Fatigue is a complex, multidimensional syndrome that is prevalent in patients with acquired brain damage and has a negative impact on the neurorehabilitation process. It presents from early stages after the injury, and may persist over time, regardless of whether sequelae have resolved. Fatigue is conditioned by upper neuronal circuits, and is defined as an abnormal perception of overexertion. Its prevalence ranges from 29% to 77% after stroke, from 18% to 75% after traumatic brain injury, and from 47% to 97% after brain tumours. Fatigue is associated with factors including female sex, advanced age, dysfunctional families, history of specific health conditions, functional status (eg, fatigue prior to injury), comorbidities, mood, secondary disability, and the use of certain drugs. Assessment of fatigue is fundamentally based on such scales as the Fatigue Severity Scale (FSS). Advances have recently been made in imaging techniques for its diagnosis, such as in functional MRI. Regarding treatment, no specific pharmacological treatment currently exists; however, positive results have been reported for some conventional neurorehabilitation therapies, such as bright light therapy, neurofeedback, electrical stimulation, and transcranial magnetic stimulation. This review aims to assist neurorehabilitation professionals to recognise modifiable factors associated with fatigue and to describe the treatments available to reduce its negative effect on patients.
La fatiga es un síndrome multidimensional, complejo y frecuente en los pacientes con daño cerebral sobrevenido, influyendo negativamente en el proceso de neurorrehabilitación. Aparece desde etapas tempranas luego de la lesión y puede permanecer en el tiempo, recuperadas o no las secuelas del daño. La fatiga depende de circuitos neuronales superiores y se define como una percepción anómala de sobreesfuerzo. Tiene una prevalencia de 29% a 77% tras el ictus, 18% a 75% tras el traumatismo craneoencefálico (TCE) y 47% a 97% tras tumores cerebrales. La fatiga se asocia a factores como sexo femenino, edad avanzada, familia disfuncional, antecedentes patológicos específicos, estado funcional (p. ej. fatiga previa a la lesión), comorbilidades, estado anímico, discapacidad secundaria y uso de ciertos fármacos. Su estudio se realiza sobre todo a partir de escalas como la Escala de severidad de fatiga (Fatigue Severity Scale). Hoy en día existen avances en herramientas de imagen para su diagnóstico como la resonancia magnética funcional. En cuanto a su tratamiento, no existe aún terapia farmacológica definitiva, sin embargo, existen resultados positivos con terapias dentro de la neurorrehabilitación convencional, terapia lumínica y el uso del neurofeedback, estimulación eléctrica y magnética transcraneal. Esta revisión tiene como objetivo ayudar al profesional dedicado a la neurorrehabilitación a reconocer factores asociados modificables, así como terapias a su alcance para disminuir sus efectos nocivos en el paciente.
Acquired brain injury (ABI) is recognised by the World Health Organization as a leading cause of disability worldwide.1 Stroke is the most frequent type of ABI, with an incidence of 150–200 cases per 100 000 person-years in Spain.2 This rate is similar to that of traumatic brain injury (TBI), with 200 cases per 100 000 person-years3; other less frequent types of ABI are brain tumour surgery, encephalopathy, and encephalitis. Fatigue is common in patients with ABI, and has a negative impact on neurorehabilitation, hindering their involvement in treatment and recovery in the long term. This review aims to summarise the available evidence on some relevant aspects of fatigue in ABI.
DefinitionFatigue is a sensation of exhaustion, lack of energy, and aversion to effort that may develop during physical or mental activity, and that usually does not decrease with rest.4,5 The difference between fatigue and tiredness is that while the latter is proportional to the effort exerted, the former is associated with an exaggerated, disproportionate perception of effort.6
Fatigue is a complex, multidimensional concept, with psychological, motivational, situational, and physical dimensions.7 As in other brain disorders following ABI, fatigue is a consequence of an abnormal perception of overexertion and the consequent disruption of descending motor pathways.8,9 Several classifications of fatigue have been proposed based on this concept.
Some researchers differentiate between central and peripheral fatigue.10–13 Others differentiate between primary and secondary fatigue, with primary fatigue being the result of alterations to a neural mechanism directly linked to the perception of fatigue, and secondary fatigue being a manifestation of neurological sequelae (eg, sleep alterations, pain, mood disorders).14 Four main concepts are widespread in the literature reviewed. The first of these is “trait fatigue,” defined as the fatigue perceived by an individual in different areas of their life. One of the most frequently used scales to measure this general fatigue is the Fatigue Severity Scale (FSS). Another widely used concept is “state fatigue,” referring to a temporary decrease in muscle strength, particularly after motor activity. It is frequently measured with maximal or submaximal motor tasks. The concept of “mental fatigue” or “cognitive fatigue” is similar to that of motor fatigue, but is assessed by measuring reaction times in cognitive tasks. Lastly, “perceived fatigue” is a subjective feeling of fatigue after physical or cognitive exertion, and is frequently measured with the Borg Rating of Perceived Exertion.
Many other concepts of fatigue (eg, emotional fatigue) have been proposed in accordance with the purposes of each study, but these lie beyond the scope of this review.
EpidemiologyThe prevalence of fatigue in the general population ranges from 3% to 23%.7,15
In the field of neurology, fatigue is highly prevalent in patients with such conditions as multiple sclerosis (80%–90%).16 In the context of acquired neurological injuries, the prevalence of fatigue in patients with spinal cord injury ranges from 50% to 57% from very early stages, persisting over the course of the disease.17
Recent studies have estimated the prevalence of fatigue in populations with ABI, although many present methodological limitations and include small samples.18,19
Around 43% of patients with ABI and reporting fatigue have not been diagnosed with or received specific treatment for fatigue,20 despite the association between fatigue and poorer prognosis in ABI.9,21–25
The prevalence of fatigue ranges from 29% to 77% in patients with stroke5,8,9,13 (despite the lower prevalence of fatigue among these patients compared to other groups26), from 18% to 75% in patients with TBI,14 and from 47% to 97% in patients with brain tumours.27,28 Studies of paediatric patients with ABI report prevalence rates of 58%–76%.12
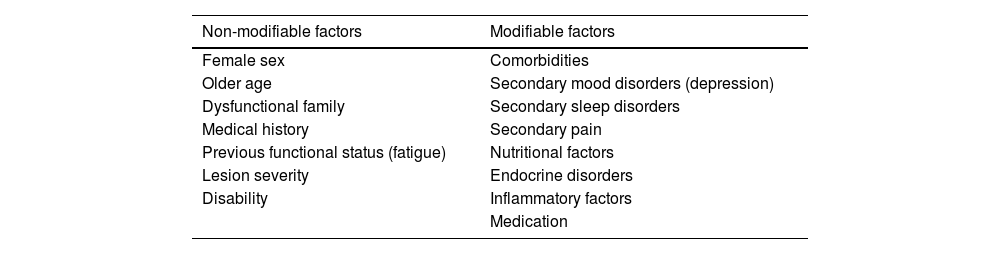
Fatigue in patients with acquired brain injurySeveral modifiable and non-modifiable factors explain not only the appearance of fatigue but also its progression or worsening over time (Table 1).29,30
Factors associated with fatigue secondary to acquired brain injury.
| Non-modifiable factors | Modifiable factors |
|---|---|
| Female sex | Comorbidities |
| Older age | Secondary mood disorders (depression) |
| Dysfunctional family | Secondary sleep disorders |
| Medical history | Secondary pain |
| Previous functional status (fatigue) | Nutritional factors |
| Lesion severity | Endocrine disorders |
| Disability | Inflammatory factors |
| Medication |
The most frequent comorbidities during the first months after ABI are mood disorders, sleep disorders, and pain (somatic, neuropathic, or mixed), which may be secondary to ABI or to the associated treatment. Depression is the mood disorder most frequently associated with fatigue in patients with ABI.26 The most frequently associated sleep disorder is insomnia (21%–23%), followed by excessive daytime sleepiness, obstructive sleep apnoea/hypopnoea syndrome, periodic limb movement disorder, narcolepsy, and such symptoms as nightmares, poor sleep efficiency, prolonged sleep latency, and early awakening.10,19,31,32
More severe disability after ABI may be linked to more severe dependence and, consequently, with presence of fatigue.8,9,15,33–37
Greater severity of cognitive impairment and of the associated alterations (eg, visuospatial neglect) has been associated with more severe fatigue; however, a systematic review conducted by Lagogianni et al.13 in 2018 found insufficient evidence to either confirm or refute this association. The cognitive impairment associated with fatigue appears to be subjective, according to the results of objective tests, which do reveal an association with attention, memory, and information processing speed.11,38 Other studies have analysed the association between visual alterations (blurred vision, diplopia, and visual field defects) secondary to ABI (50%–75%) and fatigue, due to its impact on cognitive function.39
Some inflammatory markers, such as blood IL-1β level, have been associated with greater risk of post-stroke fatigue.40 On the other hand, such nutrition-related factors as anaemia and vitamin B1, B12, and D deficiency have also been linked to greater risk of fatigue following ABI5,7,9,11; the same is true for such endocrine disorders as TSH deficiency, suggesting a neurohormonal mechanism.41–43
The medications used, both in the subacute stage and during management of brain damage (eg, antihypertensives [beta blockers], antiemetics, anxiolytics, tricyclic antidepressants, corticosteroids, antipsychotics, hypnotics [benzodiazepines], antiepileptics), may cause or exacerbate fatigue.9,28 A study conducted in the United States by Chen et al.44 showed that early post-stroke fatigue is linked to the severity and localisation of the lesion, whereas chronic post-stroke fatigue is more closely associated with comorbidities and medication use.
PathophysiologyThe pathophysiology of fatigue in ABI is unknown. However, as this is a distinct phenomenon occurring in patients with a wide range of neurological diseases, deeper understanding of its pathophysiological basis in conditions unrelated to ABI may shed light on the mechanisms of and potential treatment for fatigue linked to ABI.16
Several trigger factors have been proposed: at the neuronal and neurotransmission level, alterations in cortical excitability; at the neuroanatomical level, localisation of specific lesions; and at the genetic and epigenetic level, alterations in metabolic and inflammatory mechanisms.
From a neuropathophysiological viewpoint, the most widely accepted hypothesis is that post-ABI fatigue is caused by an imbalance between afferent sensory information and demodulated efferent motor information; this results in a perception of excessive, non-attenuated execution, which ultimately triggers a feeling of exhaustion.4,10,40,45
From a neuroanatomical viewpoint, several studies show that the neural damage associated with fatigue is not located exclusively in the brain. Lesions to spinal motor neurons following spinal cord damage or neuromuscular lesions in the peripheral nervous system may alter the balance between afferent and efferent corticosubcortical information, triggering the mechanisms that cause fatigue; this phenomenon is described by some authors as peripheral fatigue.6,46
In turn, the pathogenesis of central fatigue may also be associated with the presence of lesions to specific brain structures, including the ascending reticular formation (ARF), thalamus, hypothalamus, basal nuclei, limbic system, anterior cingulate cortex, parietal cortex, and medial frontal cortex. These structures are involved in such high-order cognitive processes as attention, concentration, response time, and executive function; therefore, these processes are interpreted as mental or cognitive fatigue.11,42,47–51
From a metabolic and inflammatory viewpoint, studies into post-TBI fatigue (especially in patients with severe TBI) show a possible neuroendocrine origin involving secondary hypopituitarism due to both a direct inflammatory lesion and a subsequent autoimmune response. This would cause hormonal alterations that would ultimately promote the persistence of fatigue over time.11,41
On the other hand, several inflammatory mechanisms have been identified that may play a role in the pathogenesis of post-ABI fatigue. Small cohort studies have observed increased levels of IL-6 and IL-1β and decreased levels of IL-1 and IL-9 receptor antagonists in patients with post-stroke fatigue. Other studies have described the mechanisms by which proinflammatory cytokines alter the activity of such neurotransmitters as hypocretin, serotonin, norepinephrine, and dopamine, leading to the formation of neurotoxic metabolites, which in turn increases the risk of neurodegeneration.52–55 Some studies describe a direct correlation between C-reactive protein level and severity of post-stroke fatigue.
Lastly, several small studies have identified genetic polymorphisms that may be involved in the decompensation of immune mechanisms observed in patients with post-stroke fatigue, which suggests more complex molecular mechanisms.5
Post-stroke fatigueStroke is the leading cause of death and disability in many countries. Due to the lack of a universal definition of fatigue, it is not possible to establish the exact prevalence and incidence of this complication.
Patients with stroke may present fatigue within 24–48 hours of hospital admission, resulting in poorer outcomes and greater risk of mortality. Fatigue is usually accompanied by anxiety and depression, and persists even after the resolution of these mood disorders.5,8
Several studies report persistence over time or slight increases in intensity within 2 years of stroke, with mental fatigue being more persistent than physical fatigue.15,21,29,30,56,57
From a demographic viewpoint, post-stroke fatigue follows the pattern of the ABI-related factors mentioned above, and does not seem to be associated with marital status or destination at discharge. It is correlated with poorer cognitive function, regardless of level of education, and lower rates of reintegration to work.5,8,25,56,58,59 Furthermore, persistence of post-stroke fatigue results in poorer adherence to rehabilitation therapy, increasing the risk of disability at discharge.60
Fatigue is more frequent after stroke (especially recurrent and haemorrhagic strokes) than after transient ischaemic attack.61 However, infarct size does not seem to influence the appearance of fatigue, which presents similar frequency and prevalence in patients with minor stroke (NIHSS ≤ 5).15 As mentioned previously, lesion localisation is also associated with the risk of presenting fatigue.62–64
Presence of leukoaraiosis or small-vessel encephalopathy, cardiovascular risk factors, other diseases associated with ABI (eg, epileptic seizures), and other comorbidities (eg, infection, metabolic imbalances) may be associated with higher prevalence of fatigue.40,65
Fatigue following traumatic brain injuryTBI is very frequent in neurorehabilitation units, which explains why it is the second most studied disease, and is associated with high prevalence rates of post-TBI fatigue.34 Although most cases of TBI are mild (80%–95%), with nonspecific symptoms resolving within 3–6 months, some patients present silent residual symptoms, known as post-concussive symptoms (eg, fatigue), which have a negative impact on the patient’s social reintegration.66–71 A study of professional rugby players reported a prevalence rate of 14.1% for fatigue; this was the most frequent symptom, followed by neck pain (11.5%).72 Fatigue is the somatic symptom most frequently reported by patients with TBI,17 and the most severe secondary symptom.73 A review of studies in paediatric populations concluded that the prevalence of post-ABI fatigue in this population ranges from 61.7% to 64.2%.12
In patients with severe TBI, self-reported fatigue is influenced by the lack of awareness of one’s own symptoms as a result of cognitive impairment secondary to diffuse axonal damage, but also by the measurement instrument used and the informant (patient or family/caregiver).
According to several longitudinal studies, 33% of patients with TBI may continue to experience significant fatigue 6 months after the event, even after the resolution of functional symptoms.7,69,73,74 One of these studies reports that up to 73% of patients may present fatigue at 5 years, which has a great impact on quality of life.73 Bushnik et al.75 performed a longitudinal study of patients with post-TBI fatigue, and observed that a large percentage improved within 6–12 months when acute TBI symptoms resolved. Other patients continued to present fatigue, which worsened at 18–24 months, in association with poorer sleep quality and lack of functional or cognitive improvement. Some studies report greater prevalence of (mental) fatigue among patients with moderate or severe TBI in populations of adolescents and young adults.76 However, a systematic review found no association between the prevalence of fatigue and the type or severity of trauma, and identified immediate onset of fatigue after injury as the best predictor of long-term post-TBI fatigue.34 A similar association has been observed with secondary disability, depression, or sleep disorders.73
A recent study conducted in the Netherlands reported post-TBI fatigue as the second most frequent complication in elderly patients (50%), after dizziness (55%) and followed by headache (44%).68 A study conducted in Denmark reported fatigue in 72% of adolescents and young adults with TBI, as compared to 29% in the general population of the same age. Despite conflicting results, fatigue appears to be more frequent among women; individuals who are divorced, separated, or widowed; and individuals with low education level and presenting disability before TBI, with no particular influence of age.14,34,35,77 The prevalence of TBI is higher among individuals with recurrent TBI and carriers of the ApoE4 allele, which is linked to other neurodegenerative diseases.34,78,79
It is well known that patients with TBI may present post-traumatic stress disorder, with hypothalamic–pituitary–adrenal axis dysregulation, a condition with a similar neuropathophysiological basis to that of central fatigue. A study of war veterans conducted in the United States found high prevalence of fatigue among participants with mild TBI and post-traumatic stress disorder; this symptom was found to have an even greater impact on cognition than sleep disorders.53,70
Fatigue in other types of acquired brain injuryThe survival rate of patients with central nervous system tumours has increased to 75%, resulting in a growing body of evidence on the alterations resulting from the disease and its treatment. The prevalence of fatigue in this population ranges from 49% to 97%, a higher rate than in other types of cancer.80 In the paediatric population, the prevalence of fatigue is 66.3%.12
In patients with primary and metastatic brain tumours, a clear association is observed between cognitive impairment and fatigue, on the one hand, and lesion size and localisation (left hemisphere), disease progression, and mood disorders, on the other.
Compared to fractionated radiotherapy, holocranial radiotherapy is more frequently associated with secondary cognitive alterations, difficulties in basic activities of daily living, and fatigue. Post-radiotherapy fatigue may be detected within 24–48 hours of the treatment, with incidence peaking at 30–35 days, and potentially persisting for months.81
The origin of central fatigue in this case may be linked to proinflammatory factors released by the tumour itself, in addition to the other factors previously mentioned. Chemotherapy and radiotherapy have also been found to cause neurological alterations, with ototoxicity, peripheral neuropathy, endocrine dysfunction, and neurocognitive changes, which influence the perception of secondary fatigue (predominantly mental rather than physical fatigue). Lastly, in the paediatric population, fatigue seems to decrease over time once treatment is completed, whereas it increases over time in the adult population (especially in the case of mental fatigue).80,82
Studies into the prevalence of fatigue in other diseases associated with ABI (eg, infectious or autoimmune encephalitis, encephalopathies) are scarce and not representative. We should mention the study by Jonasson et al.,83 who reported similar prevalence rates for fatigue in patients with stroke, TBI, and encephalitis/meningitis. However, another study of a paediatric population reported higher frequency of fatigue in patients with encephalitis and meningitis (around three-quarters of the population) than in those with other types of ABI.12
Diagnostic instrumentsThe most frequently used tools for the study of post-ABI fatigue are clinical scales. The most widely known is the Fatigue Severity Scale (FSS). This scale presents high internal consistency, stability, sensitivity to clinical changes, and correlation with other fatigue scales. It has even been shown to be useful for online surveys.5,6,8,31,84 Around 10 scales are frequently used,40 and evaluate different dimensions of fatigue, which hinders the conceptualisation and differentiation of secondary factors of this complication.10
Tools have been developed to study specific components of fatigue, including neuropsychological tests of attention, information processing speed, and working memory; these domains are affected in patients with mental fatigue.83 Some authors have used specifically designed computer-based tests to evaluate information processing speed and working memory; these include timed tests, with repetition tasks of increasing difficulty. However, none of these tests has been shown to be useful in clinical practice.85
Other instruments, such as use of a pressure dynamometer to assess strength, only quantify the motor component of physical fatigue related to the pyramidal tracts. These tools do not evaluate cognitive-affective function or other extrapyramidal structures.16,19,31
Applications in neuroradiologySome findings of conventional neuroimaging studies may help in diagnosing fatigue. For example, there is evidence of an association between the presence of leukoaraiosis or small-vessel encephalopathy and greater prevalence of fatigue; this may be explained by the greater risk of fatigue in patients with multiple cardiovascular risk factors and other comorbidities.8
Furthermore, from an experimental viewpoint, a statistically significant association has been reported between the volume of grey and white matter lesions in the right hemisphere (frontoparietotemporal network) and post-TBI fatigue.46 Likewise, a bilateral decrease in thalamic volume at the anterior and dorsomedial levels appears to be closely linked to mild post-TBI fatigue.51 Furthermore, diffusion tensor tractography demonstrated changes secondary to TBI in the inferior dorsal (circuits to the intralaminar thalamic nuclei) and ventral portions of the ARF (circuits to the hypothalamus) in patients with fatigue and hypersomnia secondary to TBI-related axonal damage.86
Other studies have used resting-state functional MRI both in diagnostic studies and for treatment follow-up.87,88
Using functional MRI, some research groups have identified a significant association between specific patterns of brain activation and self-reported fatigue. For example, some studies have detected hypoactivation of anterior circuits (prefrontal circuits at the level of the dorsolateral cortex) and their connections with the basal ganglia (frontostriatal circuits) and the thalamus as a result of the imbalance caused by the dysfunction of the AFR and other subcortical structures. A case-control study observed slower information processing speed associated with increased neuronal activity in different brain regions in patients with mild TBI as compared to controls. These findings are consistent with the increased cognitive effort reported by patients with fatigue.8,19,46,85
TreatmentFatigue is a long-lasting syndrome; therefore, early detection and appropriate management of modifiable risk factors are essential. Neurorehabilitation teams must be familiar with and apply the necessary therapies to manage this complication.8,44,89
The efficacy of the pharmacological treatments currently available is limited. In the field of conventional neurorehabilitation, however, positive results have been reported for some non-pharmacological therapies.
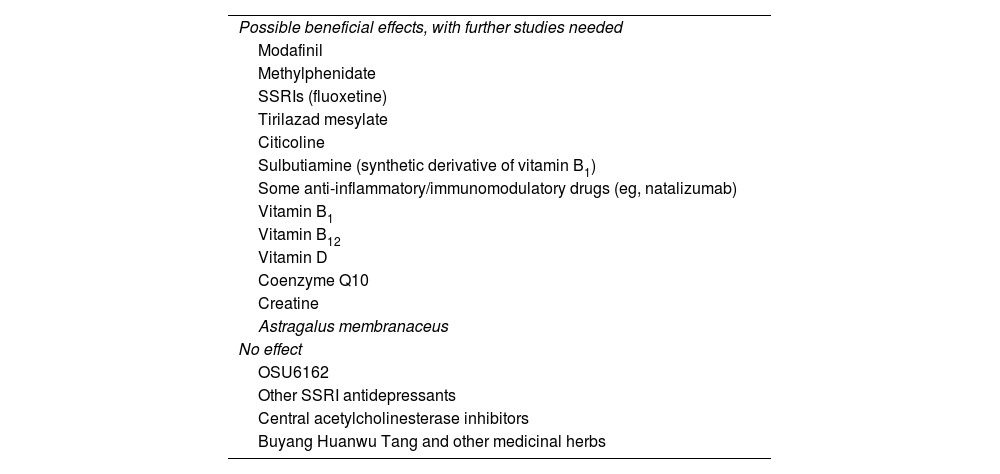
Pharmacological and non-pharmacological interventionsA Cochrane systematic review published in 2015, including 12 randomised clinical trials with a total of 703 patients, identified different types of pharmacological and non-pharmacological treatments for post-stroke fatigue.90 Although some studies reported improvements in fatigue severity, the small samples included and the great heterogeneity between studies underscore the need for further research. Other studies reviewed report no benefits. Table 2 summarises the results of studies into post-ABI fatigue. We will now summarise the most interesting findings for some treatments included in the review.
Drugs and supplements studied for the management of fatigue.
| Possible beneficial effects, with further studies needed |
| Modafinil |
| Methylphenidate |
| SSRIs (fluoxetine) |
| Tirilazad mesylate |
| Citicoline |
| Sulbutiamine (synthetic derivative of vitamin B1) |
| Some anti-inflammatory/immunomodulatory drugs (eg, natalizumab) |
| Vitamin B1 |
| Vitamin B12 |
| Vitamin D |
| Coenzyme Q10 |
| Creatine |
| Astragalus membranaceus |
| No effect |
| OSU6162 |
| Other SSRI antidepressants |
| Central acetylcholinesterase inhibitors |
| Buyang Huanwu Tang and other medicinal herbs |
SSRI: selective serotonin reuptake inhibitor.
This dopamine reuptake inhibitor, which increases the expression of hypocretin, was originally used in patients with hypersomnia/narcolepsy, sleep apnoea/hypopnoea syndrome, and circadian rhythm disorders. The study of modafinil in patients with post-stroke fatigue (200 mg/day for 6 weeks) has shown positive results, although these are dependent on the assessment tool used.5,9,11,31,48,55,88
MethylphenidateMethylphenidate is a psychostimulant that affects both the dopaminergic and the norepinephrine systems. Studies with poor methodological quality have reported positive results in patients with post-ABI fatigue. A study conducted in Sweden, including patients with mild-to-moderate TBI, reported a dose-dependent effect on mental fatigue (5–20 mg/8 h). This effect, associated with improvements in cognitive function, was found to persist over time (at 6 and 22 months).7,69,89,91,92 Used in combination with selective serotonin reuptake inhibitors (SSRI), it has been shown to be useful for the treatment of fatigue associated with cognitive alterations and/or apathy.47 However, in a prospective double-blind study evaluating its efficacy for fatigue prevention at doses of 5–15 mg in patients starting radiotherapy for primary or metastatic brain tumours, no benefits were observed at 8 weeks.28
Other pharmacological and non-pharmacological treatmentsOSU6162 (a partial agonist of both dopamine D2 and serotonin receptors) dosed at 15, 30, or 45 mg every 12 hours has shown promising results for the treatment of post-ABI fatigue.50,69,89
Studies with low statistical power have reported that tirilazad mesylate has neuroprotective effects against fatigue, although only in patients with subarachnoid haemorrhage.90
Other studies propose using targeted anti-inflammatory treatment. Such immunomodulatory drugs as natalizumab administered within 9 hours after stroke have been shown to be beneficial for fatigue and functional and cognitive recovery.55
Small, non-randomised, non-controlled studies of supplementation with vitamin B1, B12, and D and coenzyme Q10 report promising results.5,9
A randomised, prospective, open-label class III pilot study into the neuroprotective effects of 6 months’ treatment with oral creatine in young patients with severe TBI reported marked improvements in fatigue in the intervention group; however, the study has several methodological limitations.7
Regarding alternative therapies, Astragalus membranaceus, a herb used in traditional Chinese medicine to treat stroke, has been shown to be useful for reducing fatigue. Astragaloside IV, a compound occurring in the herb, has been studied as a neuroprotective agent due to its anti-inflammatory and antiapoptotic effects.54 Other studies have analysed the usefulness of Buyang Huanwu Tang, a herbal complex used in East Asia to treat stroke.93
Small studies have been conducted into SSRIs (escitalopram, citalopram, sertraline, and fluoxetine) and dual-action antidepressants (duloxetine), but their effectiveness is unclear.5,8,29,55
Finally, a study into post-TBI fatigue found no benefits with the use of central acetylcholinesterase inhibitors.69
Rehabilitation therapyHospital neurorehabilitation units must ensure regular access to fresh air, a home-like environment allowing quiet rest, and leisure areas.8
Physical activity for functional recovery must avoid strenuous intensity, whereas neuropsychological activity should seek to promote adherence to the intervention, reducing the perception of fatigue and using tools that reduce stress, anxiety, and depression. A daily rehabilitation routine should ideally schedule physical and cognitive activities in the morning, leaving less strenuous activities for the afternoon, with rest always at the same times.32,85
All these factors have been shown to improve fatigue. In order to maintain these improvements after discharge, families and caregivers should receive guidance, and the necessary home adaptations should be made to promote physical activity, proper nutrition, and rest.8,15,20,60,67,94
Regarding physical activity for fatigue prevention, a study conducted in the Netherlands found that up to 51% of young adults with mild TBI presented lower-than-average activity levels for their age.95 This was associated with greater concentration problems and fatigue than in individuals with other types of trauma, which points to lack of physical activity as a therapeutic target.96
In patients with ABI, regular, gentle physical exercise is associated with a decrease in perceived fatigue at all ages in the long term.7,10,95–97
Several studies support the hypothesis that exercise therapy protects brain function, alleviates depressive symptoms, inhibits neuronal apoptosis, decreases neuroinflammation, and promotes neuroplasticity. Functional neuroimaging studies have shown that exercise therapy is associated with activation of the prefrontal lobes, insula, and anterior cingulate cortex. This would explain why physical activity improves attention and indirectly reduces mental fatigue. Physical exercise also increases cerebral blood flow, which in turn activates the sympathetic system; at the molecular level, this modulates the changes in neurotransmission that have been identified in the development of fatigue.8
For post-stroke fatigue, several clinical guidelines recommend performing regular, gentle physical exercise, avoiding sedentary lifestyles, and establishing good sleep patterns.5,9,48
The American Cancer Society and the American Society of Clinical Oncology recommend 5 weekly sessions of 30 minutes of moderate aerobic exercise plus 2–3 sessions of strength training to prevent fatigue.28
A recent study conducted in the United States, including 123 individuals with post-TBI fatigue, found that walking for 30 minutes per day, 5 days per week, seems to be an efficient and cost-effective strategy to improve fatigue, walking performance, mood disorders, sleep disorders, and pain.98
Such activities as yoga, tai chi, and qigong have also been reported to improve fatigue in patients with different types of post-ABI fatigue.7,28
Energy conservation management is a neurorehabilitation strategy that may reduce fatigue in daily life, although a recent analysis found no statistically significant benefits.99
From a neuropsychological viewpoint, patients with post-ABI fatigue usually present cognitive alterations, with hemispatial neglect having a great impact on perceived fatigue; behavioural, mood, and sleep disorders also have a direct impact on rehabilitation.10,38,48
An inverse relationship has been observed between resilience and fatigue in patients with chronic diseases, neurodegenerative diseases, and cancer. Psychotherapy strategies aimed at improving resilience have shown benefits in other diseases, and may also be useful in the management of post-ABI fatigue.36
Cognitive-behavioural therapy has been shown to effectively reduce chronic fatigue syndrome and fatigue associated with such chronic processes as cancer and neurological diseases including multiple sclerosis.28,37 Cognitive-behavioural therapy helps individuals to recognise their cognitive deficiencies, applying behavioural strategies to compensate for the secondary effects of TBI.10,47,48 Several observational studies have analysed the effects of this therapy for the progression of post-TBI fatigue and other sleep and mood disorders, finding minimal benefits.7,100
Another frequently used strategy in this area is mindfulness. Kabat-Zinn’s mindfulness-based stress reduction programme has been studied in the context of post-stroke and post-TBI fatigue, with positive results.8,10,12,15,67,69
Other therapiesAdvances in electrophysiology have led to the development of promising tools for the management of ABI.
A small randomised, controlled study of 10 patients with TBI revealed significant improvements in fatigue, among other symptoms, in the group receiving cranial electrotherapy stimulation.7
Repetitive transcranial magnetic stimulation is frequently used for the management of drug-resistant depression, and there is growing evidence of its usefulness in the treatment of aphasia, upper limb paresis, and neuropathic pain, among other conditions. In the case of post-concussive syndrome following TBI, some studies report positive results both with repetitive transcranial magnetic stimulation and with cranial electrotherapy stimulation.71 Despite insufficient evidence, recent studies report that these techniques are useful for the management of fatigue secondary to different types of ABI.28
Finally, real-time visualisation of brain activity with EEG or evoked potentials (neurofeedback) has been used in some studies for the management of fatigue in patients with ABI. One study evaluated the effect of 25 sessions of EEG biofeedback (Flexyx Neurotherapy System) in 12 patients with moderate to severe TBI who presented symptoms including fatigue, and reported significant improvements with this treatment as compared to the control group.7
Fatigue and sleep alterations are strongly associated with ABI.
Excessive daytime sleepiness secondary to sleep disorders is different from fatigue, but may share some pathophysiological mechanisms, such as impaired homeostasis and circadian rhythm disruption.101 A recent study conducted in Denmark in a population of 71 patients with stroke admitted for inpatient rehabilitation showed significant improvements in fatigue and mood in the group receiving rehabilitation therapy in a room equipped with naturalistic lighting (colour spectrum changing over the course of the day).102
Another study conducted in Australia also reported improvements in fatigue and daytime sleepiness in patients with subacute TBI after short wavelength (blue) light therapy (45 min/day for 4 weeks).103
The United States National Comprehensive Cancer Network recommends light therapy for the management of fatigue and depression in patients with brain tumours.28
Impact on the family, social, and workplace settingsPost-ABI fatigue has been found to cause restrictions in everyday life.
Approximately 20% of stroke survivors are of working age. Between 50% and 74% of stroke survivors return to work, with many requiring workplace adaptations. A qualitative study conducted in Sweden, analysing the work status of stroke survivors 7–8 years after the event, reported “invisible” problems including cognitive issues (difficulty concentrating, processing information, and multitasking) and post-stroke fatigue.104 A one-year follow-up study of 18 patients with TBI showed an association between fatigue and subjective cognitive complaints. These patients also reported tiredness and poor coordination, difficulty making decisions and completing tasks on time, working more slowly, and difficulty following conversations and instructions. Patients’ families/caregivers also identified problems in the family and social spheres.77
In another study conducted in Sweden, a group of patients with TBI (mostly mild TBI) were followed up for 5 years; up to 39% reported chronic mental fatigue, which was correlated with lower level of employment.78 In a study conducted in Australia, a sample of patients with TBI who had been discharged from the emergency department were contacted 3 months after injury; up to 22% reported workplace fatigue and 17% were unable to maintain their previous workload/standards.105
Another study described difficulties returning to the previous social and academic activity in a paediatric population with post-ABI fatigue.12
ConclusionsFatigue is a highly prevalent syndrome in patients with such neurological diseases as multiple sclerosis. Fatigue is also highly prevalent, though less well understood, after ABI. Fatigue seems to be more frequent after stroke and TBI, although it is being increasingly detected in patients with such other conditions as neoplastic brain tumours, encephalopathy, and encephalitis.
Fatigue is defined as a feeling of exhaustion, lack of energy, and aversion to effort that may develop during physical or mental activity and that usually does not decrease with rest. It may be interpreted from a central (cognitive and mental aspects) or a peripheral perspective (motor and physical aspects), as well as from a pathophysiological perspective (central and peripheral nervous system structures). It is caused by alterations in neuronal activity secondary to injury, as well as by genetic and epigenetic changes associated with metabolic and inflammatory mechanisms.
Fatigue persists over time, even after complete or partial recovery from other limitations. It has a negative impact on quality of life, with patients presenting poorer long-term performance than individuals without the syndrome.
At present, fatigue is mainly studied with clinical assessment scales.
There is currently no consensus on the most appropriate pharmacological and non-pharmacological treatments for fatigue; however, several studies recommend a combination of strategies within a neurorehabilitation approach with a view to acting on modifiable factors.
Awareness should be raised about the presence, epidemiology, and pathophysiology of fatigue; this would promote early diagnosis and the use of appropriate treatments, preventing its negative effects in the long term.
Conflicts of interestThe authors have no conflicts of interest to declare.