This study evaluates care-related sociodemographic, clinical, and imaging factors and influences associated with outcome at discharge in patients with aneurismal subarachnoid haemorrhage.
Patients and methodRetrospective cohort study in 334 patients treated at Hospital Hermanos Ameijeiras in Havana, Cuba between October 2005 and June 2014.
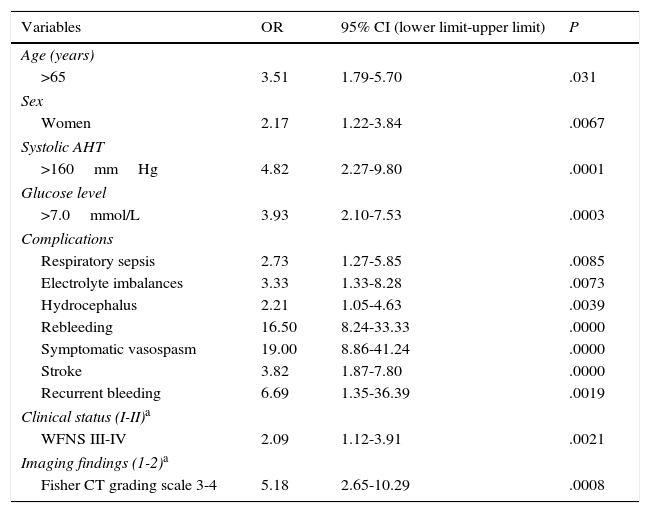
ResultsLogistic regression analysis determined that the following factors were associated with higher risk of poor outcome: age older than 65 years (OR 3.51, 95% CI 1.79-5.7, P=.031), female sex (OR 2.17, 95% CI 1.22-3.84, P=.0067), systolic hypertension (OR 4.82, 95% CI 2.27-9.8, P=.0001), and hyperglycaemia at admission (OR 3.93, 95% CI 2.10-7.53, P=.0003). Certain complications were also associated with poor prognosis, including respiratory infection (OR 2.73, 95% CI 1.27-5.85, P=.0085), electrolyte disturbances (OR 3.33, 95% CI 1.33-8.28, P=.0073), hydrocephalus (OR 2.21, 95% CI 1.05-4.63, P=.0039), rebleeding (OR 16.50, 95% CI 8.24-41.24, P=.0000), symptomatic vasospasm (OR 19.00, 95% CI 8.86-41.24, P=.0000), cerebral ischaemia (OR 3.82, 95% CI 1.87-7.80, P=.000) and multiplex rebleeding (OR 6.69, 95% CI 1.35-36.39, P=.0019). Grades of iii and iv on the World Federation of Neurological Surgeons (OR 2.09, 95% CI 1.12-3.91, P=.0021) and Fisher scales (OR 5.18, 95% CI 2.65-10.29, P=.0008) were also related to poor outcome.
ConclusionsOutcome of aneurysmal subarachnoid haemorrhage was related to age, sex, clinical status at admission to the stroke unit, imaging findings according to the Fisher scale, blood pressure, glycaemia and such complications as electrolyte disturbances, hydrocephalus, rebleeding, and multiplex rebleeding.
Evaluar los factores sociodemográficos, clínicos, imagenológicos y relacionados con la atención médica que influyen sobre el pronóstico de los pacientes con hemorragia subaracnoidea aneurismática al alta hospitalaria.
Pacientes y métodoSe realizó un estudio tipo cohorte, retrospectivo, con 334 pacientes atendidos en el Hospital Hermanos Ameijeiras en La Habana, Cuba, en el periodo comprendido entre octubre de 2005 y junio de 2014.
ResultadosEn el análisis multivariado se encontró que los factores asociados a una evolución desfavorable fueron la edad mayor a 65 años (OR 3,51, IC 95% 1,79-5,7, p=0,031), el sexo femenino (OR 2,17, IC 95% 1,22-3,84, p=0,0067), la HTA sistólica (OR 4,82, IC 95% 2,27-9,8, p=0,0001), la hiperglucemia al ingreso (OR 3,93, IC 95% 2,10-7,53, p=0,0003), las complicaciones como la sepsis respiratoria (OR 2,73, IC 95% 1,27-5,85, p=0,0085), los trastornos hidroelectrolíticos (OR 3,33, IC 95% 1,33-8,28, p=0,0073), la hidrocefalia (OR 2,21, IC 95% 1,05-4,63, p=0,0039), el resangrado (OR 16,50, IC 95% 8,24-41,24, p=0,0000), el vasoespasmo sintomático (OR 19,00, IC 95% 8,86-41,24, p=0,0000), el infarto cerebral (OR 3,82, IC 95% 1,87-7,80, p=0,0000), el resangrado múltiple (OR 6,69, IC 95% 1,35-36,39, p=0,0019), así como los grados iii y iv de las escalas de la Federación Mundial de Neurocirujanos (OR 2,09, IC 95% 1,12-3,91, p=0,0021) y de Fisher (OR 5,18, IC 95% 2,65-10,29, p=0,0008).
ConclusionesLa evolución de la hemorragia subaracnoidea aneurismática está relacionada con la edad, el sexo, el estado clínico al arribo a la unidad de ictus, así como las características imagenológicas según la escala de Fisher, las cifras de tensión arterial y de glucemia, y las complicaciones como los trastornos hidroelectrolíticos, la hidrocefalia, el resangrado, el vasoespasmo y el resangrado múltiple.
Cerebrovascular diseases have remained among the world's 3 leading causes of death for many years.1 Although aneurysmal subarachnoid haemorrhage (ASAH) is not ranked among the most frequent cerebrovascular diseases, it has one of the highest morbidity and mortality rates (between 23% and 51%).2
Multiple sociodemographic, clinical, radiological, and healthcare-related factors have been associated with poor outcome in ASAH; however, data come mainly from studies conducted in developed countries and following treatment protocols which differ considerably from the ones proposed recently with the introduction of new drugs and technologies.3,4 Identifying the variables associated with poor outcomes in our setting would help us develop and implement more effective treatment strategies focused on preventing and managing these factors. Furthermore, few studies have evaluated the factors associated with poor outcomes in our setting, or the mortality risk factors associated with the aetiopathogenic subtype of stroke.1
In light of the above, we decided to undertake a study to evaluate the sociodemographic, clinical, imaging, and healthcare-related factors linked to prognosis at discharge in patients with ASAH.
Patients and methodsWe conducted a retrospective observational cohort study initially including 357 patients with ASAH treated at the stroke unit (SU) of Hospital Clínico-Quirúrgico Hermanos Ameijeiras in La Habana, Cuba, between 1 October 2005 and 31 June 2014. Patients had to meet the following inclusion criteria: (1) a diagnosis of subarachnoid haemorrhage (SAH) confirmed by either an initial CT scan showing signs of bleeding in the subarachnoid space or a CSF analysis displaying xanthochromia; (2) CT-angiography or brain angiography revealing an aneurysm with a bleeding pattern in the initial CT scan coinciding with the location of SAH; (3) ages 18 and older; (4) a Glasgow Coma Scale5 score >8; and (5) grades IV or below on the World Federation of Neurosurgical Societies (WFNS) grading scale.6 Exclusion criteria were presence of mycotic aneurysms and incomplete medical history.
We excluded 18 patients whose histories did not include all the data necessary for our study and an additional 5 patients whose complications and early mortality made it impossible for us to complete the diagnostic protocol.
We recorded the following sociodemographic variables: age, race, and biological sex.
Clinical variables recorded were history of SAH, arterial hypertension, or diabetes mellitus. This condition was considered to have been met when either the patient or a close relative had been diagnosed with any of these conditions. We also gathered data on tobacco and alcohol use: patients smoking at least one cigarette per day were considered smokers, and alcohol users were those consuming at least 30mL rum or 2 beers 3 times per week for 2 consecutive months of the preceding 6 months. Patients were also recorded as having systolic arterial hypertension when monitoring values exceeded 160mmHg at any moment during the first 4 hours after arrival at the SU; mean systolic/diastolic blood pressure over that period was also recorded as a continuous quantitative variable. Any blood glucose levels >7.0mmol/L at admission were noted, and glucose level was recorded as a continuous quantitative variable.
We took note of any of the following complications. Respiratory sepsis: demonstrated by presence of signs of inflammation on chest radiography (anteroposterior projection). Sepsis: temperature >38°C and presence of >100000CFU/mL urine in cases of urinary infection; fever (>38°C) and a positive blood culture in cases of toxic-infectious symptoms with no definite septic focus, and >10 polymorphonuclear cells in CSF in cases of meningoencephalitis. Catheter-related sepsis and/or phlebitis was defined by the presence of specific clinical signs, reddening along the trajectory of the vein, and signs of inflammation in the catheterised vein. Patients were considered to have epileptic seizures when they experienced recurrent paroxysmal events such as tonic, clonic, myoclonic, or tonic–clonic movements, or else cognitive disorders/sensory alterations correlated with EEG findings during wakefulness. Presence of electrolyte imbalances was established based on the results of serum electrolyte tests conducted daily: hypernatraemia (sodium level >145mmol/L), hyponatraemia (sodium level <130mmol/L), hyperkalaemia (potassium level >4.5mmol/L), and hypokalaemia (potassium level <3mmol/L). Hydrocephalus: patients experiencing deterioration in the level of consciousness and displaying signs suggestive of hydrocephalus on simple cranial CT. Rebleeding: presence or worsening of headache, or sudden changes in the level of consciousness combined with simple cranial CT revealing a greater volume of subarachnoid or intraventricular blood than that found by the initial study. Recurrent bleeding: presence of clinical signs and neuroimaging alterations suggestive of haemorrhage on several occasions, as described previously. Cerebral ischaemia: hypodensity on simple cranial CT which was not seen in the initial study. Symptomatic vasospasm (arterial blood flow velocity >120cm/s in anterior circulation arteries and/or >100cm/s in posterior circulation arteries) associated with findings from the physical examination corresponding to the affected territory.
We also recorded whether patients needed mechanical ventilation, decompressive craniectomy, admission to the intensive care unit, or aneurysm treatment (embolisation or clipping). All these variables were regarded as dichotomous. We evaluated patients’ clinical and imaging status on the WFNS grading scale and the Fisher CT grading scale,7 respectively. We also recorded the number of complications per patient, and divided the sample into patients with no complications, patients with 1 or 2 complications, and patients with 3 or more complications. At discharge, patients were classified according to their scores on the modified Rankin Scale (mRS); this scale was used to measure progression. Scores of 1, 2, and 3 were regarded as suggestive of good outcomes, whereas scores 4, 5, and 6 indicated poor outcomes.
All patients were managed according to our hospital's treatment protocol, which follows recommendations in international treatment guidelines for the management of ASAH.8 Surgery (clipping or embolisation) was conducted as early as possible (preferably within the first 72hours). Treatment type was determined by reaching a consensus among vascular neurologists, vascular neurosurgeons, and neuroradiologists. Although endovascular treatment was generally applied to elderly patients and those displaying aneurysms in posterior circulation arteries, each case was evaluated individually.
All patients received 0.9% saline solution dosed at 1mL/kg/h, supplemented with 5% human albumin when necessary to raise central venous pressure to 5mmHg. Standard treatment included nimodipine (360mg/day), paracetamol (2g/day), tramadol when necessary, phenytoin (100mg twice daily) in the post-haemorrhage stage, and laxatives. Patients with hydrocephalus underwent ventricular shunt implantation. All patients underwent transcranial Doppler ultrasonography on a daily basis plus 2-hour EEG monitoring during wakefulness where necessary.
Our study was discussed and approved by the ethics committee at our hospital. Patients’ personal data were protected to preserve confidentiality. Our study complied with the ethical standards of the Declaration of Helsinki of 1975.
In the data analysis, patients were divided into 2 groups: patients with good outcomes and those with poor outcomes. Qualitative variables were expressed as absolute and relative frequencies and compared using non-parametric tests (chi-square test, Fisher exact test). Quantitative variables were expressed as means±SD and compared using the t test. A binary logistic regression analysis was conducted to determine the independent factors associated with poor outcome. P-values≤.05 were considered statistically significant. Statistical analysis was performed using SPSS® 17.0 statistical software.
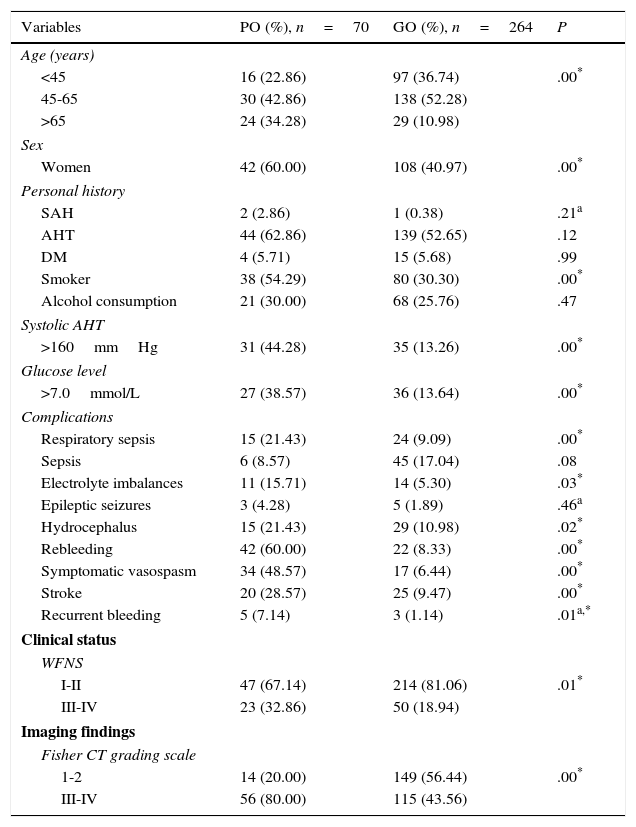
ResultsOur study included 334 patients, 70 of whom (20.95%) had poor outcomes. According to the univariate analysis (Table 1), most patients were between 45 and 65 years old (42.86% of all patients with poor outcomes and 52.28% of those with good outcomes); most patients with poor outcomes were women (60%). We found a statistically significant association between poor outcome and the following factors: more advanced age (P=.00), female sex (60% vs 40.97%; P=.00), smoking (54.29% vs 30.30%; P=.00), systolic arterial hypertension >160mmHg (44.28% vs 13.26%; P=.00), glucose levels >7.0mmol/L at admission to the SU (38.57 vs 13.64%; P=.00), rebleeding (60% vs 8.33%; P=.00), symptomatic vasospasm (48.57% vs 6.44%; P=.00), and stroke (28.57% vs 9.47%; P=.00). Poorer clinical status at admission (P=.01) and a larger bleeding volume in the initial CT scan according to the Fisher CT grading scale (P=.00) were also associated with poorer outcome.
Multivariate analysis.
| Variables | PO (%), n=70 | GO (%), n=264 | P |
|---|---|---|---|
| Age (years) | |||
| <45 | 16 (22.86) | 97 (36.74) | .00* |
| 45-65 | 30 (42.86) | 138 (52.28) | |
| >65 | 24 (34.28) | 29 (10.98) | |
| Sex | |||
| Women | 42 (60.00) | 108 (40.97) | .00* |
| Personal history | |||
| SAH | 2 (2.86) | 1 (0.38) | .21a |
| AHT | 44 (62.86) | 139 (52.65) | .12 |
| DM | 4 (5.71) | 15 (5.68) | .99 |
| Smoker | 38 (54.29) | 80 (30.30) | .00* |
| Alcohol consumption | 21 (30.00) | 68 (25.76) | .47 |
| Systolic AHT | |||
| >160mmHg | 31 (44.28) | 35 (13.26) | .00* |
| Glucose level | |||
| >7.0mmol/L | 27 (38.57) | 36 (13.64) | .00* |
| Complications | |||
| Respiratory sepsis | 15 (21.43) | 24 (9.09) | .00* |
| Sepsis | 6 (8.57) | 45 (17.04) | .08 |
| Electrolyte imbalances | 11 (15.71) | 14 (5.30) | .03* |
| Epileptic seizures | 3 (4.28) | 5 (1.89) | .46a |
| Hydrocephalus | 15 (21.43) | 29 (10.98) | .02* |
| Rebleeding | 42 (60.00) | 22 (8.33) | .00* |
| Symptomatic vasospasm | 34 (48.57) | 17 (6.44) | .00* |
| Stroke | 20 (28.57) | 25 (9.47) | .00* |
| Recurrent bleeding | 5 (7.14) | 3 (1.14) | .01a,* |
| Clinical status | |||
| WFNS | |||
| I-II | 47 (67.14) | 214 (81.06) | .01* |
| III-IV | 23 (32.86) | 50 (18.94) | |
| Imaging findings | |||
| Fisher CT grading scale | |||
| 1-2 | 14 (20.00) | 149 (56.44) | .00* |
| III-IV | 56 (80.00) | 115 (43.56) | |
PO: poor outcome (mRS scores 4-6); GO: good outcome (mRS scores 1-3); WFNS: World Federation of Neurosurgical Societies grading scale; AHT: arterial hypertension.
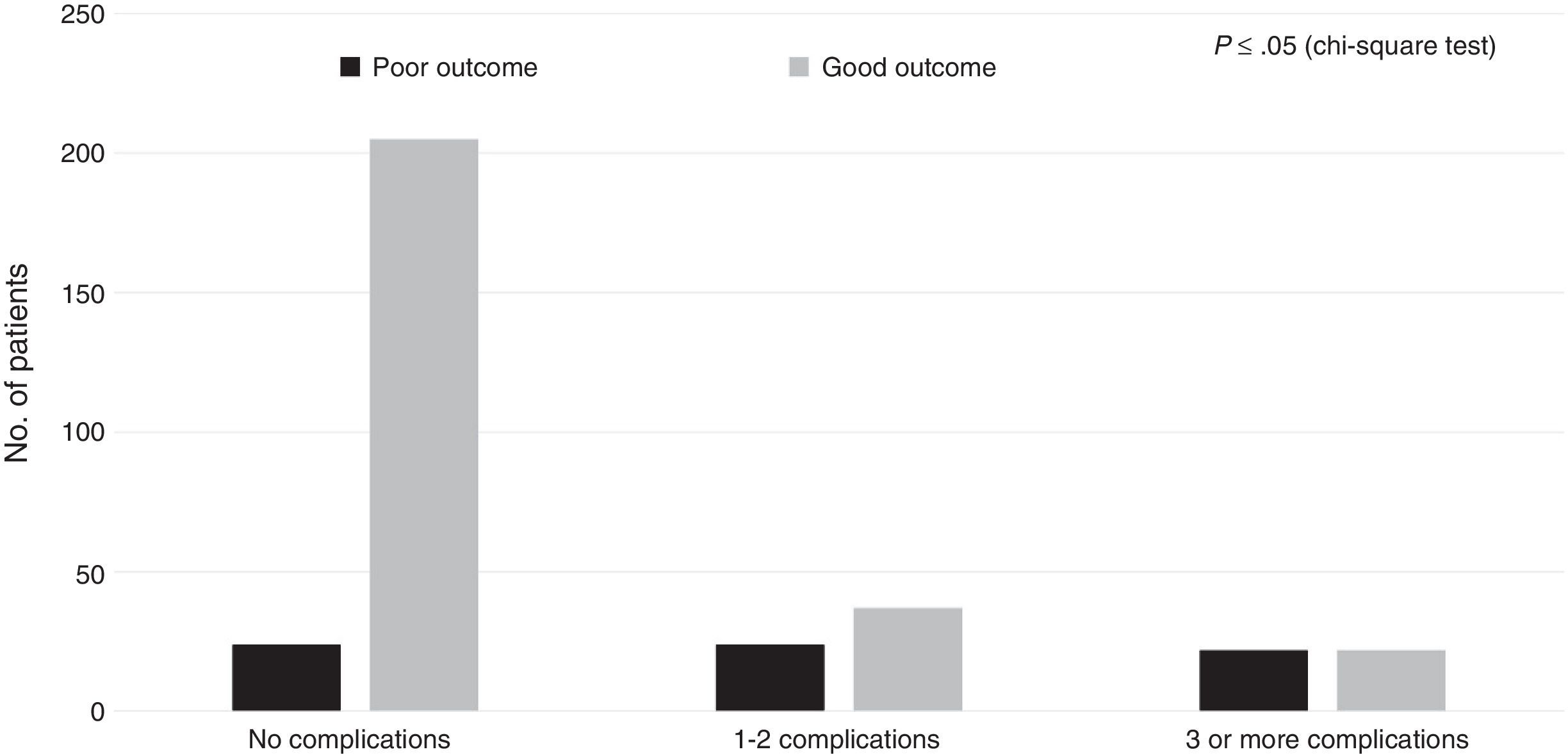
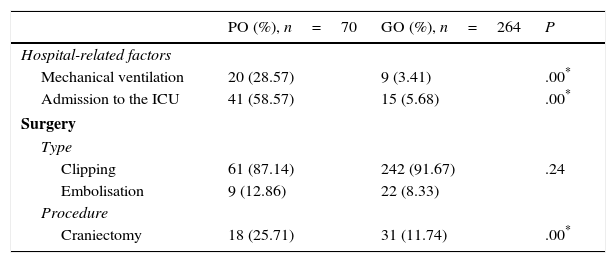
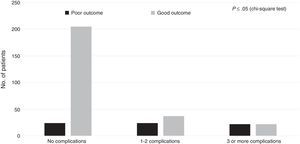
Regarding the healthcare-related factors included in our study (Table 2), mechanical ventilation (P=.00), admission to the intensive care unit (P=.00), and craniectomy (P=.00) displayed a statistically significant association with poor outcome. However, no differences were found among alternative surgical approaches. Fig. 1 shows the distribution of patients with poor or good outcomes by number of complications (no complications, 1-2 complications, 3 or more complications). Most of the patients with no complications had good outcomes; however, the number of patients with a good outcome decreased significantly among patients with 1-2 complications and 3 or more complications. On the other hand, the number of patients with poor outcomes was similar in all groups regardless of the number of complications.
Healthcare-related factors and outcomes.
| PO (%), n=70 | GO (%), n=264 | P | |
|---|---|---|---|
| Hospital-related factors | |||
| Mechanical ventilation | 20 (28.57) | 9 (3.41) | .00* |
| Admission to the ICU | 41 (58.57) | 15 (5.68) | .00* |
| Surgery | |||
| Type | |||
| Clipping | 61 (87.14) | 242 (91.67) | .24 |
| Embolisation | 9 (12.86) | 22 (8.33) | |
| Procedure | |||
| Craniectomy | 18 (25.71) | 31 (11.74) | .00* |
PO: poor outcome (mRS scores 4-6); GO: good outcome (mRS scores 1-3); ICU: intensive care unit.
According to the multivariate analysis (Table 3), poor outcome was associated with the following factors: age older than 65 years (OR 3.51; 95% CI, 1.79-5.7); female sex (OR 2.17; 95% CI, 1.22-3.84); systolic arterial hypertension (OR 4.82; 95% CI, 2.27-9.8); high blood glucose levels at admission (OR 3.93; 95% CI, 2.10-7.53); such complications as respiratory sepsis (OR 2.73; 95% CI, 1.27-5.85), rebleeding (OR 16.50; 95% CI, 8.24-41.24), symptomatic vasospasm (OR 19.00; 95% CI, 8.86-41.24), and stroke (OR 3.82; 95% CI, 1.87-7.80); WFNS grades III and IV (OR 2.09; 95% CI, 1.12-3.91), and Fisher CT grading scale grades 3 and 4 (OR 5.18; 95% CI, 2.65-10.29), among others.
Logistic regression.
| Variables | OR | 95% CI (lower limit-upper limit) | P |
|---|---|---|---|
| Age (years) | |||
| >65 | 3.51 | 1.79-5.70 | .031 |
| Sex | |||
| Women | 2.17 | 1.22-3.84 | .0067 |
| Systolic AHT | |||
| >160mmHg | 4.82 | 2.27-9.80 | .0001 |
| Glucose level | |||
| >7.0mmol/L | 3.93 | 2.10-7.53 | .0003 |
| Complications | |||
| Respiratory sepsis | 2.73 | 1.27-5.85 | .0085 |
| Electrolyte imbalances | 3.33 | 1.33-8.28 | .0073 |
| Hydrocephalus | 2.21 | 1.05-4.63 | .0039 |
| Rebleeding | 16.50 | 8.24-33.33 | .0000 |
| Symptomatic vasospasm | 19.00 | 8.86-41.24 | .0000 |
| Stroke | 3.82 | 1.87-7.80 | .0000 |
| Recurrent bleeding | 6.69 | 1.35-36.39 | .0019 |
| Clinical status (I-II)a | |||
| WFNS III-IV | 2.09 | 1.12-3.91 | .0021 |
| Imaging findings (1-2)a | |||
| Fisher CT grading scale 3-4 | 5.18 | 2.65-10.29 | .0008 |
WFNS: World Federation of Neurosurgical Societies grading scale; AHT: arterial hypertension; 95% CI: 95% confidence interval; OR: odds ratio.
Management of patients with ASAH is a true therapeutic challenge. Nevertheless, mortality has decreased in the past decade, probably as a result of early treatment and the introduction of new neuroimaging and surgical techniques. However, an estimated 20% of all surviving patients with ASAH will be partially or completely dependent (mRS scores 4 and 5) at 12 months, and this figure is in line with our results.8,9 Several factors have been associated with poor outcomes, including advanced age, female sex, and race or skin colour.3,8,10 Although results from our study were similar overall, we found no statistically significant associations between outcome and race or skin colour, a finding that may be due to Cuba's sizeable multiracial population.
Other factors associated with poor outcomes, as identified by this and other studies, are hyperglycaemia, systolic arterial hypertension, and poor clinical status at admission (WFNS grade ≤IV).3,9,11,12 Poor outcome was linked not only to hyperglycaemia as a metabolic disorder, but also to blood sugar fluctuations; a recent study found an association between glucose variability and cerebral metabolic distress and mortality.13 Systolic arterial hypertension >160mmHg was independently associated with poor outcome; however, we did not analyse whether or not this was due to its association with rebleeding. The same was true for clinical status at admission (WFNS), vasospasm, and grades 3 and 4 on the Fisher CT grading scale. In our view, poor outcomes are more likely to result from multiple factors than from single factors, considering that patients with larger haemorrhages usually have a poorer clinical status and are more likely to experience rebleeding, hydrocephalus, vasospasm, and more severe surgical complications.
The complications found to act as independent predictors of poor outcome were respiratory sepsis, electrolyte imbalances, hydrocephalus, rebleeding, symptomatic vasospasm, stroke, and recurrent bleeding. These results are in line with the literature.2,3,9,14,15 According to some studies, rebleeding is one of the complications with the highest rates of poor outcome and mortality15; surgery should therefore be carried out as early as possible. However, this is no easy task. In contrast with other studies,3,9 ours found no association between sepsis and patient outcome. We feel that having several conditions with different levels of severity (CNS sepsis, phlebitis) into the same category may have biased our results. Complications are certainly one of the factors with the most impact on patient outcomes: according to our analysis, absence of complications was associated with good outcome. In our view, poor outcome in patients requiring mechanical ventilation and admission to the intensive care unit was due to greater disease severity rather than to errors in care in this unit.
Our study has a number of limitations, including its retrospective observational design and the fact that outcome is influenced by a wide range of factors in addition to those variables included in our study. Furthermore, in our series endovascular treatment was only administered in the last 4 years, which may have biased our results. Likewise, multivariate analysis may also have introduced biases.
In conclusion, our results support the idea that the outcome of ASAH is influenced by a number of factors including age, sex, clinical status at admission to the SU, imaging features (Fisher CT grading scale), blood pressure, blood glucose levels, and such complications as electrolyte imbalances, hydrocephalus, rebleeding, vasospasm, and recurrent bleeding.
FundingThis study has received no funding of any kind, whether for research or for manuscript publication.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Rivero Rodríguez D, Scherle Matamoros C, Fernández Cúe L, Miranda Hernández JL, Pernas Sánchez Y, Pérez Nellar J. Factores asociados a una evolución desfavorable en la hemorragia subaracnoidea aneurismática. Serie de 334 pacientes. Neurología. 2017;32:15–21.