Since the relationship between chewing and cognitive functions has not been fully elucidated, this study aimed to determine the impact of chewing behaviour on spatial learning and memory in albino male BALB/c mice.
MethodsTwenty mice aged 8 weeks were divided into 2 equal groups. The regular chewing group was fed with uncrushed grains (the same diet given to all 20 mice since they were weaned) and the limited chewing group was fed with crushed grains. At 16 weeks of age, the mice were evaluated over 5 days, including a 4-day acquisition phase prior to a probe test of spatial learning and memory in the Morris water maze on the fifth day.
ResultsA comparison of the regular chewing group and the limited chewing group found no significant differences in either the acquisition phase or the probe test. However, there were significant differences in the acquisition phase for just the regular chewing group when comparing results from the first day to those from the other 3 days.
ConclusionsThe results suggest that regular chewing affects spatial learning and memory since mice in the regular chewing group decreased their times to find the hidden platform during the acquisition phase.
Debido a que la relación entre la masticación y las funciones cognitivas no está todavía completamente elucidada, se tuvo como objetivo determinar la influencia del tipo de masticación sobre la memoria y el aprendizaje espacial en ratones albinos machos de la cepa BALB/c.
MétodosVeinte ratones de 8 semanas de edad fueron divididos en 2 grupos iguales; el grupo masticación normal fue mantenido con alimentación en granos, la misma que recibieron los 20 ratones desde el destete; el grupo masticación deficiente se alimentó con granos pulverizados. Se evaluó a los ratones a las 16 semanas de edad durante 5 días, los primeros 4 días en la fase de adquisición y el quinto día en la fase de recuperación de memoria y aprendizaje espacial en el laberinto acuático de Morris.
ResultadosTanto en la fase de adquisición como de recuperación, no se hallaron diferencias significativas en los grupos masticación normal vs. masticación deficiente. Sin embargo, las diferencias sí fueron significativas en la fase de adquisición del grupo masticación normal, comparando los resultados del primer día vs. los otros 3 días.
ConclusionesEstos resultados indican que la masticación normal influye sobre la memoria y el aprendizaje espacial, al disminuir los tiempos de latencia en encontrar la plataforma oculta en la fase de adquisición dentro de dicho grupo experimental.
The cognitive functions of memory and learning are undeniably important for life processes. In humans, loss of these functions has a considerable social and economic impact on patients, their families, healthcare systems, and society at large.1,2 Cognitive impairment has been shown to arise from multiple causes. Some, such as ageing, are clearly understood; other less obvious causes of impaired memory and learning include masticatory deprivation.1–4
Chewing is classically regarded as the initial process in physiological digestion; however, we now believe that this function may also affect psychological, physical, and cognitive activities.1,3,5 Ample evidence has confirmed that the most important brain structure involved in the cognitive functions of memory and spatial learning is the hippocampus. Furthermore, normal mastication is reported to transmit an enormous amount of sensory information related to the maintenance of those functions to the central nervous system, and these afferent pathways are primarily directed to the hippocampal area.1,4–6 Studies in both humans and experimental animals have shown that impaired memory and spatial learning may be linked to masticatory deficiency.1,4,7–9
We understand that the cognitive functions of learning and memory are crucial for human and animal survival, and that these studies appear to indicate that appropriate chewing is one of the factors promoting maintenance of optimal cognitive processes. This study presents the first of a series of experiments whose main purpose is to determine the influence of chewing patterns on memory and spatial learning in male albino BALB/c mice tested with the Morris water navigation task.
Materials and methodsAnimalsSubjects were 20 male BALB/c mice acquired at the age of 8 weeks from the laboratory animal breeding facility at the National Institute of Health in Lima, Peru. Following weaning at 21 days, mice had been fed a conventional diet (commercial mouse chow). Once acquired, the animals were housed in the vivarium pertaining to the Faculty of Medicine, Universidad Nacional Mayor de San Marcos. Food and water were provided ad libitum, the light/dark cycle was 12:12, and humidity and temperature (22±2°C) were kept within the parameters stipulated in the Guide for the care and use of laboratory animals.10 After a one-week habituation period, the 20 mice, now 9 weeks old, were randomly divided into 2 experimental groups of 10 mice each. The 2 experimental groups were given different types of diet to elicit different chewing patterns. The normal mastication group continued with the same chow in pellet form; the reduced mastication group was fed crushed chow. Animals were assessed in the Morris water maze at the age of 16 weeks.
Testing deviceOur Morris water maze is similar in design to those described by Dhingra et al.11 and Kitanaka et al.12 It is a circular plastic tank measuring 60cm in diameter and 30cm deep with 4 marked quadrants. It was placed in the same location on all days of testing, in a room measuring 6m×4m with distinctive features on each of its walls. The Morris water maze was filled up to the 25cm level; innocuous dark dye was then added to the water, which was maintained at a temperature of 22±2°C. A black plastic platform rising to 1cm below the water level was placed in the tank. The platform had a surface area of 6cm×6cm and was always positioned within the same quadrant of the maze.
ProcedureThe protocol was approved by the Research Ethics Committee at the Faculty of Medicine, Universidad Nacional Mayor de San Marcos (project code 0288). Animals were evaluated in the memory/spatial learning acquisition and recall phases between the hours of 8.00 and 12.00. The acquisition phase lasted 4 days, during which every mouse completed 4 trials daily separated by 30-second rest periods. Every day, a starting point was chosen at random from among the 4 quadrants of the maze. Each trial consisted of placing the mouse facing the central part of the wall belonging to any of the 4 quadrants, releasing it, and letting it swim freely; the trial concluded when the animal came to rest on the hidden platform for at least 15seconds (escape latency in the acquisition phase). Mice were allowed to swim freely a maximum of 60seconds; if the mouse did not find the platform during this time, it was guided by the researcher and kept on the platform for 15seconds. In such cases, the escape latency was given as 90seconds. In this phase, shorter latencies to find the hidden platform were understood to be linked to better memory and spatial learning. The fifth day was set apart as the recall phase for memory and spatial learning. The single test in this phase differed from the ones previously described; in this case, researchers removed the platform before releasing each mouse from the quadrant opposite to where the platform had been. The trial lasted 60seconds, during which researchers recorded time spent swimming in the target quadrant (where the platform had been located) and time between release and arrival at the specific area where the platform had been (latency in the recall phase). In this phase, we understand longer times spent swimming in the target quadrant, and shorter latency times (out of the total 60seconds), to indicate better memory and spatial learning. All trials were filmed and subsequently processed using EthoVision XT 11.5 software by Noldus; results were gathered from processed recordings.
Data analysisStatistical analysis was conducted using SPSS version 23. Results are expressed as mean±standard deviation. Shapiro–Wilk and Kolmogorov–Smirnov tests were used to determine normal distribution. Given that data displayed a normal distribution and homoscedasticity, we used the t-test for both independent and related samples in the data collection phase. Since data from the recall phase were not homoscedastic, they were analysed using the non-parametric Mann–Whitney U test. P<.05 was considered statistically significant.
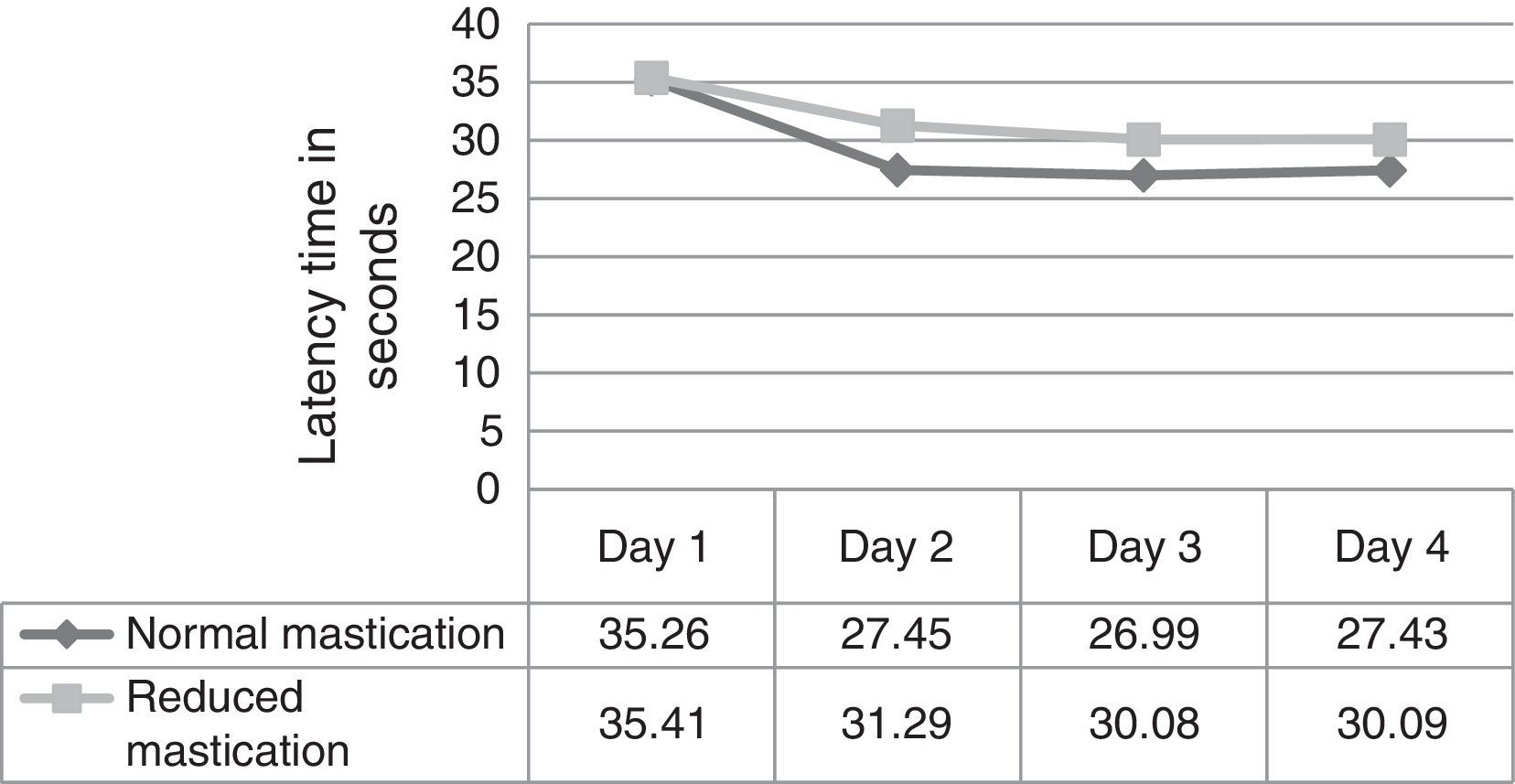
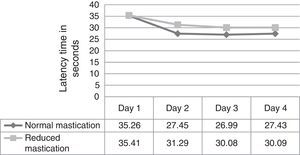
ResultsFig. 1 corresponds to the acquisition phase and shows each mouse's escape latency times (the time required to find the hidden platform); means were compared between the 2 groups for each of the 4 days of trials in this phase. Results did not show significant differences (day 1, P=.973; day 2, P=.238; day 3, P=.267; day 4, P=.317).
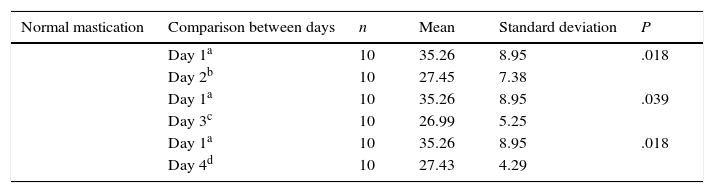
We evaluated latency times during the 4 days of trials in the acquisition phase and observed that the normal mastication group (Table 1) showed significant differences in latency time between days 1 and 2, and between days 3 and 4. In contrast, differences in latency time for other days within the same group, and differences within the reduced mastication group, were not statistically significant (P<.05).
Comparison of escape latency between day 1 and all other days in the acquisition phase for the normal mastication group.
| Normal mastication | Comparison between days | n | Mean | Standard deviation | P |
|---|---|---|---|---|---|
| Day 1a | 10 | 35.26 | 8.95 | .018 | |
| Day 2b | 10 | 27.45 | 7.38 | ||
| Day 1a | 10 | 35.26 | 8.95 | .039 | |
| Day 3c | 10 | 26.99 | 5.25 | ||
| Day 1a | 10 | 35.26 | 8.95 | .018 | |
| Day 4d | 10 | 27.43 | 4.29 |
a–d Different superscript letters indicate statistically significant results.
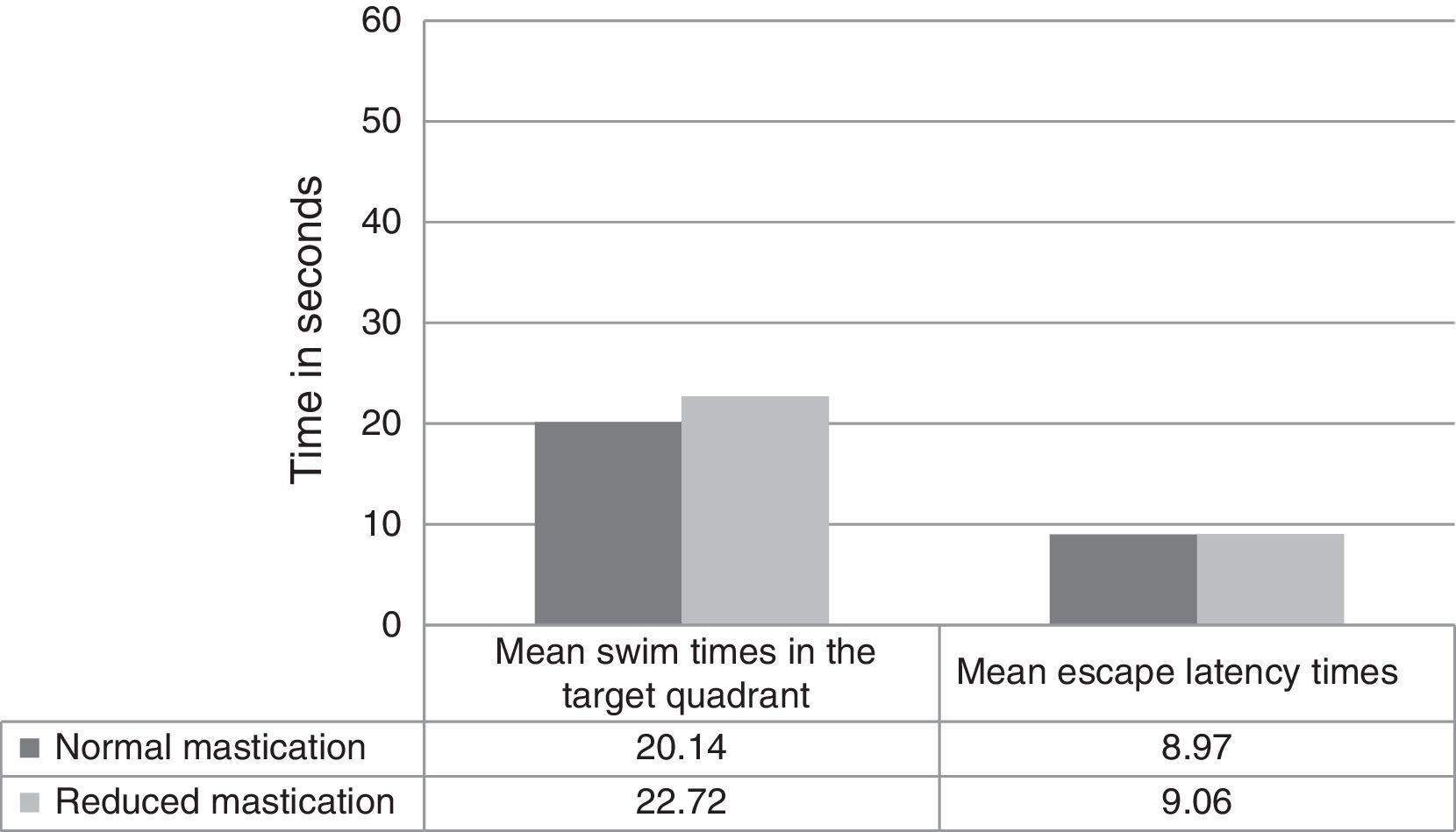
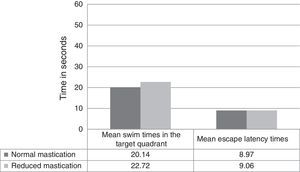
Regarding differences in recall-phase data between the normal and reduced mastication groups, Fig. 2 shows swim times in the target quadrant out of the total of 60seconds per trial (20.14±3.38 normal vs 22.72±6.09 reduced, P=.326) and the latency time in the recall phase (8.97±3.49 normal vs 9.06±3.85 reduced, P=.910). These differences were not statistically significant.
DiscussionThe concept of normal mastication refers to an efficient masticatory cycle that depends on the activity and strength of the muscles of mastication, mandibular movements, dentition, and the synchronicity and coordination of the tongue, cheeks, and lips. The time dedicated to this process determines its effectiveness, and this time may be conditioned by the consistency of available foodstuffs. On the other hand, reduced mastication as a concept refers to anomalies in the components described above, resulting in loss of an efficient masticatory cycle.13 Said loss will provide insufficient neural stimulation to the stomatognathic system, and lastly, from that system to the central nervous system (CNS).14 This being the case, rodents with reduced mastication have been found to demonstrate decreased memory and learning ability, which in turn elicits degenerative changes and abnormalities in the periodontal mechanoreceptors that indicate a drastic decrease in trigeminal afferents extending from the periodontal membrane to the CNS.6
Other researchers have also examined the influence of chewing behaviour on memory and spatial learning in mice. Animals in this study were divided into 6 groups in which 3 were fed with mouse chow pellets and 3 with crushed chow; 2 groups representing each of the diets were tested in the Morris water maze at 3, 6, and 18 months. Researchers observed significantly better memory and spatial learning among 6-month-old mice fed with pellets than in the corresponding group fed with crushed chow.4 In another study, the effect of chewing gum on the cognitive function of 2 equal groups of university graduates was tested by having subjects in one group chew gum for 5minutes prior to cognitive testing, while the other group did not chew gum. The gum-chewing group performed better than the control group on the test battery employed in this study.15 Similarly, the effect of gum-chewing on spatial tasks was examined in 100 subjects divided into 2 equal groups and evaluated on tasks selected from the Endless Loops Test (ELT). Participants in the experimental group were given sugar-free gum immediately before taking the ELT and asked to chew actively during the test; the control group did not chew gum. This study did not find that chewing gum improved performance on the spatial tasks evaluated by the ELT.16 The effects of soft-diet feeding on the expression of brain-derived neurotrophic factor (BDNF) in the murine hippocampus have also been studied. Here, mice were divided into 6 groups in which 3 were fed a hard diet (pellets) and 3 a soft diet (crushed chow). Mice from 2 groups representing each type of diet were killed at the ages of 1, 3, and 6 months. Soft diet feeding resulted in a significant decrease in BDNF in the hippocampus in the mice aged 3 and 6 months, which corresponded to a lower number of synaptic connections.17 The same research group examined the effect of soft diet feeding on neurogenesis in the hippocampus of albino mice. Animals were divided into soft-diet and hard-diet groups. Two groups representing each diet were evaluated when mice were 3 and 6 months old.
Mice fed a soft diet exhibited a significant reduction in the number of neurons in the dentate gyrus at 3 months and 6 months of age; this finding corresponded to lower levels of neurogenesis in the hippocampus than in mice fed a hard diet.8 Still other studies have reported that consuming chewing gum or sweets does not increase performance on cognitive tasks, and that changing from chewing to sucking motions during cognitive activity has no positive or negative impact on recall.18,19
In this study, latency times in the acquisition phase were shorter in the normal mastication group than in the reduced mastication group beginning on day 2. Results in the recall phase were very similar between groups. Considering the statistical tests that were applied, evidence is not sufficient to show that the differences observed in the acquisition phase would be due to the type of chewing behaviour. These results are similar to those reported by Frota de Almeida et al.4 who used a Morris water maze to test mice who at 3 months were similar in age to those in our study. As occurs in humans, these young animals are in a stage of growth and development in which they are acquiring their maximum physiological abilities. It is understood that reduced mastication is only one factor out of all of those able to alter the proper function of memory and learning; young animals have other physiological resources that influence these cognitive functions and may compensate for the effects of reduced mastication. Nevertheless, the presence of significant differences between results from day one and later trials in the normal mastication group may indicate that the neural stimulation provided over time by normal mastication (pellet-based diet) is very important for mice to be able to learn a spatial task quickly when trials are repeated over several consecutive days.
We should point out that all of the animal studies described in the preceding paragraphs used the approach of introducing dietary changes soon after the rodents were weaned and evaluating them over time. The novelty in our study is that the change of diet (in the reduced mastication group) took place when the animals were 9 weeks old, several weeks after weaning. This approach attempts to simulate what occurs in humans who experience the changes that lead to reduced mastication in later stages of life, and not when teeth begin to erupt. Lastly, it would be useful to study the same variables in animals belonging to different age groups after applying the modified methodology highlighted in the previous paragraph. The purpose of such a study would be to continue exploring the association between the variables in question so as to apply resulting knowledge to improving human quality of life; deterioration of memory and learning is an important indicator in such processes as mild cognitive impairment or Alzheimer disease, and anomalies in the chewing function are easy to correct in most cases.
FundingThis study has been completed using the authors’ own resources. The study was presented in poster format at the IX International Research Congress held by the National Health Institute of Lima, Peru.
Conflicts of interestThe author has no conflicts of interest to declare with regard to this study.
We would like to thank Reynaldo Madrid, César Franco Quino, and Eliberto Ruiz Ramirez.
Please cite this article as: Aguirre Siancas EE. Influencia del tipo de masticación sobre la memoria y el aprendizaje espacial en ratones albinos de la cepa BALB/c. Neurología. 2017;32:236–240.