; samples with an absorbance ratio between 1.7 and 2.0 were considered optimal.
Reverse transcriptase and chain reaction of semi-quantitative polymeraseWe used Moloney murine leukaemia virus reverse transcriptase to synthesise complementary deoxyribonucleic acid (cDNA). We collected 2μg of RNA from each sample and added sterile water to achieve a total volume of 6μL. Samples were denatured at 70°C for 10min. Afterwards they were immediately incubated in an ice bath with continuous agitation for 5min. We subsequently added a total volume of 14μL of the reverse transcriptase blend, consisting of 5× RT buffer; dNTP (deoxynucleoside triphosphate) 2.5mM; dithiothreitol 1.0mM; random primers 1μg/μL; and RNAse inhibitor (1U/μL). The blend was incubated with the reverse transcriptase (200U/μL) at 37°C for 1h, with a final phase of 95°C for 10min. At the end of that phase, we added 5μL of sterile water.
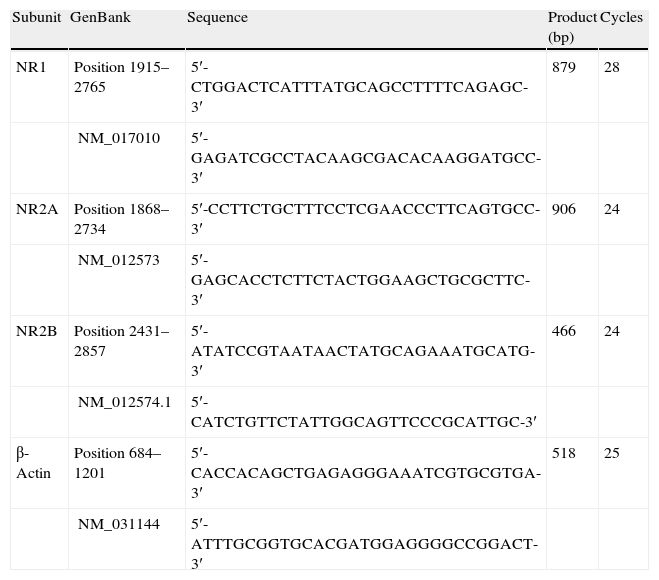
For the polymerase chain reaction, we used a reaction blend with the following composition: injectable sterile water, primers corresponding to the genes in question (Table 1) dNTP (2.5mM), 10× PCR buffer, MgCl2 (50mM), cDNA and Taq DNA polymerase (1U/μL).
Oligonucleotide sequences used for semi-quantitative PCR.
| Subunit | GenBank | Sequence | Product (bp) | Cycles |
| NR1 | Position 1915–2765 | 5′-CTGGACTCATTTATGCAGCCTTTTCAGAGC-3′ | 879 | 28 |
| NM_017010 | 5′-GAGATCGCCTACAAGCGACACAAGGATGCC-3′ | |||
| NR2A | Position 1868–2734 | 5′-CCTTCTGCTTTCCTCGAACCCTTCAGTGCC-3′ | 906 | 24 |
| NM_012573 | 5′-GAGCACCTCTTCTACTGGAAGCTGCGCTTC-3′ | |||
| NR2B | Position 2431–2857 | 5′-ATATCCGTAATAACTATGCAGAAATGCATG-3′ | 466 | 24 |
| NM_012574.1 | 5′-CATCTGTTCTATTGGCAGTTCCCGCATTGC-3′ | |||
| β-Actin | Position 684–1201 | 5′-CACCACAGCTGAGAGGGAAATCGTGCGTGA-3′ | 518 | 25 |
| NM_031144 | 5′-ATTTGCGGTGCACGATGGAGGGGCCGGACT-3′ |
We added mineral oil to the amplification reactions in order to prevent evaporation. The thermal cycler was set for an initial cycle of 95°C for 5min. The machine automatically completed the number of cycles specified in Table 1. Each cycle was set for 95°C for 1min, 60°C for 1min and 72°C for 1.5min, with a final extension cycle of 72°C for 5min. We also used the expression of the β-actin gene as a PCR control.
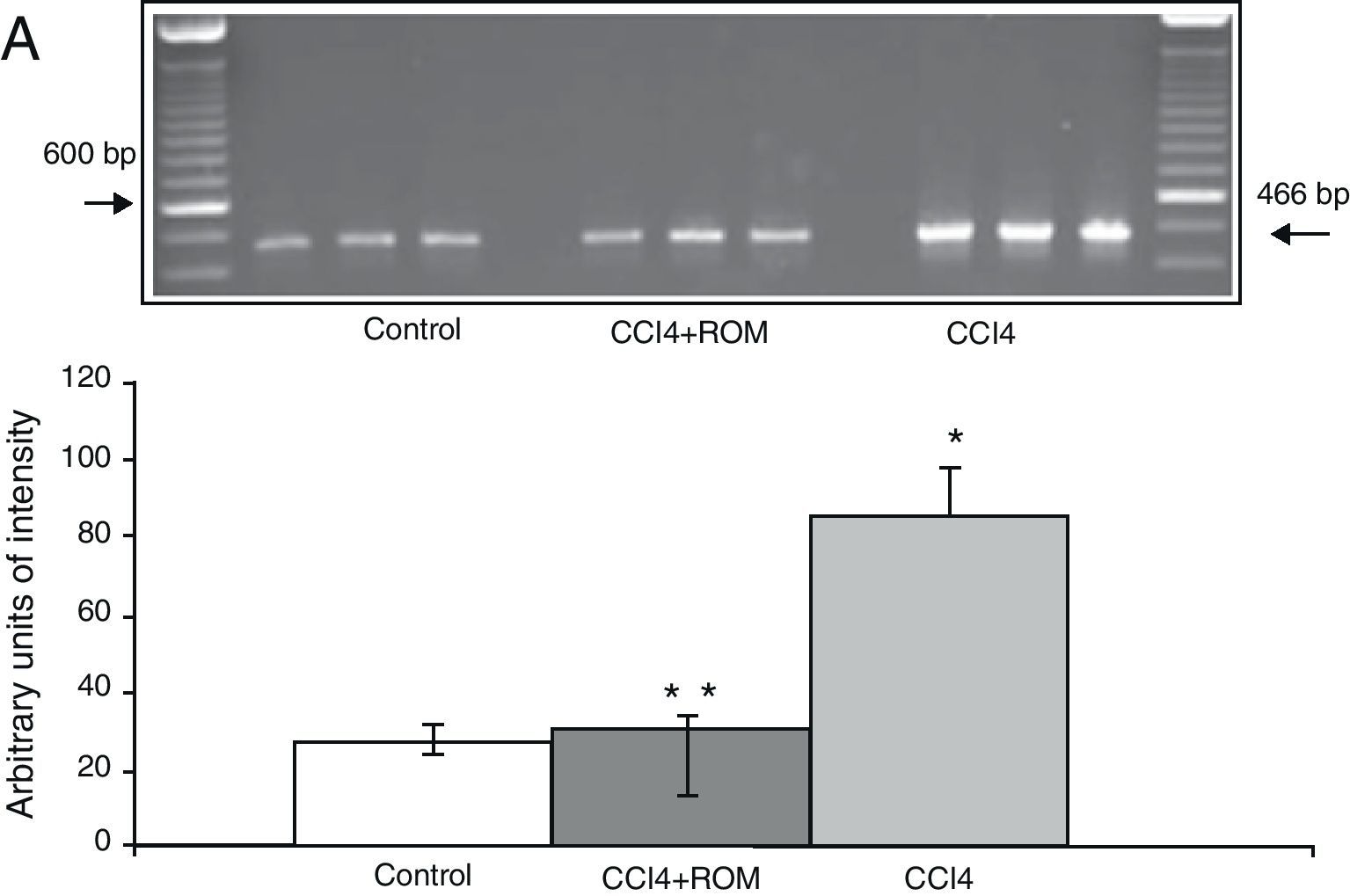
We analysed amplified PCR products in a horizontal electrophoresis chamber by using a 100bp molecular weight marker in an agarose gel (1.5%) containing ethidium bromide 0.5mg/mL for 45min at 70V in a 1× TBE (Tris-borate-EDTA) buffer. Band intensity was determined by using a photo-documentation system equipped with analysis software (Molecular Image Gel Doc XR System Quantity One 1-D Analysis Software). Levels of expression of each receptor were calculated and normalised according to the area represented by expression of a constitutive gene (β-actin). Results are expressed as arbitrary units of area by maximum intensity.
Statistical analysisWe used one-way analysis of variance (ANOVA) to perform statistical evaluation of the data. As a post hoc analysis, we used Tukey's test to compare the different study groups. The accepted level of significance was P≤.05.
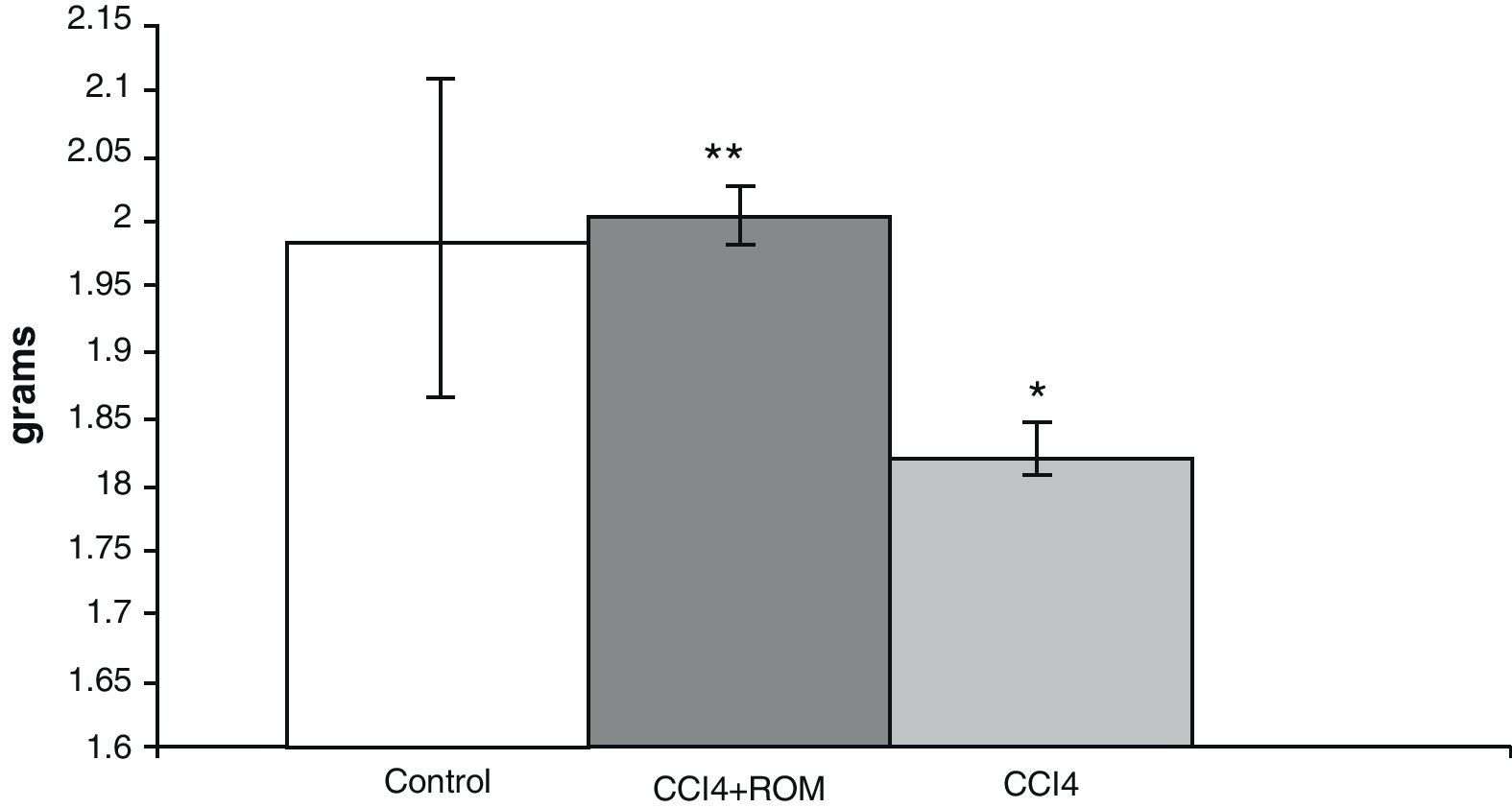
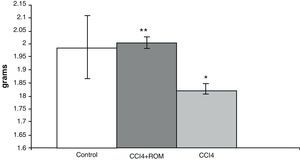
ResultsBrain weightIn animals from the CCI4 group, analysis of brain weights showed a decrease of approximately 10% (180±5) compared to animals from the C group (197±3) and cirrhotic animals who received treatment with R. officinalis L. extract (200±5) (Fig. 2).
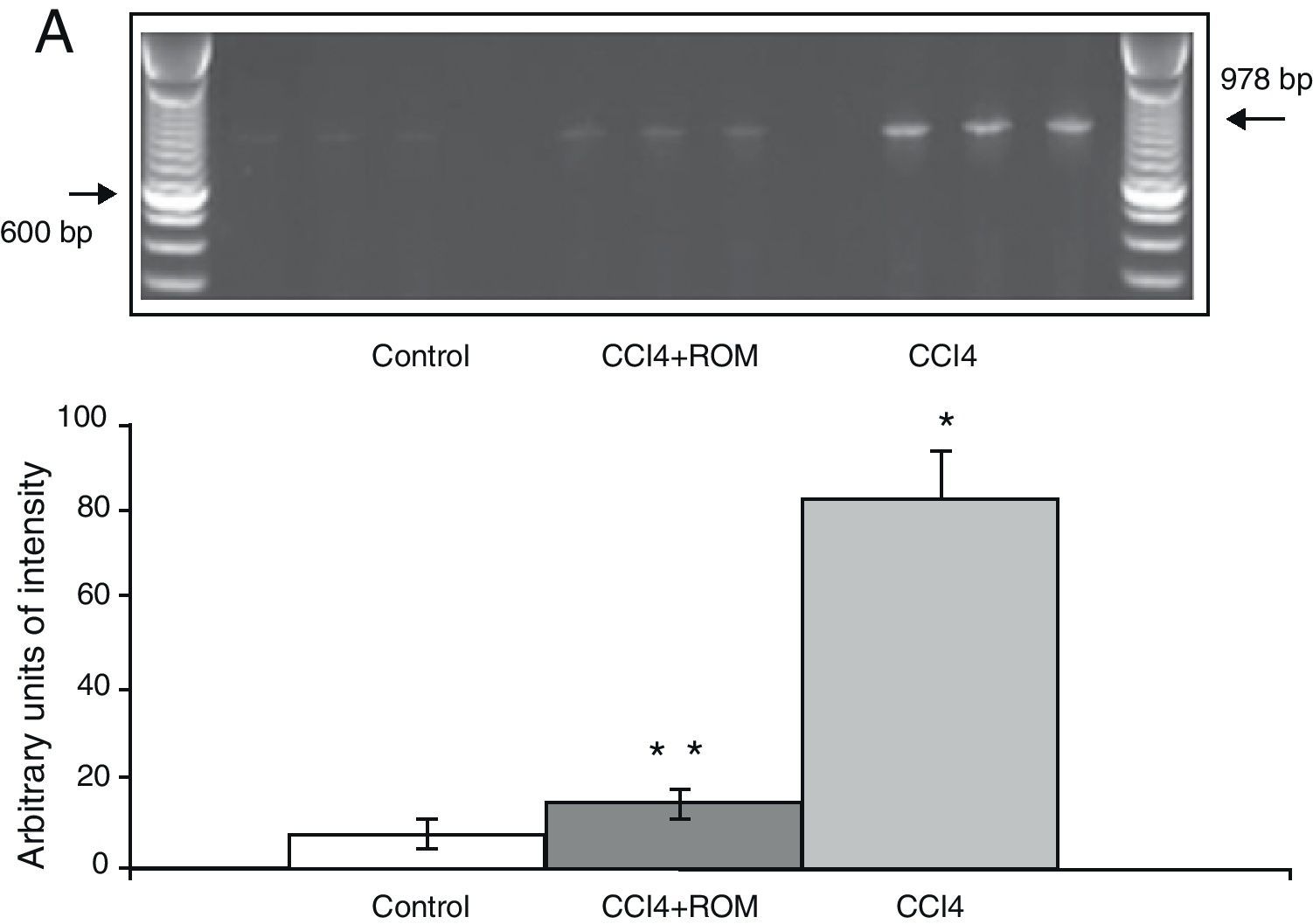
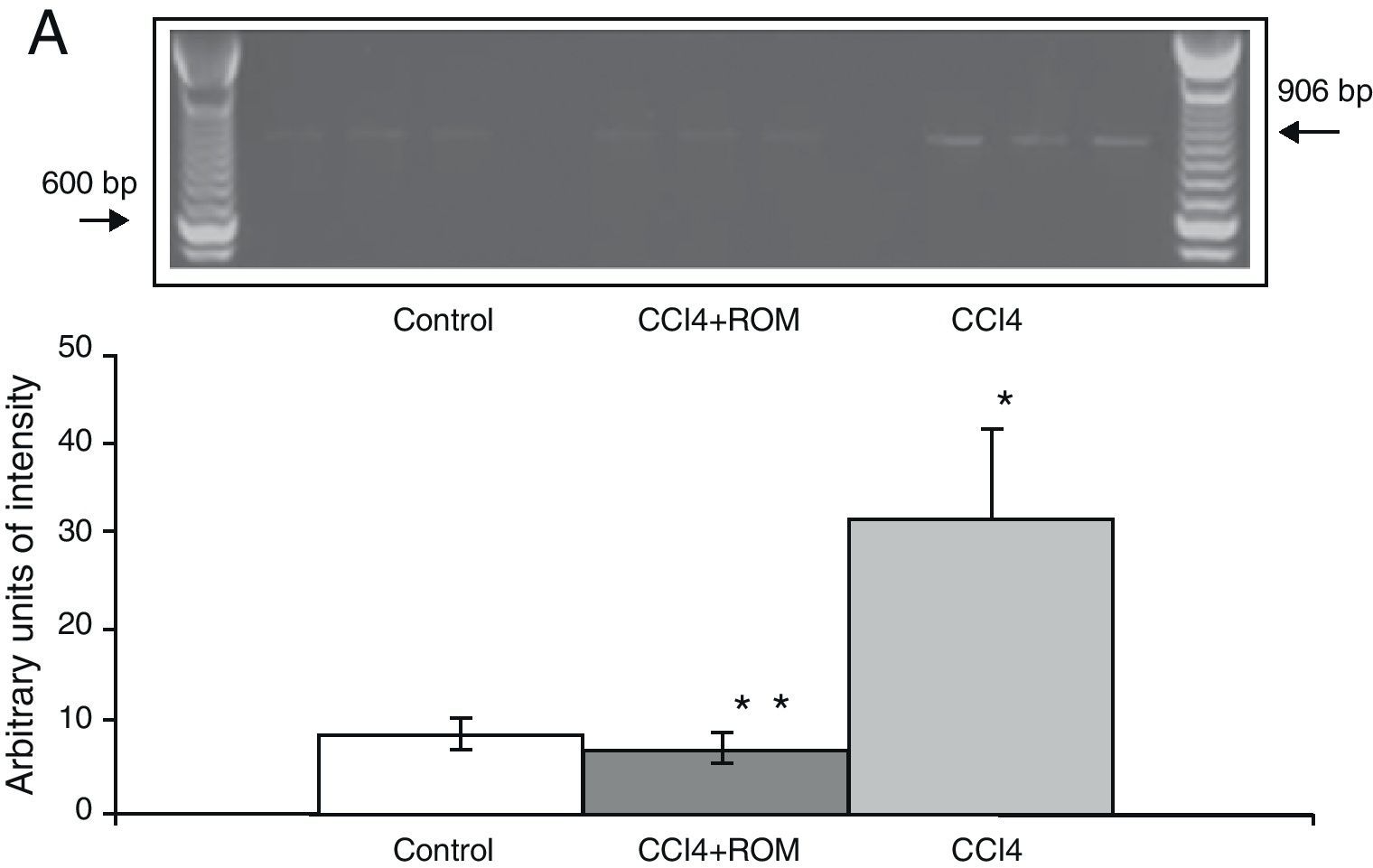
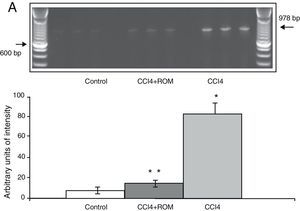
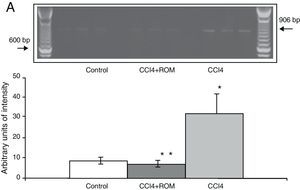
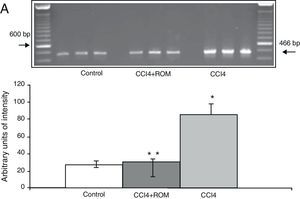
Expression of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptorThe mRNA expression of NR1 subunits in the rat prefrontal cortex was significantly higher in animals with induced hepatic damage (85±6) than in animals from the C group (10±2) and the CCI4+ROM group (12±6) (Fig. 3). At the same time, expression of the NR2A subunit was somewhat higher in the cirrhotic animals (35±6) than in animals from the C group (10±3) and CCI4+ROM (9.5±3) (Fig. 4). Lastly, the expression of the NR2B subunit of the NMDA receptor in the rat prefrontal cortex showed a statistically significant increase in those animals with induced hepatic damage (90±5) compared to animals from the C group (10±5) and the CCI4+ROM group (9.5±5) (Fig. 5). It should be noted that expression of this subunit is quite pronounced compared to other subunits in both the cirrhotic animals and in those that received the extract.
An organism's good state of health depends on the proper function of and interaction among its different organs. In particular, the liver and the brain closely interact given that the liver provides the brain with nutrients which the brain itself cannot produce, and eliminates toxic substances from the blood. This includes those substances released by the brain itself (excitotoxins) or by another organ, and which are necessarily released outside of that organ. As a consequence, liver dysfunction may elicit significant cerebral function disorders.23 Results from this study showed that the livers of animals with CCI4-induced hepatic damage show a number of traits typical of hepatic damage.20,21 They present alterations in brain weight and in mRNA expression of the NR1, NR2A and NR2B subunits of the NMDA receptor in the prefrontal cortex.
Due to this close interaction between the liver and brain, the synthesis and delivery of the nutrients necessary for maintaining cerebral function probably decrease following CCI4-induced hepatic damage.5 This may reduce the number of nerve connections that naturally occur in the brain, which is likely to be reflected as a decrease in brain weight in the CCI4 group. It is interesting to note that the extract administered to the cirrhotic animals did not alter their brain weight in comparison with animals in the C group.
The mRNA expression of the NR1, NR2A and NR2B subunits of the NMDA receptor increased significantly in those animals treated with CCI4. It has been reported that hepatic damage induced by different hepatotoxic agents, by hyperammonaemia or by bile duct ligation does not modify binding by NMDA receptors; however, their functions do not remain the same.10,24,25 One might think that some of its subunits, as structural components, could undergo changes, thereby altering receptor function as well. To date, the expression of NMDA receptor subunits in the brain in the presence of hepatic damage has not been reported.
It should be noted that the NMDA receptor, due to its functional tetrameric structure, requires the structural presence of the NR1 and NR2 subunits; the latter performs glutamate recognition. Therefore, the total function of the receptor depends on the variability of the subunits that make it up.26 It has been reported that the presence of the NR2B subunit in the receptor protects the neuron from some harmful stimuli, while the electrophysiological characteristics of NR1 and NR2A make these subunits more susceptible to damage. This is because when this combination of subunits is activated, the channel remains open for a longer amount of time,26 which promotes neuronal damage. The results for NR1 and NR2B expression were much higher than those for the NR2A subunit, in both cirrhotic animals and those receiving extract of R. officinalis L. This may suggest two things. Firstly, that the CCI4-induced hepatic cirrhosis model may develop a different adaptive mechanism from the one reported for other models of hepatic damage and HE. Secondly, that the increase in NR2B expression, both in cirrhotic animals and after treatment with the extract, may protect the neuron from toxic substances that may trigger neuronal death or damage mechanisms. However, in this CCI4-induced hepatic damage model, we did observe neuronal damage in the prefrontal cortex.20 Changes in the expression of the receptor subunits were very likely not sufficient to exert a protective effect on the neuron. However, we should not rule out the possibility of there being a response mechanism specific to the model employed, or potential participation by other types of receptors. It has been shown that hepatic damage decreases the expression of both GLT-1 protein and its mRNA. It also increases the extracellular concentration of Glu,9,11,20 which may over-activate NMDA receptors, increase calcium influx into the postsynaptic neurons, and cause neuronal damage.
Alterations in the expression of NMDA receptor subunits may be related to the increase in the number of astrocytes, given that we know that ammonium levels in the bloodstream are elevated during hepatic damage.27 This may be the cause of the increased expression of NR1, NR2A and NR2B subunits of astrocyte NMDA-type receptors.28
We do not completely understand the mechanism of action of R. officinalis L. extract, but the presence of antioxidants and flavonoids may provide an explanation. For instance, it has been reported that one of its components, CA, has neuroprotective effects and is able to penetrate the blood–brain barrier. It has been shown that CA activates the Keap1/Nrf2 pathway and protects neurons from oxidative stress and excitotoxicity.29 The results of this study suggest that, in addition to the hepatoprotective effect caused by its high polyphenol content and antioxidant activity,8 the extract could have a neuroprotective effect which would be useful in pathologies involving altered brain function as a result of liver failure. Nevertheless, more research is needed in order to evaluate the activity of certain enzymes involved in glutamate metabolism, and investigate other enzymes with an antioxidant activity that may activate the Keap1/Nrf2 pathway. This would allow us to determine the molecular mechanism of action of R. officinalis L. extract in the brain.
FundingPartial funding for this study was provided by COECYTJAL-UDG as project leaflet PS-209-515.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Soria Fregozo C, et al. Expresión de las subunidades del receptor NMDA en la corteza prefrontal de la rata con daño hepático inducido con CCL4 después del tratamiento con Rosmarinus officinalis L. Neurología. 2012;27:261–7.