We present the guidelines for pain management in neurorehabilitation of brain injury in adults of the Spanish Society of Neurorehabilitation based on the review and analysis of the available literature on the subject. We establish recommendations according to the level of evidence offered by the reviewed studies.
DevelopmentThe methodology followed by the Spanish Society of Neurorehabilitation for the elaboration of the present guide is based on the analysis of the national and international clinical practice guidelines of the last seven years, carried out according to the management considerations dictated by the evidence for the usual types of pain in the population of people who have suffered an acquired brain injury.
ConclusionsPain is a common symptom in patients who have suffered brain damage, having a negative impact on quality of life and adherence to the rehabilitation process. When classifying the type of pain according to etiological characteristics, in order to optimize the type of therapeutic approach, we usually refer to pain as nociceptive, and neuropathic pain, although pain in such patients often has “mixed” characteristics. The most common type of pain is nociceptive ahead of neuropathic. The most common pain syndromes in this population are headache, hemiplegic shoulder pain and poststroke central pain. As pain is a subjective experience, people with impaired level of consciousness, severe cognitive impairment and/or severe language problems may have greater difficulty or even being unable to communicate it. An adequate clinical history and a directed physical examination, as well as the use of specific scales for its correct diagnosis are therefore important. Finally, many of the drugs used for its management have a negative impact on rehabilitation, affecting cognitive processes, and/or worsening other neurological symptoms. Furthermore, these patients often have several comorbidities and are frequently on several drugs which means that the approach to pain management must be carefully elaborated by a multidisciplinary team approach.
Guía para el manejo del dolor en neurorrehabilitación del daño cerebral sobrevenido de personas adultas de la Sociedad Española de Neurorrehabilitación basada en la revisión y análisis de la bibliografía disponible sobre el tema. Estableciéndose recomendaciones según el nivel de evidencia que ofrecen los estudios revisados.
DesarrolloLa metodología seguida por la Sociedad Española de Neurorrehabilitación para la elaboración de la presente guía se basa en el análisis de las guías de prácticas clínicas nacionales e internacionales de los últimos siete años, llevado a cabo en función de las consideraciones de manejo que dicta la evidencia para las clases de dolor habituales en la población de personas que han sufrido un daño cerebral sobrevenido.
ConclusionesEl dolor es un síntoma habitual en personas que han sufrido daño cerebral teniendo un impacto negativo en la calidad de vida y en la adherencia al proceso rehabilitador. A la hora de clasificar el tipo de dolor según las características etiológicas, para optimizar el tipo de abordaje terapéutico, se suele hablar de dolor nociceptivo y dolor neuropático, aunque con frecuencia el dolor en estos pacientes presenta características “mixtas”. El tipo de dolor más habitual es el nociceptivo frente al neuropático. Los cuadros sindrómicos dolorosos que aparecen con más frecuencia en esta población son la cefalea, el hombro doloroso del hemipléjico y el dolor central postictus. Al ser el dolor una experiencia subjetiva, las personas con afectación del nivel de consciencia, alteraciones cognitivas severas y/o problemas severos de lenguaje pueden tener muchas dificultades o ser incapaces de comunicarlo. Es importante, por tanto, una adecuada anamnesis y exploración física dirigida, así como el uso de escalas específicas para su correcto diagnóstico. Finalmente, muchos de los fármacos utilizados para su manejo tienen un efecto negativo en la rehabilitación, afectando a procesos cognitivos, y/o empeorando otros síntomas neurológicos. Este hecho, junto a la circunstancia de que muchas veces se trate de personas con otras patologías y polimedicadas, hace que el abordaje del dolor deba ser especialmente meticuloso y desde un enfoque multidisciplinar.
In 2020, the International Association for the Study of Pain (IASP) revised its definition of pain, previously established in 1979. The new wording is as follows: “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage.”1 The same IASP study group emphasise various points of interest in their latest publication, such as the differences between the concepts of pain and nociception. They also stress the fact that pain is always a personal experience, influenced to a greater or lesser extent by biological, social, and psychological factors; therefore, all manifestations of pain must be respected. Furthermore, the authors note that, though pain serves an adaptive function, it may have adverse effects on an individual’s functioning and social and psychological well-being. Another recent historical consideration is that the latest revision of the International Classification of Diseases (ICD-11), published by the World Health Organization (WHO) in May 2019, establishes different types of chronic pain, according to the intensity, organic alterations, and cognitive and functional impact of pain on the patient. These revisions of concepts in the field of pain give us a sense of the importance of managing pain as a complex symptom in diverse areas of medicine, including neurorehabilitation.
Given the prevalence and the impact of pain in patients with acquired brain injury (ABI), this study is intended to guide clinical practice in the management of pain and its different manifestations in patients with ABI undergoing neurorehabilitation. The recommendations made are based on the latest and best available evidence to date on pain in adult patients (> 16 years) with stroke (whether ischaemic or haemorrhagic) or moderate-severe traumatic brain injury (TBI). These guidelines, developed on behalf of the Spanish Society of Neurorehabilitation (SENR), aim to improve the quality of care provided to these patients. To that end, the authors extracted and reviewed data and levels of evidence on the diagnosis and treatment of different types of pain occurring in the neurorehabilitation of ABI from clinical practice guidelines and consensus statements published by different national and international organisations between 2013 and 2020 (Fig. 1).
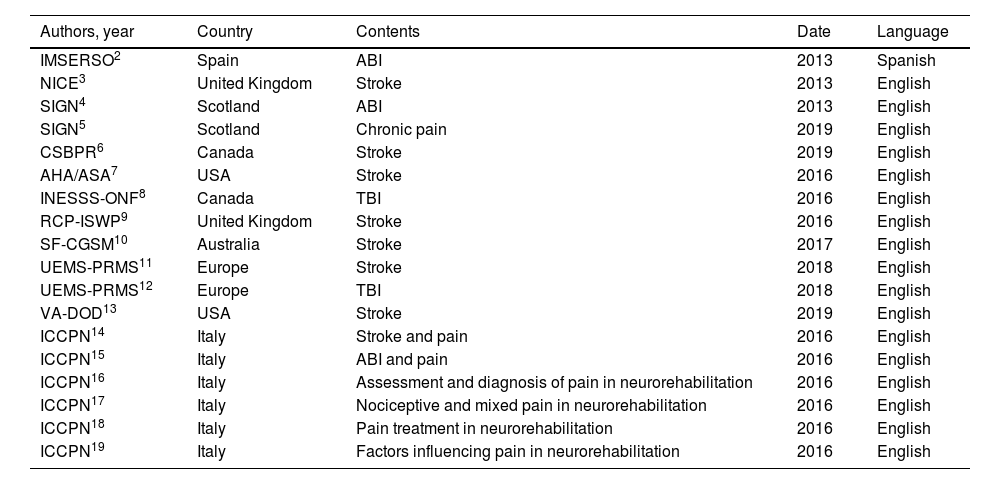
A total of 18 clinical practice guidelines were finally included in the systematic review.2–19Table 1 summarises the 18 intervention programmes meeting the inclusion criteria for the review and highlights the most relevant aspects of each. Fig. 2 shows the grades of recommendation and level of evidence for each recommendation.
Guidelines included in the study.
| Authors, year | Country | Contents | Date | Language |
|---|---|---|---|---|
| IMSERSO2 | Spain | ABI | 2013 | Spanish |
| NICE3 | United Kingdom | Stroke | 2013 | English |
| SIGN4 | Scotland | ABI | 2013 | English |
| SIGN5 | Scotland | Chronic pain | 2019 | English |
| CSBPR6 | Canada | Stroke | 2019 | English |
| AHA/ASA7 | USA | Stroke | 2016 | English |
| INESSS-ONF8 | Canada | TBI | 2016 | English |
| RCP-ISWP9 | United Kingdom | Stroke | 2016 | English |
| SF-CGSM10 | Australia | Stroke | 2017 | English |
| UEMS-PRMS11 | Europe | Stroke | 2018 | English |
| UEMS-PRMS12 | Europe | TBI | 2018 | English |
| VA-DOD13 | USA | Stroke | 2019 | English |
| ICCPN14 | Italy | Stroke and pain | 2016 | English |
| ICCPN15 | Italy | ABI and pain | 2016 | English |
| ICCPN16 | Italy | Assessment and diagnosis of pain in neurorehabilitation | 2016 | English |
| ICCPN17 | Italy | Nociceptive and mixed pain in neurorehabilitation | 2016 | English |
| ICCPN18 | Italy | Pain treatment in neurorehabilitation | 2016 | English |
| ICCPN19 | Italy | Factors influencing pain in neurorehabilitation | 2016 | English |
Authors: author, society, or authority that published the guidelines (reference). Country: country or region where the guidelines were published.
Contents: patient population addressed in the recommendations.
Date: year of publication of the latest update of the document.
ABI: acquired brain injury; AHA/ASA: American Heart Association/American Stroke Association; CSBPR: Canadian Stroke Best Practice Recommendations: Stroke Rehabilitation Practice Guidelines; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; IMSERSO: Instituto de Migraciones y Servicios Sociales-Fundación Reintegra; INESS-ONF: Institut National d’Excellence en Santé et en Services Sociaux – Ontario Neurotrauma Foundation; NICE: National Institute for Health and Care Excellence; RCP-ISWP: Royal College of Physicians Intercollegiate Stroke Working Party; SF-CGSM: Stroke Foundation – Clinical Guidelines for Stroke Management; SIGN: Scottish Intercollegiate Guidelines Network; TBI: traumatic brain injury; UEMS-PRMS: European Union of Medical Specialists-Physical and Rehabilitation Medicine Section; VA-DOD: Clinical Practice Guidelines for the Management of Stroke Rehabilitation. Department of Veterans Affairs-Department of Defense.
The brain controls all vital functions, from the most basic (breathing, blood pressure, heart rate, etc.) to the most sophisticated (artistic creation, abstraction, and emotions, among others). Due to this great functional involvement, ABI affecting different brain structures can give rise to a broad range of alterations. Currently, the most prevalent type of ABI is stroke, followed by TBI; less frequent forms include sequelae following brain tumour surgery, as well as encephalitis and encephalopathy of various aetiologies. Pain is a subjective experience, and patients with TBI affecting level of consciousness or with severe cognitive alterations and/or communication problems may have great difficulty communicating painful sensations or experiences.20,21 According to the IASP, inability to communicate verbally does not imply the inability to feel pain, and appropriate assessment and treatment of pain is needed in these cases. Interpretation of indirect signs entails a risk of under- or overestimating the presence of pain, and it is unethical to apply potentially nociceptive stimuli to individuals who are unable to consent to this. Therefore, instruments for assessing pain in patients who are unable to communicate verbally are based on behavioural signs, such as the localisation of pain, restlessness, agitation, specific movements, and facial expressions and emotional reactions (grimacing, moaning, sobbing, etc.). The Nociception Coma Scale, Nociception Coma Scale-Revised, and Pain Assessment in Advanced Dementia scales are routinely used to assess pain in patients with impaired consciousness or advanced dementia, respectively.15 Pain is a frequent problem in patients with stroke (19%–74% of patients). It tends to be more frequent in the subacute (42.7%) and chronic (31.9%) stages than in the acute stage (14.1%),14 can negatively impact patient quality of life,22 and can present in a range of forms, including neuropathic pain, musculoskeletal pain (including spasticity), and mixed pain. Pain may also be secondary to pre-existing problems not directly related to stroke.17 Several variables have been identified as independent risk factors for pain following stroke; these include female sex, spasticity, depression, presence and severity of upper limb paresis, ischaemic lesions, and certain pre-existing conditions (e.g., alcohol use, statin use, peripheral vascular disease). The role of age is controversial, with some evidence suggesting younger age may be a risk factor and other studies reporting greater risk among older patients.16 In TBI, during the acute phase and early stages of rehabilitation, nociceptive pain often appears as the result of associated lesions in the context of polytrauma (e.g., bone fractures, thoracic-abdominal injuries, skin lesions) and/or surgical wounds or invasive therapeutic interventions (tracheostomy, central venous catheters, nasogastric tubes, gastrostomy, urinary catheters, etc.). In the subacute and chronic stages, the characteristics of pain are often mixed, with both nociceptive and neuropathic features, and the causes are varied, including diffuse spasticity, joint restriction, pressure ulcers, peripheral nerve damage, paraosteoarthropathy, urinary tract infections, respiratory infections, constipation, post-traumatic headache, critical illness polyneuropathy, direct brain lesions, central neuropathic pain (CNP), and/or thalamic pain syndromes.15 Several cases have been reported of pain after TBI showing no association with any pre-existing factor, in which the lesion mechanism of TBI is considered to have activated a series of circuits involved in the pain mechanism.23 Another symptom secondary to ABI that is frequently associated with pain is spasticity. The pathophysiology of spasticity is similar in stroke, TBI, and other aetiologies of ABI, with damage to the upper motor neuron and its descending motor pathways, which regulate spinal reflex connections to the peripheral motor neuron. Spasticity affects limb and trunk biomechanics, resulting in abnormal postures and a progressive loss of range of motion. Painful spasms and dystonic postures may also appear in some of these patients.14 Therefore, spasticity may be painful in and of itself, in addition to causing conditions that are painful. On the other hand, pain often acts as a noxious stimulus, exacerbating the existing spasticity. The management of pain associated with spasticity requires a multidisciplinary approach, which may be focused on treating spasticity and/or pain.14,16 Thus, pain is a frequent symptom in the neurorehabilitation process, and has a negative impact on adherence to rehabilitation and on the results achieved. In general terms, pain negatively affects quality of life after brain injury, as well as rehabilitation treatment, in up to 25% of cases.16 Many of the drugs used to control pain negatively affect the recovery process, affecting cognitive processes and/or exacerbating other neurological symptoms. Therefore, pain management during neurorehabilitation constitutes a challenge in which we must achieve an appropriate use of effective pharmacological and non-pharmacological strategies.16,18 To that end, it is fundamental to correctly diagnose the different manifestations of pain in these diseases, and to recognise their presentation in order to address and/or prevent their appearance in each stage of rehabilitation after ABI. Although there is controversy about whether a single harmful mechanism can trigger pathophysiological mechanisms associated with both nociceptive and neuropathic pain (“mixed pain”), objective separation of these types of pain in clinical guidelines is helpful in optimising treatment strategies and minimising undesired effects.16Table 2 shows the recommendations on pain in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
The diagnostic tools (clinical scales) used should be appropriate for the type of lesion and level of consciousness.
- 2
Detailed clinical history is essential to gathering information on premorbid conditions; the presence, characteristics, and treatment of pain prior to brain injury are particularly relevant.
- 3
Correct neurological examination should be performed.
- 4
Pain management in patients with ABI should follow a specialised, interdisciplinary approach.
- 5
Different treatment tools for pain should be indicated and prescribed according to the level of evidence and type of pain.
- 1
Recommendations on general and nociceptive pain in acquired brain injury.
| Recommendation | Grade of recommendation |
|---|---|
| Pain intensity and treatment response should be evaluated with the VAS, NPRS, and Neuropathic Pain Symptom Inventory. | High A (ICCPN) |
| Botulinum toxin infiltration is a first-line treatment for painful spasticity, either in isolation or combined with rehabilitation therapy. | High A (ICCPN) |
| Routine use of hand and wrist orthoses (splints) is not effective in improving function, pain, or range of motion. | High A (SF-CGSM) |
| Mindfulness interventions, and particularly the use of mindfulness techniques in cognitive therapy or stress reduction strategies, are recommended for chronic pain syndromes with heterogeneous pathophysiology. | High A (ICCPN) |
| Cognitive-behavioural therapy, whether administered individually, in a group, or by computer, is also recommended. | Moderate B (ICCPN) |
| The available evidence does not support the use of transcutaneous electrical nerve stimulation to treat pain in patients with neurological alterations, but it may be considered to complement pharmacological therapy and exercise. | High A (ICCPN) |
| Other physiotherapy and exercise techniques may also be considered in patients undergoing neurorehabilitation, although the available evidence is preliminary. | Low C (ICCPN) |
| Low D (ICCPN) | |
| Potential trigger factors (pain, infection, constipation) should be eliminated in all patients with ABI who develop spasticity and/or tendon shortening. | Moderate II (UEMS-PRMS) |
| Specialist physicians should closely and regularly monitor patients with stroke to identify such complications and secondary conditions as malnutrition, deep vein thrombosis, pressure ulcers, bladder/bowel dysfunction, infections (especially urinary and respiratory tract infections), depression, HSP, central pain, CRPS, spasticity, contractures, fatigues, falls, osteoporosis, and seizures, which may affect or worsen the initial level of disability. | Low IV (UEMS-PRMS) |
| In all patients with TBI who are conscious, the following common alterations should be specifically assessed: | Low C (INESS-ONF) |
| Motor alterations including weakness, altered muscle tone, impaired balance, and loss of coordination; | |
| Wounds and fractures not previously diagnosed; | |
| Pain; | |
| Bulbar alterations affecting speech or swallowing; | |
| Sphincter control problems (bladder/bowel); | |
| Cognitive alterations: attention, orientation, and/or memory; | |
| Behavioural dysregulation, including potential behavioural/emotional problems. | |
| The presence of pain should always be considered in patients with TBI who present agitation or cognitive/communication problems, non-verbal psychomotor restlessness, or worsening of spasticity, paying particular attention to non-verbal signs of pain (e.g., grimacing). | Low C (INESS-ONF) |
| Pain should be assessed on a VAS (scored 0 to 10). | Low C (VA-DOD) |
| A pain management plan should be established that includes assessment of: probable aetiology (musculoskeletal or neuropathic) and pain location, quality, quantity, duration, intensity, and aggravating and relieving factors. | Low C (VA-DOD) |
| The impact of pain on quality of life and rehabilitation outcomes should be assessed in patients with stroke and other disorders associated with spasticity. | Low C (ICCPN) |
| Musculoskeletal pain syndromes may respond to correction of the underlying condition, through reducing spasticity or preventing or correcting shoulder subluxation. | Low Insufficient evidence (VA-DOD) |
| Rehabilitation programmes for patients with TBI should include protocols for the assessment and management of pain, which include: | Low C (INESS-ONF) |
| Mechanisms for periodic review and adjustment | |
| Management, support, and pain relief methods adjusted to the needs of each individual | |
| Training of healthcare professionals and caregivers in the appropriate handling of the paretic upper limb during transfers, and on hypersensitivity and neuropathic pain. | |
| Clinical specialists should adjust the target treatment to the type of pain. | Low C (VA-DOD) |
| NSAIDs may be helpful in treating musculoskeletal pain. | Low Insufficient evidence (VA-DOD) |
| Opioids or any other medication that may affect cognition should be used with caution. | Low Insufficient evidence (VA-DOD) |
| Centrally-acting analgesic drugs should be administered at low doses, as they may cause confusion, impairment of cognitive execution, and interference in rehabilitation processes. | Low C (VA-DOD) |
| The balance between benefits and potential adverse effects should be considered for analgesic medications that may affect patients’ ability to participate and benefit from rehabilitation. | Low Insufficient evidence (VA-DOD) |
| When possible, support from a clinical psychologist should be used to address psychological aspects of pain and improve adherence to the pain treatment plan. | Low C (VA-DOD) |
| When appropriate, non-pharmacological analgesic treatments should be used, such as biofeedback, massage, imaging therapy, and physiotherapy. | Low C (VA-DOD) |
| The NCS-R and Pain Assessment in Advanced Dementia Scale are currently the gold standard scales for pain assessment in patients with impaired consciousness or dementia, respectively. | Low C (ICCPN) |
| Analgesic treatment should be administered to patients in a minimally conscious or vegetative state. | Low D (ICCPN) |
| Sex should be taken into account in neurorehabilitation, as it plays a role in: | Low D (ICCPN) |
| Epidemiology | Low C (ICCPN) |
| Outcomes | |
| Pain management | |
| The treatment of nociceptive pain in movement disorders, amyotrophic lateral sclerosis, severe ABI, disorders of consciousness, dementia, oncological processes, and neurological infections should be based on the WHO “analgesic ladder.” | High A (ICCPN) |
| Non-opioid analgesics, NSAIDs, COX-2 inhibitors, and opioids may be used to treat nociceptive pain in neurorehabilitation. | High A (ICCPN) |
ABI: acquired brain injury; CRPS: complex regional pain syndrome; HSP: hemiplegic shoulder pain; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; INESS-ONF: Institut National d’Excellence en Santé et en Services Sociaux – Ontario Neurotrauma Foundation; NCS-R: Nociceptive Coma Scale Revised; NPRS: Numerical Pain Rating Scale; NSAID: non-steroidal anti-inflammatory drug; SF-CGSM: Stroke Foundation – Clinical Guidelines for Stroke Management; TBI: traumatic brain injury; UEMS-PRMS: European Union of Medical Specialists-Physical and Rehabilitation Medicine Section; VA-DOD: Clinical Practice Guidelines for the Management of Stroke Rehabilitation. Department of Veterans Affairs-Department of Defense; VAS: visual analogue scale; WHO: World Health Organization.
Nociceptive pain is caused by painful stimulation of nociceptors in non-neuronal tissues, with preserved somatosensory activation mechanisms. Depending on the place of origin, pain is classified as somatic or visceral. Somatic pain is caused by activation of nociceptors located in the skin and mucous membranes (superficial somatic pain), or in the bones, ligaments, tendons, muscles, fasciae, and blood vessels (deep somatic pain). Visceral pain results from the activation of receptors at the level of the visceral internal organs, and is related with the autonomic or vegetative system. There is no more specific definition of this type of pain that is applicable to neurorehabilitation.16 Nociceptive pain is more frequent than neuropathic pain in patients with stroke.22 Shoulder pain is the most frequent type of musculoskeletal pain after stroke.17 Nociceptive pain after stroke may be caused by prolonged immobilisation and abnormal postures, which exacerbate pre-existing painful musculoskeletal conditions, such as osteoarthritis. Table 2 shows the recommendations related to the management of nociceptive pain in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
HeadacheConceptHeadache and other craniofacial pain syndromes, whether primary or secondary, are extremely frequent in the general population, both in healthy individuals and in the context of various diseases, including ABI. In the context of ABI, headache is typically secondary (caused by the head injury), although we must consider the possibility that patients may have history of primary headache. The most recent edition of the International Classification of Headache Disorders (ICHD-3) classifies headache as primary; secondary; and neuropathies, facial pains, and other headaches.17,24 Headache is a target symptom in haemorrhagic stroke (34%–65%) and in other associated vascular disorders, such as venous sinus thrombosis (80%–90%), cervical artery dissection (55%–100%), reversible cerebral vasoconstriction syndrome (95%–100%), and vasculitis; however, it is also observed in 6%–44% of cases of ischaemic stroke.25,26 New-onset headache at the time of ischaemic stroke has been described as an indicator of persistent headache up to 6 mo after stroke,25 in 23% of cases.26 In stroke, headache may appear in the subacute to chronic stages of brain injury, as a result of damage to higher centres responsible for pain neuromodulation. Headache can also be secondary to medication use (e.g., overuse of analgesic medication, or as an adverse effect of some antipsychotic drugs); this is most frequent in the acute stage (5.3%), and becomes less prevalent in the subacute (1.8%) and chronic stages.14 In the case of TBI, post-traumatic headache is the most common form of secondary headache, and is more frequent in mild TBI (90%) (and even post-concussion syndrome) than in moderate or severe TBI (71%).27–32 It typically presents in the acute phase, up to 7 days after injury. However, pain persists beyond 3 mo in 33%–58% of patients (and for years in 15%); this chronic pain has a significant impact on patient quality of life.24,28,29,31,33 Approximately 90% of patients with brain tumours require craniotomy for resection of the tumour, with a view to improving survival. In addition to pain during the procedure, patients often present post-craniotomy headache (50%–96%). This pain is attributed to muscle dissection, inflammation of bone remnants, dural retraction, cerebrospinal fluid (CSF) leakage, and direct lesions to the nerves of the scalp.34 Treatment of this symptom is often inadequate, frequently due to concern that analgesics may conceal neurological changes, particularly in the context of neurocritical care units. In many cases, this headache can become chronic (56%).34,35 Like the craniotomy procedures performed in some patients with TBI, surgical treatment and secondary pain are associated with longer hospital stays and poorer cognitive and functional outcomes.35 The correct management of headache secondary to ABI requires consideration of various aspects related to its pathophysiology, prevention, assessment, diagnosis, and treatment.
Aetiology and pathophysiologyThe most common types of headache and craniofacial pain in patients undergoing neurorehabilitation after ABI are tension-type headache and migraine; depending on the type of injury, less frequent types of headache include craniocervical pain, facial and orofacial neuralgia (e.g., trigeminal neuralgia), temporomandibular disorder, and burning mouth syndrome, among others.17,24 In ischaemic stroke, tension-type headache is the most frequent (50%–80%), with migraine being less common (around 30%, depending on the study).25,26 In haemorrhagic stroke, headache tends to precede other focal neurological signs; it typically presents as thunderclap headache, with moderate to severe intensity.26 In TBI, the most frequent type of headache is migraine (23%–49%), presenting specific characteristics; it usually responds poorly to anti-migraine treatment, suggesting that its pathophysiology does not involve the same structures and mechanisms as primary migraine.27–29,33 Less frequent types of headache after TBI include occipital neuralgia (with major occipital neuralgia being common), cervicogenic headache, trigeminal neuralgia (particularly involving the supra- and infraorbital nerves), local dysaesthesia due to lacerations to extracranial tissue, and non-specific pain related to intracranial bleeding.29,30,32 The pathophysiology of post-stroke headache is not fully understood. There is thought to be a relationship with the vascular pathogenesis of primary migraine (despite differences in the form of expression), with mechanical or chemical activation of trigeminovascular afferent fibres, meningeal inflammation, physical mechanisms, neuroelectrical events with abnormal depolarisation, the effect of proinflammatory derivatives of the coagulation process, and/or serotonergic mechanisms, among others.25,26
In the case of post-traumatic headache, pathophysiology in the acute stage is probably directly related to different types of somatic and visceral nociceptive damage due to tissue lesions caused by the trauma. These lesions include damage to both extracranial and intracranial protective structures (dermal and subcutaneous tissues, muscle, bone, meninges), associated cervical lesions, and lesions secondary to neurosurgical interventions (e.g., epidural haematoma drainage in the acute stage, or treatment of secondary hydrocephalus).24,27,30 It is also important to consider the pathophysiology of other associated lesions that may provoke pain, such as cervical artery dissection, venous thrombosis, intracranial hypotension due to CSF leakage through fistulae following cranial fracture, or oedema due to inflammation, resulting in intracranial hypertension.28 Patients undergoing surgical drain placement or brain tumour surgery may experience pain due to the manipulation of tissues during the procedure. In the chronic stage, pain pathophysiology involves direct and indirect changes due to damage to peripheral and central neurons of the ascending sensory pathways, as well as changes in various descending pain control centres and modulatory pathways; these structures are also affected by neuroinflammatory, neurodegenerative, hormonal, and epigenetic mechanisms. Many of these centres may, in turn, be affected by comorbidities after ABI, including depression, fatigue, and sleep disorders, which are therefore thought to share a common pathophysiological mechanism.25–27,30,32,33
PreventionAs noted above, physicians treating patients with ABI must be aware of the potential presence of primary headache associated with presentation of a new secondary headache following the injury. In patients with stroke, posterior circulation involvement, history of some type of primary headache (20%), and female sex present a greater risk of headache. Some studies report greater incidence of headache after stroke in patients younger than 50 years, and there seems to be no association with stroke severity.25,26,36 A systematic review observed greater prevalence in European and North American populations than in Asian and Middle-Eastern populations.25 Risk factors for headache after haemorrhagic stroke include young age, female sex, haematoma volume, and cerebellar or lobular involvement.26 Factors associated with persistent headache, even years after stroke, include antidepressant use, absence of atrial fibrillation, and right hemisphere involvement.26 Regarding migraine-type pain, whether primary or secondary, it is important to take into account intrinsic factors, such as hormonal factors among women of childbearing age24 or damage to the hypothalamic-pituitary axis secondary to ABI. Localisation of the brain lesion is also a relevant factor in ABI, given the potential for involvement of centres participating in pain pathophysiology. For instance, in patients with stroke, infarctions involving the insular region, somatosensory cortex, and parietal and medial temporal regions are associated with the development of headache. This type of headache also appears to be more frequent in patients with cortical involvement than in those with subcortical or deep lesions.25 In ischaemic stroke, the aetiology of the cerebrovascular event also influences headache prevalence. Strokes due to cardioembolism (9%–39%) and large-artery disease (15%–41%) are more frequently associated with headache than those caused by small-vessel disease (13%–33%) or lacunar stroke or transient ischaemic attack (16%–36%).25,26 In headache after TBI, onset between 3 h and 7 days after injury, migraine-type headache, and female sex are associated with slower recovery. Certain genetic conditions, history of headache, and younger age are also risk factors for the development of headache after TBI, even in mild cases.30,32,33 Similarly, in a study into TBI in athletes, presentation of other associated post-concussion symptoms (e.g., dizziness, vertigo, anxiety, early amnesia regarding the injury, impaired level of consciousness), younger age, and female sex were associated with development and persistence of headache, and with slower recovery.27,29,30,32 In post-craniotomy headache, it is unclear whether pain presentation or intensity are influenced by sex or age, with studies reporting contradictory results. However, frontal craniotomy, as well as preoperative nerve blocks, do seem to be associated with less pain. In general, longer duration of the surgical procedure is associated with greater headache prevalence and intensity.34,35 Furthermore, in the immediate postoperative period, it is important to take into account the management of associated symptoms, such as dizziness, fatigue, nausea and vomiting, respiratory depression, constipation, and changes in blood pressure, which may affect the risk of increased intracranial bleeding, pain, and agitation.35 Similarly, it is essential to identify other triggers of pain, such as mood/emotional disorders (depression, anxiety, post-traumatic stress disorder), fatigue, and sleep disorders, which tend to appear as comorbidities during neurorehabilitation.17,24,26,27,30,32,35,37 Finally, many patients with ABI present loss of functional control of movement, with a tendency to poor postural control and the appearance of new musculoskeletal pains that may contribute to the development of secondary headache.24 Comorbidities present prior to ABI (alcohol abuse, smoking, diabetes, peripheral artery disease, etc.) also influence the presence of pain; furthermore, multimorbidity requires large amounts of medication, with an increased risk of adverse events, which may promote the appearance of headache.26,36 However, we must always seek to administer multimodal anaesthesia, taking into account synergism in acute treatment, in order to avoid potential adverse effects associated with dose reduction.38,39
- -
SENR recommendations
- 1
In stroke and TBI, young age, female sex, and primary headache are non-modifiable risk factors for headache after ABI.
- 2
In stroke, predisposing factors must also be considered: posterior circulation involvement, large-vessel disease in ischaemic stroke, and haematoma volume in haemorrhagic stroke.
- 3
In TBI, greater numbers of post-concussion symptoms are associated with the risk of headache after injury.
- 4
In post-craniotomy headache, longer duration of the surgical procedure, due to its greater complexity, is associated with a greater risk of headache.
- 5
In all types of post-ABI headache, mood and sleep disorders, fatigue, and postural and musculoskeletal alterations, whether immediate or subacute as a consequence of motor sequelae, are modifiable risk factors that must be considered.
- 1
The diagnostic criteria of the International Headache Society (IHS) are recommended as the gold standard for the diagnosis of patients with headache.17,26,28,31 On many occasions, experience and skill are required for headache diagnosis and for the identification of secondary symptoms of brain damage that may prevent appropriate collection of information from the patient; such symptoms include reduced level of consciousness, aphasia or severe dysarthria, hemispatial neglect, or pre-existing neurodegenerative disorders, as well as other symptoms such as fatigue and mood disorders, which may conceal the presence of headache.36 It is important to establish the time of headache onset with respect to the brain injury. For instance, several studies define headache associated with ischaemic stroke as headache presenting simultaneously with or within 24–72 h before or after the cerebrovascular event, whereas others establish a period of up to 7 days.25,28 The ICHD-3 criteria reflect the temporal relationship with other symptoms of stroke, and speak of sentinel headache in cases occurring 72 h before stroke symptoms, acute stroke-attributed headache, whose symptoms resolve in the first 3 mo after injury, and persistent post-stroke headache in cases that do not resolve within 3 mo after ischaemic stroke or other cranial or cervical vascular disorders, with the exception of cerebral venous thrombosis.26 In the case of post-TBI headache, it is important to establish whether headache is de novo or an exacerbation of a pre-existing headache, appearing within 7 days after brain injury. Similarly, the ICHD-3 criteria classify headache after TBI as acute (< 3 mo) or persistent (> 3 mo), and by severity of the TBI (mild or moderate/severe). With respect to the level of consciousness and withdrawal of medications (which may be concealing pain), post-TBI headache is defined as that appearing within 7 days after brain injury.27,28 Thorough examination and clinical history are necessary to identify the type of headache or facial pain. In patients with TBI, proper examination of the spinal column and occipital area and the associated muscles is recommended.27 Diagnostic tests including imaging studies should be considered in order to rule out potential secondary complications that may explain the headache, such as CSF leakage through cranial fissures or fractures.27,30 After the type of pain is established, its severity (both at onset and after initiation of treatment) can be periodically assessed with the visual analogue scale (VAS) and the Numerical Pain Rating Scale (NPRS) by the neurorehabilitation team.26,35 In tension-type headache after stroke, pain intensity is characterised as mild to moderate.26 In headache with migraine-like characteristics, the Migraine Disability Assessment (MIDAS) questionnaire may be suitable for identifying related characteristics; however, as in other types of pain, it is advisable to use other tools to measure factors associated with mood disorders, sleep quality, etc.17 It is equally important to record the duration of symptoms (acute or chronic) and their intensity over time.28 Headache diaries are helpful for the periodic collection of data on headache frequency and duration, premonitory or accompanying symptoms, and the long-term efficacy of the treatment indicated.17,24 For instance, persistent post-stroke headache is considered to present greater daily frequency and pain intensity than headache attributed to acute stroke, which is typically of mild-to-moderate intensity and low frequency, decreasing over time.26 All types of secondary pain should be reviewed periodically, even during long-term follow-up after discharge from the neurorehabilitation unit.36 Analgesic medication overuse (defined as ≥ 15 days per month for non-steroidal anti-inflammatory drugs [NSAID], > 2 times per week for triptans, and ≥ 8 days per month for opioids) is a common situation that constitutes another predisposing factor for paradoxical presentation of headache (19%–42%).26,27,29,30Table 3 shows the single recommendation related to the assessment and diagnosis of ABI identified in the main guidelines reviewed and the corresponding level of evidence.
- -
SENR recommendations
- 1
Secondary pain should be classified according to the IHS guidelines.
- 2
Physicians should have experience with the diagnostic tools, physical examination, and complementary tests needed to identify the type and time of presentation of headache, differentiating between acute and persistent headache, for instance.
- 3
Such pain rating tools as the VAS and NPRS should be considered; follow-up tools including headache diaries are helpful for ensuring proper management of this symptom and avoiding analgesic medication overuse.
- 1
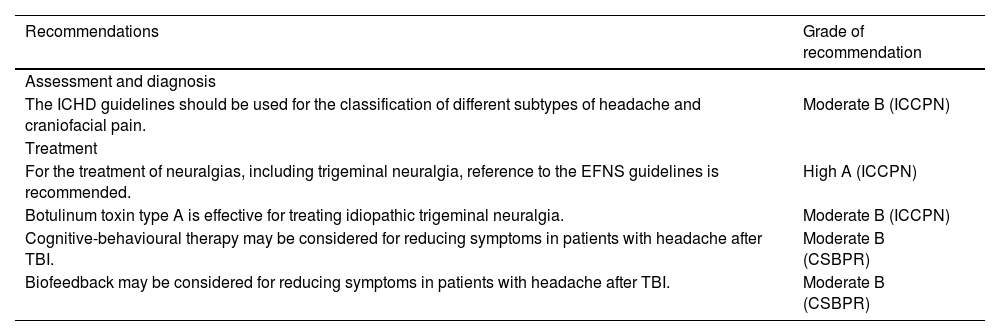
Recommendations on headache secondary to acquired brain injury.
| Recommendations | Grade of recommendation |
|---|---|
| Assessment and diagnosis | |
| The ICHD guidelines should be used for the classification of different subtypes of headache and craniofacial pain. | Moderate B (ICCPN) |
| Treatment | |
| For the treatment of neuralgias, including trigeminal neuralgia, reference to the EFNS guidelines is recommended. | High A (ICCPN) |
| Botulinum toxin type A is effective for treating idiopathic trigeminal neuralgia. | Moderate B (ICCPN) |
| Cognitive-behavioural therapy may be considered for reducing symptoms in patients with headache after TBI. | Moderate B (CSBPR) |
| Biofeedback may be considered for reducing symptoms in patients with headache after TBI. | Moderate B (CSBPR) |
CSBPR: Canadian Stroke Best Practice Recommendations: Stroke Rehabilitation Practice Guidelines; EFNS: European Federation of Neurological Societies; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; ICHD: International Classification of Headache Disorders; TBI: traumatic brain injury.
According to a series of studies, patients with headache following stroke, the most frequent type of ABI, often require more analgesia than patients with other types of post-stroke pain (32% vs. 17%).26 This underscores the importance of treating this type of pain. Similarly, early management of headache reduces the likelihood of future persistence of the headache.32,35 To date, no specific clinical guidelines have addressed the pharmacological treatment of headache after ABI.25,26,35 Firstly, in the hyperacute stage after ABI, to prevent the onset of headache following craniotomy (in patients undergoing the procedure), it is important to consider such approaches as preoperative nerve block; administration of intravenous NSAIDs, paracetamol, or metamizole when closing the surgical wound; or infusion of dexmedetomidine during or immediately after the procedure. Physicians must be experienced in the identification and management of headache in patients under sedation or with impaired level of consciousness during their stay at the neurocritical care unit, without administering excessive amounts of opioids, and monitoring for secondary effects of other drugs.32,34 For tension-type headache, migraine, and other types of headache appearing after ABI, pharmacological treatment should be based on the guidelines of the European Federation of Neurological Societies (EFNS) and the Spanish Society of Neurology’s 2020 clinical practice guidelines for headache.30,33,40 Generally, acute-onset headache can be controlled with simple or first-line analgesics. Management of persistent or chronic headache is more complex, and must be established on an individual basis, accounting for the possible comorbidities and diseases present in each patient.26,31 It is helpful to be aware of the profiles of tension-type headache and migraine, in order to indicate the most appropriate symptomatic treatments, taking TBI into account. For instance, in patients presenting migraine after stroke, triptans and dihydroergotamine cannot be used due to the cardiovascular risk associated with these drugs; in the case of headache after TBI, medications indicated for anxiety or insomnia, as well as muscle relaxants, may affect the recovery of cognitive symptoms.26,27,32 Regarding local pharmacological treatment, the use of botulinum toxin A, as in primary headaches, has been shown to be useful in the treatment of chronic daily headache and chronic migraine, but not for cervicogenic headache.24,29 Nerve block with local anaesthetics, either in isolation or combined with corticosteroids, has been shown to be useful for occipital neuralgia and for the prevention of migraine.29 The combination of pharmacological and non-pharmacological interventions is more effective for managing pain after ABI than the use of either treatment in isolation.31,32 Regarding non-pharmacological treatment, there is some evidence supporting the use of osteopathic manipulative treatment and chiropractic therapy.24 Non-pharmacological treatment also includes manual therapy, a physical approach used by healthcare professionals to manage musculoskeletal disorders and pain. This treatment includes massage, dry needling, soft tissue mobilisation, myofascial release therapy, and spinal manipulation, among other techniques. Several published studies have documented the usefulness, alongside pharmacological treatment, of meditation, mindfulness-based stress reduction, cognitive-behavioural therapy, acceptance and commitment therapy, other biofeedback-based psychological therapies, relaxation therapies, hypnosis, eye movement desensitisation and reprocessing therapy, and yoga in the management of pain following stroke or TBI, and specifically to treat primary headache reported by patients during rehabilitation. The best level of evidence was observed for cognitive-behavioural therapy, acceptance and commitment therapy, and biofeedback (grade of recommendation: A).24,26,27,29,31 In patients with persistent or chronic oromandibular pain, it is helpful to rule out other aetiologies, including periodontal alterations, vascular disorders, temporomandibular joint lesions, or neoplasms. Beyond ruling out these aetiologies, no specific guidelines are available on the management of oromandibular pain after ABI. Regarding neuralgia, and particularly trigeminal neuralgia, it is helpful to refer to such guidelines as those published by the EFNS. In general, pharmacological management should include oral antiepileptic treatment with carbamazepine and oxcarbazepine, and botulinum toxin A infiltration, which achieves a positive response in up to 85% of patients. Other topical treatments, such as 5% lidocaine patches or 8% capsaicin patches, play a role in reducing general neuropathic pain, although no clear evidence is available on their use for orofacial or trigeminal neuropathic pain. Invasive treatments include glycerol rhizolysis and percutaneous radiofrequency thermocoagulation, with pulsed or continuous application (mixed application is best). These treatments are considered to be helpful, despite low levels of evidence, in patients with pain refractory to previous treatments. Unlike trigeminal neuralgia, glossopharyngeal neuralgia responds better to interventional treatment than to pharmacological approaches.17Table 3 shows the recommendations related to treatment of headache and other craniofacial pains in ABI, and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
The treatment algorithms presented in the guidelines of the EFNS and in the Spanish Society of Neurology’s 2020 clinical practice guidelines for headache should be followed, taking into account the specific medical history and comorbidities present in patients with ABI.
- 2
Pharmacological treatment should be combined with non-pharmacological interventions (e.g., cognitive-behavioural therapy).
- 3
In patients with persistent or chronic headache, botulinum toxin A and nerve blocks are useful for headache prevention.
- 4
In patients with facial and orofacial neuralgias, invasive procedures should be considered if headache is refractory to systemic or local pharmacological treatment.
- 1
Shoulder pain in the paretic side of the body is common after stroke, and can have significant repercussions on various aspects of functioning and rehabilitation. The term “hemiplegic shoulder pain” (HSP) is often used to refer to the multiple diseases underlying and potentially causing shoulder pain in these patients. Shoulder pain is reported to significantly impact patients’ participation in rehabilitation activities, contribute to poorer functional recovery, and hinder the recovery of movement and function, and is related to a decrease in passive joint range of motion.16,41,42 HSP is also associated with poorer quality of life, higher rates of depression, longer hospital stays, and lower likelihood of living independently after discharge.16,41–43 Various publications on HSP after stroke report incidence rates ranging from 1% to 84%, depending on the study.41 This broad range is probably due to the heterogeneity of studies in terms of the time since stroke, the measurement instruments used, communication difficulties, and/or differences in the clinical manifestations of stroke in each patient.41 It is broadly acknowledged that the incidence and severity of HSP increase over time.14,41,42 Several prospective studies have established the incidence of shoulder pain at approximately 33% in the first months after stroke; in 65% of cases, pain persisted several months later.43 Another study reported that up to 72% of adult patients present at least one episode of shoulder pain in the first year after stroke.16 A study published in 2019 detected HSP at 72 h after stroke in 35% of patients in the sample.42 Clinical risk factors or predictors of shoulder pain include age, tactile and proprioceptive sensory alterations, pain appearing upon passive joint mobilisation at 2 weeks after stroke, reduced passive range of motion in shoulder abduction and external rotation, muscle imbalance in the periscapular region, increased biceps and supraspinatus muscle tone, hemispatial neglect, and left hemiplegia.7,16,22,42,43 Several authors consider the severity of paresis and National Institutes of Health Stroke Scale (NIHSS) score at baseline to be the most relevant predictors of the development of shoulder pain.41–43 The direct association between shoulder subluxation and HSP is controversial: while some authors describe the presence of subluxation as a predictor of HSP, others have found no significant correlation.9,41–43 This is probably partly due to methodological differences between studies.42,43 The pathogenesis of HSP is probably multifactorial,16,41 with both neurological and mechanical factors playing a role.42,43 The underlying aetiology or aetiologies include such diseases as adhesive capsulitis, shoulder subluxation, rotator cuff injury, and shoulder-hand syndrome, among others. Central sensitisation has also been linked to this type of pain.41
PreventionAccording to experts, the prevention of HSP is an important objective that should be prioritised from the first day after stroke as part of rehabilitation treatment.3 Interventions that may be performed to prevent the appearance of HSP include: identifying its presence from the acute stage through targeted examination; strapping techniques; electrical muscle stimulation (although these techniques are mainly used in the prevention and treatment of shoulder subluxation); paying attention to the positioning of the affected upper limb; promoting proper maintenance of shoulder range of motion; informing the patient or their caregivers about how to prevent shoulder pain or trauma if there is a risk of HSP; and targeting the shoulder in training to recover movement or function.3,7,10,42 Several authors have stressed the need to reduce the risk of HSP in patients with functional loss in the arm after stroke, with careful positioning of the arm, supporting its weight, but avoiding the use of slings and pulleys.9 Nonetheless, there is no firm consensus on the methods for the prevention of HSP, possibly due to the large number of possible underlying causes.3 Some authors note that shoulder pain does not interfere in upper limb rehabilitation if it is effectively treated or prevented.22Table 4 shows the recommendations related to the prevention of shoulder pain after ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
Healthcare staff and patients and their families should be instructed about the protection, positioning, and handling of the affected arm.
- 2
Strapping techniques and electrical muscle stimulation are recommended; in patients using wheelchairs, arm support devices such as trays/hemi-trays, arm troughs, and cushions are recommended.
- 3
The use of pulleys should be avoided. Slings are also not recommended (except during the flaccid phase). Passive mobilisation of the arm must not exceed 90° of flexion or abduction without proper fixation of the scapula.
- 1
Recommendations on shoulder pain secondary to acquired brain injury.
| Recommendations | Grade of recommendation |
|---|---|
| Prevention | |
| Stroke | |
| Pulleys should not be used. | High A (CSBPR) |
| Healthcare staff and patients and their families should be appropriately instructed about protecting, positioning, and handling the affected arm. | High A (CSBPR) |
| For instance, the arm should be carefully positioned or supported during transfers, preventing pulling on the arm. | Low C (CSBPR) |
| In patients with flaccid arm (i.e., Chedoke-McMaster Stroke Assessment Impairment Inventory score < 3), electrical muscle stimulation should be considered. | Moderate B (CSBPR) |
| Passive mobilisations of the arm should not exceed 90° flexion or abduction, unless the scapula is upwardly rotated and the humerus is laterally rotated. | Moderate B (CSBPR) |
| Shoulder mobility should be monitored and maintained during rehabilitation. | Moderate B (VA-DOD) |
| Joint protection strategies should be applied in the early or flaccid stage of recovery to prevent or minimise HSP or shoulder injury. | |
| These include: | |
| 1) Positioning and support of the arm during rest; | Moderate B (CSBPR) |
| 2) Protection and support of the arm during functional movement, avoiding pulling on the affected arm; | Low C (CSBPR) |
| 3) Protection and support of the arm while the patient is using a wheelchair, for instance with trays/hemi-trays, arm troughs, or cushions; | Low C (CSBPR) |
| 4) The use of slings is not recommended, except during the flaccid phase, as they may discourage use of the arm, inhibit arm swing, promote contractures, and affect body image. | Low C (CSBPR) |
| It is reasonable to consider the use of support devices and slings in patients with shoulder subluxation. | Low C (AHA/ASA) |
| Joint protection strategies, such as appropriate positioning, support, and proper handling, should be applied during the flaccid and early stages of recovery to prevent or minimise shoulder pain and subluxation. | Low IV (UEMS-PRMS) |
| Assessment and diagnosis | |
| Stroke | |
| Ultrasound should be considered for diagnosing soft tissue lesions. | Moderate B (AHA/ASA) |
| It may be helpful for clinical assessment to include musculoskeletal evaluation, degree of spasticity, regional sensory changes, and subluxation. | Low C (AHA/ASA) |
| Diagnosis of HSP may include assessment of: tone, active movement, changes in length of soft tissues, alignment of shoulder girdle joints, trunk posture, pain level, orthopaedic changes in the shoulder, and the impact of pain on physical and emotional well-being. | Low C (CSBPR) |
| Patients with severe paresis, diabetes, and left hemiplegia should be asked about shoulder pain. | Low C (ICCPN) |
| Treatment | |
| Stroke | |
| Neuromuscular electrical stimulation (with either surface of intramuscular electrodes) may be considered in the treatment of shoulder pain. | High A (AHA/ASA) |
| Intramuscular electrical stimulation should be considered in patients with shoulder pain after stroke. | High A (IMSERSO) |
| Botulinum toxin injections may be helpful in reducing severe hypertonia in hemiplegic shoulder muscles. | High A (AHA/ASA) |
| Combination treatment with aromatherapy and acupressure is recommended to treat pain in HSP. | High A (IMSERSO) |
| Taping of the hemiplegic shoulder has been shown to reduce pain. | High A (CSBPR) |
| In patients with painful spasticity in the shoulder, botulinum toxin injection to the pectoralis major muscle is recommended. | High A (IMSERSO) |
| Passive, continuous mobilisation of the shoulder is recommended to treat HSP. | High A (IMSERSO) |
| The use of neuromodulatory analgesics is a reasonable approach in the management of HSP in patients with clinical signs of neuropathic pain, manifesting as sensory changes in the shoulder area, allodynia, or hyperpathia. | High A (AHA/ASA) |
| For the treatment of HSP: | |
| Intra-articular corticosteroid infiltration is indicated, although its effect is short-lasting. | High A (ICCPN) |
| Botulinum toxin is indicated. | Moderate B (ICCPN) |
| Acupuncture may be helpful. | Moderate B (ICCPN) |
| Such oral agents as tizanidine and baclofen should be considered to treat spasticity, especially if it is associated with pain, poor skin hygiene, or decreased function. Tizanidine should be specifically used in patients with chronic pain after stroke. | Moderate B (VA-DOD) |
| Functional electrical stimulation of the shoulder girdle can be used to reduce subluxation and pain. | Moderate B (VA-DOD) |
| Botulinum toxin, either alone or associated with oral medication, is recommended in patients with focal spasticity that is painful, limits function, decreases the ability to participate in rehabilitation, or compromises proper positioning or skin care. | Moderate B (VA-DOD) |
| Infiltration of botulinum toxin in the subscapular and pectoral muscles can be used to treat HSP associated with spasticity. | Moderate B (CSBPR) |
| Suprascapular nerve block can be considered as a coadjuvant treatment for HSP. | Moderate B (AHA/ASA) |
| The usefulness of subacromial or glenohumeral corticosteroid injection in patients with inflammation in these areas is unclear. | Moderate B (AHA/ASA) |
| Subacromial corticosteroid injection can be used when HSP is considered to be related to a lesion of inflammation in the subacromial area (rotator cuff or bursa). | Moderate B (CSBPR) |
| The usefulness of acupuncture as a coadjuvant treatment for HSP is unclear. | Moderate B (AHA/ASA) |
| Dry needling in trigger points is recommended to treat HSP. | Moderate B (IMSERSO) |
| Treatment of HSP associated with limited range of joint motion may include gentle stretching and mobilisation techniques, and typically involves increasing external rotation and abduction. | Moderate B (CSBPR) |
| The active range of motion should be progressively increased at the same time as joint realignment and strengthening of weak muscles in the shoulder. | |
| In patients with shoulder pain after stroke, strapping can be used to reduce pain. | Low C (SF-CGSM) |
| Patients’ family members should receive education/training (e.g., on range of motion, positioning, etc.), particularly before discharge or care transitions. | Low C (AHA/ASA) |
| In patients with shoulder pain and upper limb spasticity after stroke, botulinum toxin A can be used to reduce pain. | Low C (SF-CGSM) |
| Management of HSP (after comprehensive assessment of underlying causes) may include subacromial or glenohumeral corticosteroid injection, suprascapular nerve block, electrical stimulation of shoulder muscles, botulinum toxin injections targeting the subscapular and/or pectoral muscles, shoulder orthoses, oral anti-inflammatory drugs, massage, gentle mobilisation of the shoulder muscles, and acupuncture. | Low IV (UEMS-PRMS) |
| In patients with shoulder pain after stroke, injections (subacromial corticosteroids or suprascapular nerve block with methylprednisolone and bupivacaine) may be used to reduce pain. | Low C (SF-CGSM) |
| In patients with shoulder pain after stroke, strapping can be used to reduce pain. | Low C (SF-CGSM) |
| Tenotomy of the pectoralis major, latissimus dorsi, teres major, or subscapularis muscles can be considered in patients with severe hemiplegia and restricted range of motion in the shoulder. | Low C (AHA/ASA) |
AHA/ASA: American Heart Association/American Stroke Association; CSBPR: Canadian Stroke Best Practice Recommendations: Stroke Rehabilitation Practice Guidelines; HSP: hemiplegic shoulder pain; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; IMSERSO: Instituto de Migraciones y Servicios Sociales; SF-CGSM: Stroke Foundation - Clinical Guidelines for Stroke Management; UEMS-PRMS: European Union of Medical Specialists-Physical and Rehabilitation Medicine Section; VA-DOD: Clinical Practice Guidelines for the Management of Stroke Rehabilitation. Department of Veterans Affairs-Department of Defense.
Subjective scales are used to detect and establish the severity of shoulder pain, including the vertical VAS (to avoid confusion in patients with hemispatial neglect), Ritchie Articular Index (RAI), NPRS, and the ShoulderQ shoulder pain questionnaire, among others.41–43 Exploratory manoeuvres are often also performed to establish the extent of subacromial involvement; these include the Neer test and/or palpation of painful points or the glenohumeral joint.41,42 In general, patients with arm weakness after stroke should be asked regularly about the presence of shoulder pain.9 Both the vertical VAS and the RAI are considered to be valid and reliable for rating the severity of shoulder pain after stroke.41 However, some authors have questioned the reliability of the VAS in patients with stroke due to its inability to reflect the potential complexity of this symptom.43 With respect to structural alterations to soft tissue and mechanical functioning of the shoulder, one study reports that one-third of patients with stroke present ultrasound alterations in the hemiplegic shoulder during the acute stage, including signs of inflammation of the subacromial bursa and biceps tendon, and biceps and rotator cuff tendinopathy. Presentation of these alterations was associated with the presence of secondary shoulder pain, but not with the severity of this pain.7Table 4 shows the recommendations related to the assessment and diagnosis of shoulder pain in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
Patients with risk factors for HSP should be asked regularly about shoulder pain.
- 2
Validated pain questionnaires and rating scales should be used and specific shoulder examination manoeuvres should be performed.
- 3
Ultrasound should be considered as a complementary study for diagnosing soft tissue lesions.
- 1
A precise aetiological diagnosis must be established in order to prescribe the most targeted, effective treatment possible; pain severity should be evaluated with validated pain scales.9,43 Treatment typically includes NSAIDs, analgesics, massage therapy, strapping, slings, electrical stimulation, vibration therapy, specific exercises, nerve blocks, and intra-articular injection of corticosteroids and/or botulinum toxin; there is no consensus on which is the best treatment approach.3,9,22,43 Treatment of HSP is probably the most extensively studied area within the field of pain management after ABI, with research analysing different treatment approaches including conventional rehabilitation (neurophysiological and robotic techniques), systemic and local pharmaceutical treatment (intra-articular and peritendinous injections), and/or alternative therapies (e.g., acupressure or acupuncture).7 In some cases of chronic, poorly controlled shoulder pain, systemic opioids may be considered, in accordance with the recommendations of the United States Centers for Disease Control and Prevention (CDC) on the control of non-cancer pain,44 and always taking into account the lowest effective dose, as well as the risks and benefits of treatment and drug interactions during up-titration, on an individual basis. Some studies support the use of intramuscular electrical stimulation, dry needling, taping, specific passive mobilisation exercises, acupressure, or administration of botulinum toxin or corticosteroids; or specify techniques that they consider not to be useful, including immobilisation of the paretic shoulder with bandages, and static stretching.2Table 4 shows the recommendations related to the treatment of shoulder pain after ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
The specific aetiology or aetiologies and the severity of HSP must be established; this information should be used to select the most appropriate therapeutic tool or tools in each case.
- 2
Intra-articular corticosteroid injection is particularly useful, especially in patients whose shoulder pain is mainly caused by underlying arthritis. Its effect is generally short-lived.
- 3
Infiltration of botulinum toxin in the subscapularis and pectoralis muscles should be considered, especially if HSP is associated with spasticity.
- 4
Neuromodulatory analgesics should be used to treat HSP in patients with clinical signs of neuropathic pain.
- 5
Neuromuscular electrical stimulation (with either surface or intramuscular electrodes) should be considered.
- 6
Therapy techniques with passive/continuous shoulder movement should also be applied.
- 7
Taping should be considered to secure the muscles of the paretic shoulder.
- 1
In addition to headache and shoulder pain, patients with ABI may present other types of nociceptive or mixed pain; these are generally less frequent, but may appear during the neurorehabilitation process. Particularly relevant symptoms include low back pain, mechanical knee pain (typically related to hyperextension of the knee during the weight acceptance phase of gait), postoperative pain, myofascial pain syndrome, pain associated with periarticular ossification, and/or chronic pelvic pain.17,26 Musculoskeletal pain is observed in up to a quarter of survivors of stroke. A multicentre study conducted in Italy observed an overall prevalence rate of 24.6%, with rates of 37.3% in the subacute stage and 28.5% in the chronic stage.14 Many of the alterations responsible for musculoskeletal pain after ABI may have existed prior to the neurological injury (e.g., degenerative joint diseases), or may result directly from the neurological damage (for instance, in patients with polytrauma as part of the aetiology of TBI) or surgical procedures performed after polytrauma. Whatever the cause, the aetiology must be taken into account for the proper pharmacological and non-pharmacological management of pain, and to prevent poor adherence to rehabilitation therapy. Some cases of musculoskeletal pain are caused by mechanisms involving a combination of inflammatory nociceptive pain, peripheral neuropathic pain, and central dysfunction. For instance, chronic low back pain (more than 3 mo’ progression) is classified as a mixed pain syndrome, with 50%–70% of patients showing a nociceptive component (lumbar intervertebral disc impairment, facet or sacroiliac joint disease, ligament injury) and 1%–15% presenting a neuropathic component (compressive or non-compressive radiculopathy associated with the inflammatory process).17 This is also the case for chronic pelvic pain and other visceral nociceptive pain conditions with associated neuropathic mechanisms; in the chronic stage, these disorders can lead to a dysfunction of central perception and may be associated with a range of complex pain disorders, such as fibromyalgia.17 Some studies report that stroke is frequently associated with chondrocalcinosis, in which deposition of calcium pyrophosphate at the cartilage junctions of the joints leads to episodes of acute pain days after stroke, with inflammatory reactions in such joints as the knee and hand on the hemiplegic side of the body; in elderly patients, this pain can be chronic.36 To prevent the appearance of these types of pain symptoms, certain risk factors may be considered, such as obesity (body mass index > 30) and age (greater risk from the third decade of life, with risk increasing in line with age), as well as genetic, hereditary, and occupational factors, which influence the appearance of pain after ABI, and chronic transformation of pain. Avoiding modifiable risk factors is helpful for preventing chronic transformation of pain after it is detected.17 In the assessment of these other types of pain in patients with ABI, it is important to evaluate associated signs and symptoms that may indicate another aetiology of pain; complementary studies (e.g., laboratory and imaging studies) should be considered, particularly if pain is chronic (> 3 mo). Pain rating tools used for the initial diagnosis, such as the VAS (generally displayed vertically), are also useful for the correct follow-up of these types of nociceptive pain. The functional impact of pain can be assessed with global clinical scales, such as the Pain Disability Index, or with such specific scales as the Oswestry Disability Index or the Roland Morris Disability Questionnaire for low back pain.17 Treatment depends on the type of pain and whether pain is chronic or acute. In patients with acute pain, symptomatic treatment with medical counselling and recommendations on prevention measures is typically sufficient. In patients with subacute and chronic pain, and taking into account the factors mentioned above, interdisciplinary management should include pharmacological treatments (indicated according to pain characteristics), physical therapy techniques, and/or psychotherapy.17 The first-line symptomatic treatment is paracetamol, with NSAIDs being the second-line treatment. Tramadol and other opioids may constitute a third line of treatment; the risks and benefits of opioid treatment must always be assessed in these patients. In patients with chronic pain, the drugs typically used to treat neuropathic pain (e.g., tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, and certain antiepileptic drugs [AED]); local anaesthetic, corticosteroid, and/or botulinum toxin injections; and electrical stimulation should be administered in accordance with the specific guidelines for each type of pain, always taking into account the individual characteristics of each patient.
- -
SENR recommendations
- 1
Besides headache and HSP, the most frequent type of nociceptive pain after ABI is joint pain, and particularly low back pain.
- 2
These pain syndromes may appear as a result of ABI or be related to prior risk factors (age, body weight, genetic and environmental factors) and/or the rehabilitation process.
- 3
Such tools as the VAS, NPRS, and generic and specific disability scales are helpful for diagnosis and follow-up.
- 4
Treatment should include both pharmacological and non-pharmacological measures, such as physiotherapy techniques and psychological approaches. Escalation of pharmacological treatment should follow the recommendations of the specific guidelines for each type of pain.
- 1
Neuropathic pain is defined as a phenomenon resulting from dysfunction of the nervous system, particularly the somatosensory nervous system, due to a nervous lesion, disease, or alteration involving Aβ or Aδ fibres, unmyelinated C fibres, or central neurons.45 It may be triggered by damage to the peripheral nervous system or to the central nervous system (CNS), with a wide range of possible aetiologies. It features in a diverse range of syndromic diagnoses, with a discrepancy between the objective signs of tissue damage, its clinical magnitude, and the associated disability; this sometimes results in underdiagnosis or hinders its therapeutic management. The classic definition of neuropathic pain, published by the IASP in 1994, is “pain initiated or caused by a primary lesion or dysfunction in the nervous system.” Due to the poor specificity of this definition in terms of the pathophysiology of this pain in other neurological processes, in 2008 the IASP Special Interest Group on Neuropathic Pain proposed the following definition: “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system.” In 2011, the IASP Taxonomy Committee expanded the definition: “pain caused by a lesion or disease of the somatosensory system.” The latter 2 definitions are better suited to the study of neuropathic pain in neurorehabilitation.16 Peripheral nervous system alterations, which are sometimes observed in patients with ABI, may progress with neuropathic pain. Such is the case of plexopathies, radiculopathies, and mononeuropathies, which may present in patients with a previous condition (e.g., diabetes-associated neuropathy) or as the direct result of polytrauma (e.g., in the acute and subacute stages of TBI, during hospitalisation). This type of pain causes disability and affects quality of life, which has an impact on patients’ adherence to neurorehabilitation treatment. These guidelines focus on neuropathic pain of central origin, as we refer exclusively to pain secondary to ABI. On the management of peripheral neuropathic pain, we recommend the guidelines of the Italian Consensus Conference on Pain in Neurorehabilitation; management of peripheral neuropathic pain shares the same principles as management of central pain, which are reviewed below.46 It is possible for ABI to involve direct lesions to the somatosensory system and regions participating in the perception and efferent modulation of pain, frequently resulting in the expression of neuropathic pain as a symptom. A specific problem in neurorehabilitation is the fact that alterations to cognition, language, and awareness in patients with stroke, TBI, and neurodegenerative diseases can be an obstacle to a detailed clinical history interview, as described in the first 2 steps of the Neuropathic Pain Grading System.
Central neuropathic painConceptCNP can be defined as a pain syndrome resulting from an acute lesion to some structure of the CNS, developing due to an altered secondary response by multiple structural modulatory mechanisms, which are distorted by the damage.47 This pain does not play a protective role and has no biological benefit. On the contrary, it causes maladaptation and abnormal perception of painful and non-painful impulses, resulting in various alterations in CNS function. Stroke is one of the most frequent causes of central pain. In patients with stroke, this type of pain is typically known as thalamic pain syndrome or Dejerine-Roussy syndrome. Other CNS diseases that can trigger this central pain include spinal cord lesions, multiple sclerosis, and Parkinson’s disease, with CNP showing an overall prevalence rate greater than 7%–10%.48 In addition to those mentioned above, CNS conditions with a lower prevalence of central pain include TBI and spinal cord injury, lesions secondary to neurosurgical interventions, CNS tumours and arteriovenous malformations, brain and spinal cord infections, autoimmune diseases progressing with CNS inflammation, and epilepsy. In Spain, the prevalence of CNP is estimated at 2.4%.49 The incidence of central post-stroke pain (CPSP) is unclear, with estimates varying between 5% and 46% of patients with stoke; this pain is frequently underdiagnosed. This variable incidence rate is attributed to differences in aetiology, lesion location, study design, and time since stroke.50 CPSP onset is generally days after stroke, with the majority of cases being symptomatic in the first month.7 According to another article, approximately 25% of cases may present during the acute or subacute stage after stroke, although CPSP typically develops at 3–6 mo.14 Prospective studies report that 2.7% of patients with stroke presented CPSP at one year, with another 25% reporting this pain in the first 6 mo after brainstem infarction.51 The delayed onset of pain suggests that it is caused by the reorganisation of neural circuits, rather than the lesion itself. This type of pain is frequently associated with lesions to the ventral caudal nucleus of the thalamus secondary to lesions in the territory of the thalamogeniculate artery, or brainstem lesions (the lateral region of the medulla in the case of Wallenberg syndrome).16 Lesions in the opercular/insular or posterior perisylvian regions are other possible causes.52 The pons, supratentorial structures, and practically any territory involving ascending and descending pain pathways may also be affected,53 with studies reporting different prevalence rates in each location. It should be noted that the appearance of central pain is not associated with the type of stroke (in the case of CPSP) or lesion extension; the only association is with lesion location. Pain may present with or without accompanying sensory alterations, in the area of the body controlled by the affected brain region. Central pain is often associated with or mistaken for painful spasticity. It is different from musculoskeletal pain.9 Pain is described by patients as a loss of normal sensitivity associated with burning or piercing pain, frequently presenting with allodynia and dysaesthesia triggered by tactile stimulation, cold, or movement in the affected area. Pain is usually continuous or intermittent, and may be exacerbated by heat, cold, or stress.16 CPSP can seriously impact an individual’s ability to perform basic activities of daily living, disrupt sleep, affect mood, trigger psychiatric alterations and even suicidal ideation (CPSP is associated with a three-fold increase in suicidal risk, compared to stroke without CPSP), and reduce quality of life.16
Aetiology and pathophysiologyThe pathophysiology of CNP can be explained according to 3 theories:
- -
Central disinhibition theory. According to this hypothesis, the spinothalamic tract is disinhibited due to selective apoptotic cell loss in the γ-aminobutyric acid (GABA) pathway secondary to a lesion to the ventral posterolateral nucleus of the thalamus.
- -
Central imbalance theory. This theory posits that pain is caused by lesions that cause greater activation of the insula and spinothalamic tract (lateral pathway) than the anterior cingulate cortex and medial lemniscus (medial pathway)47,53–55 due to incorrect processing of signals secondary to the interruption of specific circuits; this results in maladaptation of the immune system to brain damage.
- -
Central sensitisation theory. According to this theory, pain is caused by cortical and thalamic hyperexcitability, with greater activation of N-methyl-D-aspartate (NMDA) receptors and calcium channels, as CNP can be regulated by NMDA receptor antagonists, calcium or sodium channel blockers, and GABA agonists.47,54
The prevention of CNP is a long process, requiring a comprehensive, multidimensional, individualised approach based on understanding of the aetiopathogenic characteristics of the pain syndrome, its clinical manifestations, the presence of comorbidities, potential secondary effects and complications of any invasive or surgical treatments, and the complexity of pharmaceutical management.
- -
SENR recommendations
- 1
Is important to be familiar with the manifestations of neuropathic pain and to be alert to the appearance of pain, particularly in the first month after ABI.
- 2
Previous and subsequent comorbidities and secondary effects of pharmacological or invasive/surgical treatments should be considered as possible triggers of neuropathic pain.
- 1
As noted above, the clinical symptoms of CNP are heterogeneous and may change over time. Some patients with CPSP complain of pain induced or exacerbated by triggers including tactile stimulation, changes in temperature (particularly cold), and stress.53 Pain is most frequently triggered by movement, and is characterised by feelings of tension, pressure, or burning of the skin. On occasions, this syndrome may present with paroxysms, with stabbing, shooting, or piercing characteristics. Not all patients are able to fully and correctly describe their pain. When this is the case, physicians must help them to find the terms that best describe their pain: burning, piercing, pressing, throbbing, cutting, dull, electric, numbing, etc.; though they are not pathognomonic, these characteristics should arouse clinical suspicion of CNP. Pain intensity is moderate to severe, typically scoring 3–8 on the VAS, and is diffuse or involves large areas of the body. Pain is generally circumscribed to the affected brain areas, although this is not always the case.56 A recent study reported that pain most frequently affects the upper limbs, followed by the lower limbs, head and face, upper and lower limbs simultaneously, and entire contralesional hemibody.57 Other reports describe mixed areas of hypo- and hyperaesthesia in regions affected by pain, a clear sign of the diverse sensory anomalies than can appear alongside CPSP. In this regard, such positive sensory phenomena as allodynia, hyperalgesia, paraesthesias, and dysaesthesias have been reported in up to 85% of patients with CNP. The diagnostic criteria for CNP are based on those proposed by Klit et al.54; though they were initially proposed for CPSP, they can be extrapolated to CNP in general. A grading system for CPSP was proposed by Treede et al.58 Over time, the latter set of criteria have become the most widely recognised and the most convenient in clinical and research settings.
Klit et al.54 diagnostic criteriaMandatory criteria
- -
Pain within an area of the body corresponding to the lesion of the CNS
- -
History suggestive of a stroke and onset of pain at or after stroke onset
- -
Confirmation of a CNS lesion by imaging or negative or positive sensory signs confined to the area of the body corresponding to the lesion
- -
Other causes of pain are excluded or considered highly unlikely.
Supportive criteria
- -
No primary relation to movement, inflammation, or other local tissue damage
- -
Descriptors such as burning, painful cold, electric shocks, aching, pressing, stinging, and pins and needles, although all pain descriptors can apply
- -
Allodynia or dysaesthesia to touch or cold
Certainty of neuropathic pain is classed as definite if all 4 criteria are met, probable if the first 2 plus either the third or fourth are met, and possible if the first 2 are met with no confirmatory evidence of the third and fourth.
- -
Pain with a distinct neuroanatomically plausible distribution: either pain localised unilaterally in the body and/or face or unilaterally on one side of the body with contralateral involvement of the face
- -
A history suggestive of a relevant lesion or disease affecting the peripheral or central somatosensory system. This must also display the typical temporal relationship.
- -
Demonstration of the distinct neuroanatomically plausible distribution by at least one confirmatory test
- -
Demonstration of the relevant lesion or disease by at least one confirmatory test: these confirmatory tests depend on which lesion or disease is causing neuropathic pain.
Table 5 shows the recommendations related to the assessment and diagnosis of CNP in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
A clinical history interview targeting the characteristics of neuropathic pain and an appropriate neurological examination should be performed; appropriate complementary testing should be performed to establish a feasible relationship between the brain injury and the pain characteristics reported by the patient.
- 2
The relationship between clinical signs and the neuroanatomical areas affected should be taken into account, and particularly those reported to be involved in the appearance of CNP.
- 1
Recommendations on central neuropathic pain secondary to acquired brain injury.
| Recommendations | Grade of recommendation |
|---|---|
| Assessment and diagnosis | |
| Stroke | |
| Wherever possible, the NPGS58 should be used to diagnose neuropathic pain after stroke. Screening tools and questionnaires may be used when the NPGS cannot be used, but should not replace clinical assessment and the grading system for diagnosing neuropathic pain. | Moderate B (ICCPN) |
| Patients with stroke should be asked about CPSP, especially if stroke involves the thalamic-geniculate artery territory, ventral posterior nucleus–pulvinar border zone, lateral medulla, and insula. | Moderate B (ICCPN) |
| In patients with stroke, CPSP and CRPS should be diagnosed using the NPGS and the Reflex Sympathetic Dystrophy Syndrome Association criteria, respectively. | Moderate B (ICCPN) |
| Diagnosis of CPSP should be based on established diagnostic criteria after other causes of pain are ruled out. | Low C (AHA/ASA) |
| Treatment* | |
| Stroke | |
| Regarding neuropathic pain in general, the practice guidelines indicate: | High A (ICCPN) |
| a) first line: tricyclic antidepressants, SSRIs, pregabalin, and gabapentin; b) second line: 5% lidocaine patches, 8% capsaicin patches, tramadol; c) third line: strong opioids, botulinum toxin. | |
| Only a minority of patients respond to pharmacological treatment. | |
| For the treatment of CPSP: | |
| Amitriptyline and lamotrigine are indicated as the first choice, with slow titration in the case of the latter drug. | Moderate B (ICCPN) |
| Duloxetine, pregabalin, and gabapentin are second-line drugs. | Low C (ICCPN) |
| Levetiracetam is not indicated. | Moderate B (ICCPN) |
| Naloxone is not indicated. | High A (ICCPN) |
| In refractory cases, opioids, rTMS, or motor cortex stimulation can be considered. | |
| For neuropathic pain in general, combined therapy with pregabalin/gabapentin + opioids/tricyclic antidepressants/duloxetine should be considered in order to reduce secondary effects. | Moderate B (ICCPN) |
| In selected patients, motor cortex stimulation may be a reasonable approach for intractable CPSP that does not respond to other treatments. | Moderate B (AHA/ASA) |
| Deep brain stimulation has not been established as an efficacious treatment. | Moderate B (AHA/ASA) |
| Amitriptyline and lamotrigine are reasonable first-line drugs. | Moderate B (AHA/ASA) |
| Pregabalin, gabapentin, carbamazepine, and phenytoin may be considered as second-line drugs. | Moderate B (AHA/ASA) |
| Patients with persistent CPSP should receive oral treatment with centrally acting analgesics (trial at low doses). | Low C (CSBPR) |
| AEDs (e.g., pregabalin or gabapentin) should be administered as the first-line treatment. | Low C (CSBPR) |
| Tricyclic antidepressants (e.g., amitriptyline) or SSRIs/SNRIs (particularly duloxetine) should be used for second-line treatment. | Low C (CSBPR) |
| If first- and second-line treatments are ineffective, opioids or tramadol may be used. Opioids should be used with caution due to the risk of physical dependence. | Low C (CSBPR) |
| Pregabalin can be considered to reduce CNP secondary to brain or spinal cord lesions. | Low C (INESS-ONF) |
| Interprofessional pain management is probably useful alongside pharmacotherapy. | Low C (AHA/ASA) |
| Interdisciplinary pain management including pharmacotherapy and physical exercise is recommended; psychosocial support may be considered, and motor cortex stimulation can be tried in CPSP. | Low IV (UEMS-PRMS) |
| Standardised measures may be helpful for monitoring treatment response. | Low C (AHA/ASA) |
| For the management of CPSP, an individualised approach should be implemented by an interdisciplinary team including healthcare professionals with experience in mental health and the management of CPSP. | Low C (CSBPR) |
AED: antiepileptic drug; AHA/ASA: American Heart Association/American Stroke Association; CNP: central neuropathic pain; CPSP: central post-stroke pain; CRPS: complex regional pain syndrome; CSBPR: Canadian Stroke Best Practice Recommendations: Stroke Rehabilitation Practice Guidelines; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; INESS-ONF: Institut National d’Excellence en Santé et en Services Sociaux – Ontario Neurotrauma Foundation; NPGS: Neuropathic Pain Grading System; rTMS: repetitive transcranial magnetic stimulation; SF-CGSM: Stroke Foundation – Clinical Guidelines for Stroke Management; SNRI: serotonin and norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; UEMS-PRMS: European Union of Medical Specialists-Physical and Rehabilitation Medicine Section.
Currently, pregabalin is the only drug whose summary of product characteristics includes the indication for central neuropathic pain. However, based on the experience of the authors and the suggestions of the references reviewed, we may also use these recommendations (with levels of evidence) for the treatment of neuropathic pain in general.
Limited evidence is available on the efficacy of pharmacological and non-pharmacological approaches in the treatment of CNP. It is reasonable to combine both treatment approaches, and include psychological support.7 It should be noted that pregabalin is the only drug currently indicated for controlling CNP. However, in general terms, we may infer that pharmacological treatment for CNP includes the same classes of drugs as those used for peripheral neuropathic pain. The Grading of Recommendations (GRADE) guidelines and the EFNS guidelines on pharmacological treatment of neuropathic pain are recommended. Non-invasive neuromodulation techniques have attracted growing attention in the management of this type of pain as coadjuvants to pharmacological and physical therapy. Patients with stroke stress the importance of educating family members and caregivers about CPSP and its management. These patients themselves also require education about CPSP, in order to assess themselves and recognise potential symptoms.16Table 5 shows the recommendations related to the treatment of CNP after ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
The recommendations for CPSP may be extrapolated to other aetiologies of CNP.
- 2
Selection of treatments for neuropathic pain must be considered on an individual basis, according to the patient, the type of ABI, and existing comorbidities and medications, as well as potential secondary effects.
- 3
The most appropriate combination of pharmacological and non-pharmacological treatments should be identified.
- 4
Pharmacological treatment should begin with amitriptyline (a tricyclic antidepressant) or lamotrigine (an AED), with subsequent escalation (always seeking the minimal effective dose), adding or switching to second-line drugs (other AEDs and/or antidepressants).
- 5
As far as possible, adverse effects threatening proper adherence to neurorehabilitation treatment should be avoided.
- 6
Caution should be exercised when using opioids due to their adverse effects on neurological and cognitive symptoms, as well as the risk of dependence.
- 7
Adjuvant treatment with non-invasive neuromodulatory therapies should be considered when results are not achieved with initial pharmacological treatment.
- 8
CNP management should be conducted by an interdisciplinary team and involve the participation of the patient and their family/caregivers.
- 1
Complex regional pain syndrome (CRPS) is a painful clinical entity with diverse signs and symptoms, whose detection can be a challenge. It is classified as a type of neuropathic pain, and may affect one or more limbs.59 CRPS is characterised by a combination of burning pain, hyper- and/or hypoaesthesia, sudomotor alterations, decreased range of motion, patchy bone demineralisation, and vasomotor changes, including oedema and changes in skin temperature and colour.59–62 The Reflex Sympathetic Dystrophy Syndrome Association identifies 3 types of CRPS: type I (also known as reflex sympathetic dystrophy), defined as CRPS that appears without a significant nervous lesion; type II (previously known as causalgia), in which there is clinical or electrophysiological evidence of a nervous lesion; and type III, in which symptoms do not fully meet criteria for types I or II.63 A frequent pain syndrome after stroke is type I CRPS in the paretic upper limb, commonly referred to as shoulder-hand syndrome (SHS). Only one limb is typically affected, although pain occasionally extends to another limb.60 The reported incidence rates after stroke vary greatly, ranging from 1.5% to 61% of patients62,63; this probably reflects the heterogeneity of diagnostic criteria and the high frequency at which signs typical of type I CRPS appear in hemiparetic patients without SHS. Several authors note that CRPS in hemiplegic patients, or SHS, can be a considerable obstacle to rehabilitation, interfere in basic activities of daily living, lead to loss of function of the affected upper limb, and cause depression and deterioration of quality of life.19,59,65–67 However, other authors report that few data are available on the impact of CRPS on functional recovery and rehabilitation, and that larger studies are needed.63
Aetiology and pathophysiologyThe aetiology and pathophysiology of SHS are not well understood. We do know that the factors involved in CRPS pathogenesis include certain biomechanical conditions or microtrauma,63 neurogenic inflammation, nociceptive sensitisation, vasomotor alterations, genetic factors, maladaptive brain plasticity, psychological factors, and/or hyperactivity of the sympathetic autonomic nervous system.59,61,65–67 A key role is played by central mechanisms that can trigger maladaptive plasticity in the sympathetic, sensory, and motor systems.59 With regard to the inflammatory processes involved, neurogenic inflammation in CRPS has been shown to be triggered by such neuropeptides as substance P, neuropeptide Y, vasoactive intestinal peptide, and bradykinin.65 In patients with type I CRPS after stroke, research using bone scintigraphy and shoulder ultrasound studies suggests that the key trigger factors in SHS may be frozen shoulder and soft tissue injuries in the wrist, possibly related to degenerative processes present prior to stroke, traction injury to the weakened limb, and passive shoulder mobilisations.64 Associations have also been detected between SHS and presence of spasticity, shoulder subluxation, severity of paresis, and limited joint range of motion.66 In fact, weakness, spasticity, sensory deficits, and coma have been proposed as predictors of CRPS.63 It should be noted that some of these findings are controversial, with some authors questioning the association between SHS and shoulder subluxation or between SHS and spasticity, for example.67
PreventionPrevention of SHS is challenging due to the syndrome’s multifactorial nature. In general, recommendations seek to avoid or minimise the risk of microscopic soft tissue lesions in the upper limb, for example by limiting the range of motion in passive or active joint mobilisations to the non-painful range, avoiding mobilisation of the fingers by the patient themselves, and using devices or manoeuvres to recentre the shoulder. A strict protocol to prevent shoulder subluxation, avoiding painful shoulder postures and positions and trauma, reduced the incidence of CRPS.61 Regarding pharmacological treatment, prophylaxis with calcitonin was also found to be useful in a group of patients with stroke.61Table 6 shows the recommendations related to the prevention of CRPS after ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
Measures should be established to minimise the risk of microlesions around the shoulder joint during active or passive mobilisation.
- 2
Specific mobilisation exercises should be performed by a professional.
- 3
Mobilisation manoeuvres should be limited to the non-painful range of motion, provided that the patient is able to communicate pain.
- 1
Recommendations on complex regional pain syndrome secondary to acquired brain injury.
| Recommendations | Grade of recommendation |
|---|---|
| Prevention | |
| Active, active assisted, and passive mobilisation exercises can be used to prevent CRPS. | Low C (CSBPR) |
| Assessment and diagnosis | |
| In patients with stroke, CPSP and CRPS should be diagnosed using the NPGS and the Reflex Sympathetic Dystrophy Syndrome Association criteria, respectively. | Moderate B (ICCPN) |
| The diagnosis of CRPS should be based on clinical findings, including pain and hypersensitivity/hyperalgesia in the metacarpophalangeal and interphalangeal joints, which may be associated with oedema on the dorsum of the fingers, trophic skin changes, hyperaesthesia, and limited joint range of motion. | Low C (CSBPR) |
| Treatment | |
| An initial schedule of oral corticotherapy, starting at 30–50 mg/day over 3–5 days with tapering over 1–2 weeks, may be used to reduce pain and swelling. | Moderate B (CSBPR) |
| With regard to mirror therapy, 2 studies report positive outcomes for reducing pain in patients with CRPS. | Low C (SF-CGSM) |
CPSP: central post-stroke pain; CRPS: complex regional pain syndrome; CSBPR: Canadian Stroke Best Practice Recommendations: Stroke Rehabilitation Practice Guidelines; ICCPN: Italian Consensus Conference on Pain in Neurorehabilitation; NPGS: Neuropathic Pain Grading System; SF-CGSM: Stroke Foundation - Clinical Guidelines for Stroke Management.
Early diagnosis and timely onset of multidisciplinary treatment are essential to the proper management of CRPS.65,67
Clinical diagnosisDiagnosis of CRPS is essentially clinical, based on signs and symptoms. CRPS is a neuropathic pain disorder with autonomic manifestations; pain may be associated with allodynia, hyperalgesia, oedema, changes in skin temperature and colour, motor dysfunction, or reduced range of motion.59,63,67 In the specific case of type I CRPS after stroke, pain typically manifests simultaneously in the hand and shoulder of the affected arm, with the elbow remaining largely free of pain (SHS).64 Various classifications and criteria have been published for the characterisation of CRPS. The IASP proposed a set of criteria for the diagnosis of CRPS in 1994. These are frequently used as the reference criteria, although some authors have criticised their lack of specificity and internal validity.67 Several sets of criteria for more precise identification of hemiparetic patients with SHS have been published, such as the Budapest criteria, whose development included more rigorous assumptions to increase their specificity in diagnosing type I CRPS. However, there is controversy regarding their sensitivity and their appropriateness for diagnosis SHS.67 Diagnosis of type I CRPS can be a challenge in patients with stroke, as many signs of SHS coincide with those observed in hemiparesis. For instance, HSP is very frequent after stroke, and may have multiple causes besides SHS. In general terms, we may assert that SHS tends to appear 2–3 mo after the brain injury, and over a year later in rare cases,62 whereas shoulder pain due to hemiparetic muscle weakness and muscle tone alterations tends to appear earlier, within the first 8 weeks. It should also be noted that distal oedema in the hand in isolation is much more frequent than in SHS, and is mainly related to immobility and the Trendelenburg position. Oedema in early stages of SHS, which is not lymphoedema, presents poorer response to drainage and elevation of the limb. As in CRPS in general, SHS after stroke is generally classified into 3 stages: stage 1 (acute, hyperaemic), stage 2 (dystrophic, ischaemic), and stage 3 (atrophic). The inflammatory response seems to play a key role in the early stages, with clinical signs including oedema/swelling, pain, and heat.65
Complementary testsWhile diagnosis is fundamentally clinical, complementary tests are occasionally used, with a view to improving the sensitivity or specificity of clinical suspicion or to rule out similar processes, etc. For instance, blood analysis can rule out rheumatic disorders that present specific markers; patients with CRPS are also reported to present elevated levels of inflammatory cytokines (tumour necrosis factor α, interleukin 6, tryptase).65,66 Three-phase bone scintigraphy is also used in some cases. Simple radiography can reveal the classic pattern of patchy osteoporosis, and bone density scanning may show reduced mineral density in patients with SHS. Other tests include digital infrared thermal imaging, among others.67Table 6 shows the recommendations related to the assessment and diagnosis of CRPS in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
The diagnosis of CRPS is essentially clinical, based on recognisable signs and symptoms.
- 2
CRPS should be suspected in patients with neuropathic pain and autonomic signs, with such symptoms as allodynia, hyperalgesia, motor dysfunction, oedema, and/or changes in skin temperature and colour.
- 3
CRPS should be suspected in patients with hemiplegia and symptoms affecting the shoulder and hand on the affected side of the body (SHS); symptoms appear from 2–3 mo after the brain injury, and respond poorly to drainage and elevation of the limb.
- 1
A broad range of treatments can be used for CRPS after stroke, although insufficient evidence is available to support any individual technique, treatment protocol, or group of techniques as being the most appropriate in all stages and all patients. In fact, our literature search identified no protocol with consensus support from the majority of the scientific community for the treatment of CRPS in general.59,65 Approaches that have been trialled and are frequently used for type I CRPS (including in patients with stroke) are:
- -
Oral corticosteroids. These drugs have been shown to reduce pain, oedema, and other aspects of CRPS in general, probably due to their anti-inflammatory and/or immunomodulatory effects. In SHS, they are reported to be efficacious with short schedules (2 weeks) and with longer schedules (2 mo with tapering).66
- -
Topical analgesic and anti-inflammatory treatment.
- -
AEDs. Gabapentin or carbamazepine (studied in CRPS unrelated to stroke).61
- -
NMDA receptor antagonists, memantine, or ketamine (studied in CRPS in general, not after stroke).61
- -
Vitamin C. Some studies propose that vitamin C be included within the treatment of CRPS, as antioxidants seem to augment the benefits of aerobic exercise.65
- -
Bisphosphonates (oral or intravenous). These drugs have an analgesic function in bone-related diseases, as well as in diseases that do not affect the osteoarticular system, possibly through inhibition of cytokine and neuropeptide production and the transportation of calcium and other ions.63 They also inhibit bone resorption, which may prevent osteoporosis induced by osteoclast hyperactivity and immobility in CRPS.63 In CRPS, intravenous pamidronate presents similar efficacy to oral corticotherapy in pain control, but not in reducing oedema.63
- -
Aerobic exercise (focusing on the upper limb). The benefits of this treatment are probably related to the general benefits of aerobic exercise, which is associated with anti-inflammatory, hormonal, and antioxidant effects on the autonomic nervous system and immune system.65 In type I CRPS, aerobic exercise can contribute to better sensorimotor recovery, promoting neuroplasticity and motor learning.65
- -
Physiotherapy and occupational therapy techniques. Joint mobilisations, compression therapy, splinting/orthoses, massage therapy, contrast baths, electrotherapy, thermotherapy, etc.
- -
Mirror therapy.59,61 Mirror therapy may have benefits in patients with hemispatial neglect, and pain in CRPS.10
- -
Acupuncture. Electroacupuncture and traditional Chinese manual acupuncture may improve the clinical outcomes (pain and motor function) of rehabilitation therapy in patients with mild CRPS. This evidence is classed as low quality according to the GRADE system.60
- -
Sympathetic nerve blocks. This technique typically uses local anaesthesia. These nerve blocks were frequently used in the past for CRPS in general; today, there is some controversy about their efficacy.61–63
- -
Posterior root radiofrequency therapy, with short pulses applied to the nerves. This is probably a safer alternative than radiofrequency neurolysis.62
- -
Invasive neurostimulation. In cases of chronic pain due to CRPS that is refractory to other treatments, spinal stimulation has been trialled, with stimulation of the dorsal columns and more recently the dorsal roots; the results reported to date are promising.62,68
Table 6 shows the recommendations related to the treatment of CRPS in ABI and the corresponding grades of recommendation in the main guidelines reviewed.
- -
SENR recommendations
- 1
Pharmacological treatment should include corticotherapy and, on an individual basis, bisphosphonates and AEDs.
- 2
Non-pharmacological treatments include mirror therapy, occupational therapy, and specific physiotherapy techniques. On an individual basis, invasive techniques with specialised devices may also be considered.
- 1
This study received no funding of any kind.