Virus herpes simplex 1 (HSV-1) encephalitis is the most common and severe type of acute sporadic encephalitis.1 About 90% of the population is seropositive for HSV-1.2 Reactivation of the latent virus on the trigeminal ganglion most frequently manifests as lip or eye eruptions, and rarely as encephalitis. Acute encephalitis is classified as a medical emergency, and this diagnosis should be suspected when the patient presents fever, headache, and neuropsychological alterations; however, clinical manifestations are non-specific.3,4 We present a case of this rare complication in which the patient recovered well despite the late diagnosis and treatment onset.
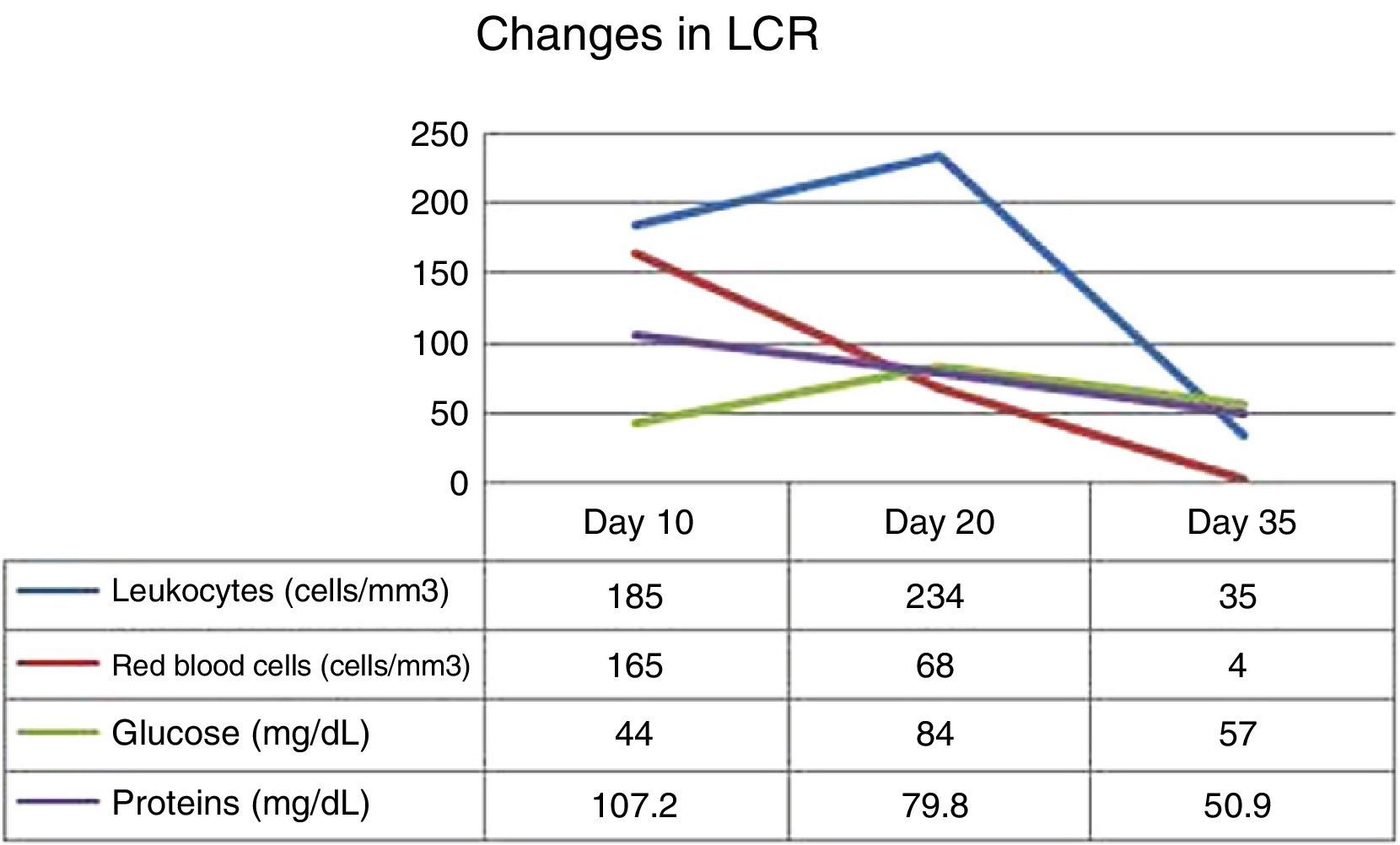
Our patient was an immunocompetent 72-year-old woman with a personal history of arterial hypertension and high cholesterol levels which were treated with diet alone. She visited our hospital due to bitemporal hemianopsia that had progressed over 6 months; a magnetic resonance imaging (MRI) study had diagnosed a meningioma 2.5cm in diameter and located on the sphenoidal tubercle. She underwent surgery to excise the lesion by left fronto-orbital craniectomy. The surgery had no complications. Results of the histological study confirmed diagnosis of conventional mixed meningioma. Five days after the surgery, we observed a cerebrospinal fluid (CSF) fistula caused by a dural tear. A lumbar drain was placed to close the fistula. At 10 days after surgery, our patient reported dizziness, nausea, headache, and disorientation. We also observed low-grade fever. CSF analysis yielded positive results for infection (leukocytes 185cells/mm3, 58% polymorphonuclear; 165red blood cells/mm3; glucose 44mg/dL; proteins 107.2mg/dL). Broad-spectrum antibiotic treatment was started intravenously: vancomycin (1g IV/12h) and meropenem (2g IV/8h), due to the suspicion of postsurgical bacterial meningitis. The lumbar drain was removed 2 days after treatment onset. Laboratory analysis performed 15 days after surgery revealed moderate hyponatraemia, which was attributed to a syndrome of inappropriate antidiuretic hormone secretion. The computed tomography performed the same day showed normal results. Following that time, the patient remained bradypsychic. Twenty days after the surgery, she started to present visual hallucinations and seizures predominantly affecting the left side of the body. Treatment with valproic acid was started and we requested an MRI study and an electroencephalography (EEG).
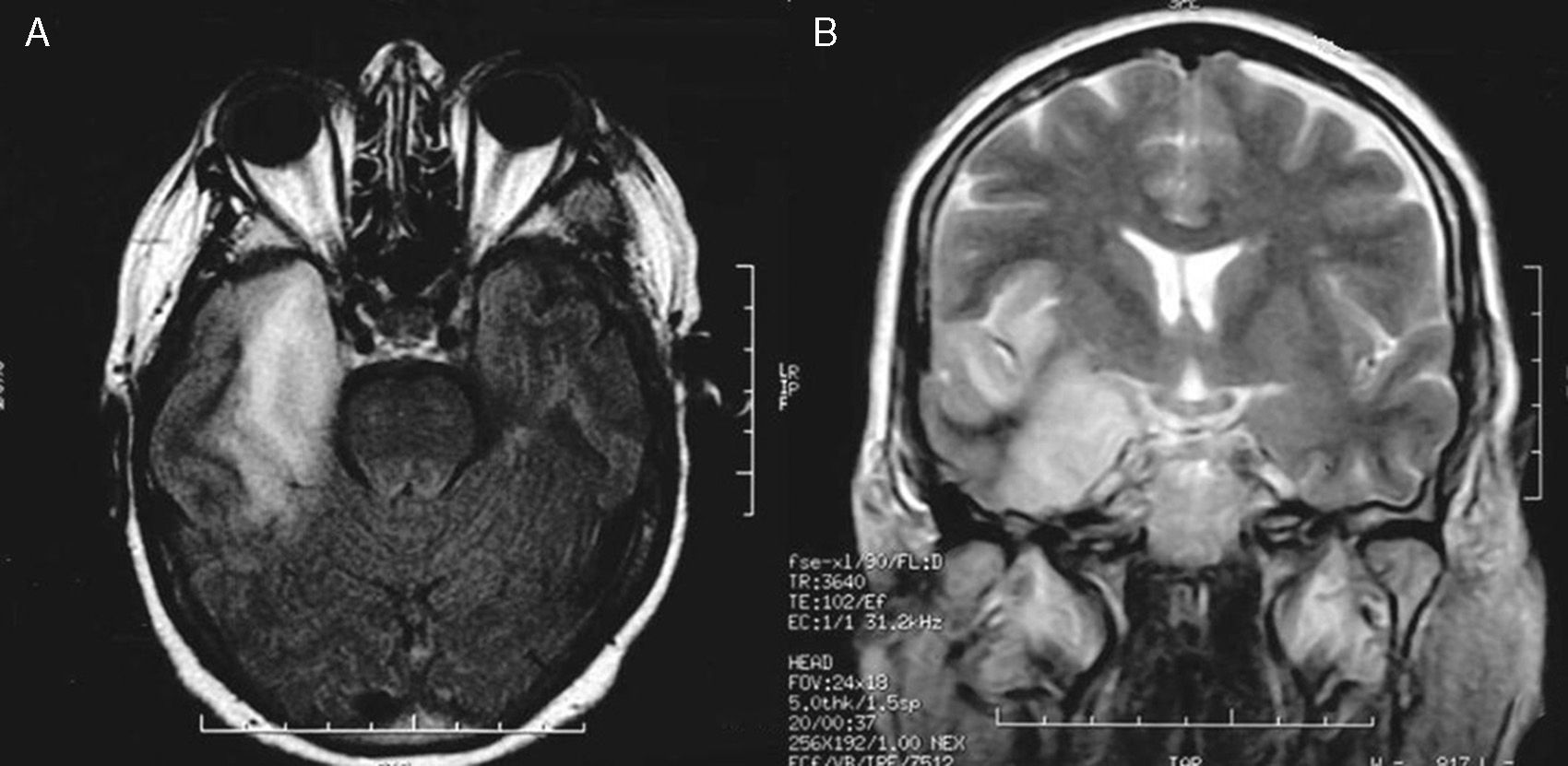
The MRI study showed a right temporal lesion with hyperintensities on T2- and FLAIR weighted sequences, a finding compatible with encephalitis (Fig. 1). The EEG showed slower baseline activity with temporal lobe spikes. We performed a second lumbar puncture yielding a sample that did not indicate bacterial infection due to the increased glucose and decreased protein levels (Fig. 2). On this basis, encephalitis treatment with acyclovir (750mg IV/8h) and dexamethasone (8mg IV/8h) was started. We added levetiracetam to control seizures. The polymerase chain reaction (PCR) test showed positive results for HSV-1 in CSF, which confirmed the diagnosis of herpesviral encephalitis.
MRI resulting in probable diagnosis of encephalitis on the right temporal lobe. Axial T1-weighted and coronal T2-weighted MR images show a hyperintense lesion on the right temporal lobe and insular cortex. The lesion displays the typical pattern of herpes virus encephalitis. MR imaging study was acquired 20 days after resection of the parasellar meningioma.
Treatment with IV acyclovir continued for 21 days. Fever resolved and the patient's level of consciousness improved. In addition, results from the CSF analysis reverted to normal. Mild bradypsychia and behavioral disorders were the only remaining sequelae.
Infections occurring after neurosurgery are most commonly bacterial, and these include meningitis, empyema, and abscess.5 Herpesviral encephalitis is an unusual complication. To the best of our knowledge, only one similar case has been described in the literature.6 Some other cases have been described in association with other tumors: acoustic neuroma,7,8 oligodendroglioma,9 oligoastrocytoma,10 glioblastoma multiforme,11 and relapsing medulloblastoma.12
Diagnosis is important since the condition may result in severe sequelae if not treated early. Ramirez-Bermudez et al.4 described 83 patients diagnosed with viral encephalitis whose main symptoms were neuropsychiatric alterations. Motor agitation, disorientation, drowsiness, visual hallucinations, and aggressiveness were the most frequent symptoms. Other authors, such as Boyapati et al.13 also described such neuropsychological symptoms as psychosis and delirium. Median time to symptom onset after surgery is 6 days,9 which together with the non-specific symptoms in encephalitis can resemble the most typical postoperative complications. The development of a viral PCR test has made lumbar puncture the most important diagnostic tool.14 However, we should not forget that CSF analysis can sometimes provide false positives, since craniectomy frequently causes alterations such as monocyte-predominant aseptic meningitis.1
The mortality rate of untreated HSV-1 encephalitis reaches 70%, and among survivors, less than 3% will show no sequelae.4,15 Early treatment with IV acyclovir reduces mortality to 30%.5 Ramirez-Bermudez et al.4 described memory changes in up to 22% of the patients with viral encephalitis and found that neuropsychiatric complications (memory changes and language disorders) were more frequent than neurological disorders. Furthermore, certain patients experience a clear life-threatening increase in intracranial pressure, which may have to be treated with decompressive craniectomy.6
Our patient presented a more insidious clinical progression than that described in the literature, since low-grade fever started 10 days after surgery and never reached 38°C. Twenty days later, she started to present more obvious symptoms, which helped establish a suspected diagnosis. Although treatment with acyclovir at high doses was not started until 20 days after surgery, the patient progressed favorably. The only sequelae that remained were bradypsychia and a slight tendency toward mutism. These findings resembled those described in the literature.4 Subsequent measurements of PCR in CSF confirmed the diagnosis.
According to the latest published articles,1,3,13 herpes virus encephalitis after neurosurgical procedures is a rare complication, but it must be taken into account if there is clinical suspicion since early treatment with IV acyclovir may prevent fatal complications. Considering that there is no evidence of the benefits of acyclovir administered prophylactically, further studies are needed to assess this indication in patients with a history of herpes virus encephalitis or those undergoing intracranial surgery at locations where the virus can persist or disseminate.
Please cite this article as: Álvarez de Eulate-Beramendi S, Santirso-Rodríguez D, Piña-Batista KM, Gutiérrez-Morales JC. Encefalitis por virus herpes simple 1 tras extirpación de meningioma. Neurología. 2015;30:455–457.