Pupil assessment is a fundamental part of the neurological examination. Size and reactivity to light of each pupil should be recorded periodically since changes in these parameters may represent the only detectable sign of neurological deterioration in some patients. However, there is great intraobserver and interobserver variability in pupil examination due to the influence of many factors, such as the difference in ambient lighting, the visual acuity and experience of the examiner, the intensity of the luminous stimulus, and the method used to direct this stimulus.
In recent years, digital cameras have incorporated infrared devices allowing the development of user-friendly portable devices that permit repeated, non-invasive examinations of pupil size and its reactivity to light with an objective, accessible and inexpensive method.
DevelopmentThe purpose of this review is to describe the fundamentals of infrared pupillometry and discuss potential applications in the monitoring of neurocritical patients. We also present some recommendations in the routine assessment of pupils in neurocritical patients.
ConclusionsThe possibility of evaluating the changes in pupil reactivity in an early, objective and almost continuous way provides a new non-invasive monitoring method. This method could improve the predictive factor of neurological deterioration and the bedside monitoring of the neurological state of the patient, avoiding unnecessary examinations and enabling early therapeutic intervention.
La exploración de las pupilas constituye una parte fundamental de la exploración neurológica. El tamaño y la reactividad a la luz de ambas pupilas deben registrarse de forma individual y periódica, dado que una alteración en estos parámetros puede constituir el único signo detectable de deterioro neurológico en determinados pacientes. Sin embargo, existe una gran variabilidad intra-observadores e inter-observadores al analizar estos parámetros, debido a la influencia de una serie de factores tales como la variabilidad en la luz ambiental, la agudeza visual y experiencia del propio observador, intensidad del estímulo luminoso y el método utilizado para dirigir este estímulo.
En los últimos años las cámaras digitales han incorporado dispositivos de infrarrojos que han permitido desarrollar equipos digitales portátiles y de fácil manejo, que permiten realizar estudios repetidos y no invasivos del tamaño y reactividad pupilar a la luz con un método objetivo, accesible y económico.
DesarrolloEl objetivo de esta revisión es describir los fundamentos de la pupilometría por infrarrojos y discutir sus posibles aplicaciones en la monitorización del paciente neurocrítico. En esta revisión también se incluyen una serie de recomendaciones metodológicas a considerar en la exploración de las pupilas.
ConclusionesLa posibilidad de evaluar los cambios en la reactividad pupilar de forma precoz, objetiva y quasi-continua aporta un nuevo método de monitorización no invasivo que podría mejorar el factor predictivo del deterioro neurológico y monitorizar el estado neurológico a la cabecera del paciente, evitando así exámenes innecesarios y permitiendo intervenciones terapéuticas precoces.
Pupil assessment is a fundamental part of the neurological examination process. In spite of technological advances and substantial progress in our understanding of central nervous system (CNS) pathophysiology, routine pupil examination with a conventional light source has undergone no significant changes in the last 100 years. Pupillary examination involves recording the size, symmetry, and light reactivity of both pupils. Nevertheless, analysis of these parameters is affected by significant interobserver variability due to the influence of factors such as differences in ambient lighting, the examiner's own visual acuity and experience, the intensity of the light stimulus, and the method used to direct that stimulus.
Different diagrams or schemas are employed in order to reduce interobserver variability, and these are usually included in the patient's chart. However, in spite of using methods such as comparison of Rosenbaum card pupillometry,1 subjective assessments of pupil diameter may lead to errors when used to measure pupils in both healthy patients2 and in those meeting brain-death criteria or under the effects of anaesthetic agents, or when doctors are determining the severity of head trauma (HT).4 This probably explains why testing pupillary reaction to light is generally considered a late-stage marker with a low sensitivity to neurological deterioration.5 Clinical practice guidelines issued by the Brain Trauma Foundation (BTF) recognise that pupil size and reaction to light are early indicators of prognosis for patients with severe HT. However, these guidelines also include the following statement: “Accurate measurement of pupil diameter or the constrictor response or the duration of the response has not been performed in studies on traumatic brain-injured individuals—for lack of a standardised measuring procedure”.3
Over the last few years, infrared devices included in digital cameras have led to the development of portable easy-to-use digital systems which enable researchers to carry out non-invasive repeatable studies of pupil size and light reactivity using an objective, accessible, and cost-efficient method. The availability of these devices gives rise to a host of potential uses for them, such as predicting neurological deterioration (neuroworsening) in certain patients, assessing the depth of anaesthesia, and monitoring the effects of different drugs. Pupillary examination with the help of such devices allows professionals to distinguish Horner's syndrome from physiological anisocoria, obtain new information about the physiology and pathophysiology of the pupillary response, and observe the result of bedside procedures. These devices may be used in the future as an additional tool for non-invasive neuromonitoring in severely brain-injured patients.6
The aim of this review is to describe the fundamentals of infrared pupillometry (IP) and discuss its potential uses for monitoring severely brain-injured patients.
Basic concepts and pathophysiology of pupillary responsePupillary size and light reflex have traditionally been used as a validated clinical parameter for detecting cerebral herniation, especially central transtentorial and uncal herniation. Furthermore, pupillary abnormalities help detect expansive processes and are indicators of prognosis in patients with severe head injuries or requiring cardiopulmonary resuscitation.3,7–14 Pupillary response is also the most important clinical sign of a structural or metabolic coma, which explains why correct pupillary assessment is such a crucial step.
From conventional light stimulus to infrared pupillometryStandard pupillary examination consists of recording the pupil's baseline condition and exposing the pupil to a source of light intense enough to trigger a direct photomotor reflex and a consensus response.15 In the case of severely brain-injured and comatose patients, pupillary examination is characterised by a response to light which is slower and somewhat less marked than a normal response. Pupillary examination should therefore be carried out with low ambient lighting and a good light source (for example, a halogen lamp). Exposure to light should last at least 10seconds, with both of the patient's eyes remaining open. Use of a magnifying ophthalmoscope is recommended in difficult cases as it provides a magnified image of the pupils. Nevertheless, pupillary response is difficult to describe in some cases. For that reason, using alternative systems permitting more objective examination of the pupils may be necessary.
In 1800, the German scientist Frederick William Herschel discovered what were once called ‘calorific rays’ while working with glass prisms. Herschel's calorific rays are currently known as ‘infrared radiation’. Wavelengths of infrared radiation are longer than those of visible light (380–780nm), but shorter than those of radio waves. The word “infrared” comes from the Latin infra (below) and the colour red, which is at the edge of the visible light spectrum. Since infrared was first described, and especially since the Second World War, advances in this type of technology have been considerable. Military use of the properties of infrared light was a key factor in those advances. The military developed night-vision systems which emitted low-luminosity or invisible radiation. Such systems were later transformed into cathode ray tubes which allowed images to be viewed on a screen.
Use of this technology for pupillary examination dates back to the 1950s. In 1958, Lowenstein and Loewenfeld published the first study on pupillary imaging with the help of an infrared camera. They highlighted the possibility of employing their technique for pharmacological and physiological research and as a general screening method.16 At present, there are numerous clinical applications of IP, including ophthalmological research (pupil diameter measurement in refractive surgery, assessment of visual function) sleep medicine, testing the effects of drugs on the autonomic nervous system, assessment of autonomic neuropathies,17–19 and recently, assessment of severely brain-injured patients.20–23
Alterations of the photomotor reflex and causes of pathological pupil dilationCranial nerve III is closely linked to the medial part of the temporal lobe (uncus of the hippocampus) and to areas of the brainstem which control consciousness. Therefore, any lesion in the midbrain or in the efferent fibres forming cranial nerve III, resulting from compression of the temporal lobe or adjacent structures, causes pupil dilation which is usually ipsilateral to the lesion. However, there are recorded cases of paradoxical dilation of the contralateral pupil, which is a false localising sign.24–26 When compression of cranial nerve III is significant, the pupil partially or completely loses its reactivity to light and subsequently dilates. Nevertheless, we should not forget that loss of pupillary response to light is not always related to compression of cranial nerve III. Hypoperfusion of brainstem structures may also evoke these kinds of changes.27
This stereotypical progressive pattern of changes in pupil size and light reactivity seems to indicate that early detection of such anomalies is very important in order to assess clinical progress in severely brain-injured patients.10,11,28 In theory, lower reactivity to light may indicate compromised function of cranial nerve II or III. In the latter case, it would be the first sign of compression of vital structures. Early detection of pupillary abnormalities as a sign of neuroworsening may help reduce the time interval during which compression is present and irreversible brain injury takes place. Early detection could therefore be associated with better clinical progress and facilitate starting treatment.29
Drugs and specific treatment methods affecting pupillary functionIn severely brain-injured patients who are sedated and under the influence of multiple drugs, it is important to consider the impact of those drugs on pupillary response. For example, drugs that block muscarinic receptors in the iris, such as atropine, increase response latency and reduce contraction amplitude, whereas drugs that decrease the impulses of the sympathetic nervous system (such as clonidine) reduce the dilation velocity of the pupillary sphincter.25,30,31 However, for many substances commonly administered to these patients, it is difficult to determine which arm of the autonomic nervous system is affected the most. This review lists the drugs, clinical conditions, and treatment methods which are most commonly associated with severely brain-injured patients and may modify pupillary size and response.
HypothermiaUsing an infrared pupillometer, Larson et al. assessed pupillary response in patients in a state of moderate hypothermia (32.2±0.5°C) with or without general anaesthesia with isoflurane. They observed that moderate hypothermia does not modify pupillary reflex, whereas use of anaesthetics compromises pupillary response in a dose-dependent manner by up to 80%.32 However, deep hypothermia (<28°C) may abolish pupillary and brainstem reflexes.6,15,33–36
HyperthermiaHyperthermia induced in healthy volunteers, whether or not it was associated with inhalation anaesthetics (isoflurane, enflurane, and nitric oxide), significantly increases pupillary diameter and percentage of constriction (difference between the maximum and minimum diameter). This partially reverts the inhibition of the photomotor reflex induced by anaesthetics. The 3 inhalation anaesthetics used by these researchers significantly decreased both parameters but did not abolish the photomotor reflex at doses commonly used in clinical practice.37
Muscle relaxantsGray et al. assessed the effect of vecuronium and pancuronium on pupillary response at a dose evoking complete block of muscle response to peripheral stimulation. Their pupillometry study showed no changes in patients’ pupillary response.32
Barbiturates and benzodiazepinesLowenstein et al. showed that high doses of barbiturates for the treatment of status epilepticus completely abolish brainstem reflexes except for the photomotor reflex.38 However, others have described cases in which pupillary reflexes were absent and the patient appeared to be brain dead.39,40 In a recent study, Taylor et al. used IP to show that use of barbiturates modifies pupillary function parameters until they reach ranges considered to be pathological and a burst-suppression pattern appears in the patient's electroencephalogram.22 Although use of propofol and midazolam significantly reduced pupillary response, pathological ranges were either not reached or the effects caused by these drugs were short-lived. In our experience, patients with intracranial hypertension treated with barbiturates frequently experience an increase in pupil size and a decreased pupillary response to light stimulation.
Vasoactive drugsHigh doses of dopamine (α-1 adrenergic agonist) may cause non-reactive mydriasis during general anaesthesia.41 It has been observed that patients with phaeochromocytoma present anomalous pupillary responses similar to those in brain death. This is probably due to adrenergic hyperstimulation of the iris.42
AntidepressantsSome researchers show an interest in the effects of tricyclic antidepressants on the kinetics of the pupillary light reflex. It is a well-known fact that these drugs affect both the sympathetic and the parasympathetic response. The most common effect of tricyclic antidepressants is enhancement of the sympathetic response by blocking noradrenaline reuptake in the synapse (α-2 receptors) and inhibition of the parasympathetic response through a postsynaptic muscarinic blockade.43 Researchers have observed that use of therapeutic doses of desipramine and reboxetine increases latency time and reduces contraction amplitude and pupillary dilation time.43
To summarise, we can affirm that barbiturates, high doses of tricyclic antidepressants, and profound hypothermia (<28°) are the most frequent causes of altered pupillary response and increased pupil size, even to the point that alterations may resemble indicators of brain death.41,44–48
Definitions and methodological recommendations for assessing photomotor reflexAssessment of pupil size and photomotor reflex is part of routine care for all neurological patients including those with severe brain injuries. In such patients it is important to perform a standardised evaluation of pupil size and response to light so as to assess the patient properly and compare results with those in the literature. The clinical practice guidelines recently published by the BTF for the treatment of patients with HT currently provide the clearest normative data. The BTF's definitions and recommendations for pupillary examination are as follows3:
- 1.
Pupillary assessment should always be carried out after cardiopulmonary resuscitation.
- 2.
Prior to pupil assessment, doctors should correct any hypotension or hypoxia and rule out the possibility of direct ocular trauma.
- 3.
Pupils should be reassessed after surgical evacuation of intracranial haematomas.
- 4.
Photomotor reflex should be used as a prognostic indicator, since its absence in both eyes has a positive predictive value of more than 70% (class I) for a poor neurological result (death, vegetative state, or severe disability).
- 5.
A mydriatic pupil is defined as one having a diameter exceeding 4mm.
- 6.
A fixed or non-reactive pupil is defined as one that does not present a constrictor response to bright light.
- 7.
The laterality of an abnormal pupil should be reported.
- 8.
Pathologic anisocoria is defined as pupillary inequality >1mm.
We should stress that definitions of non-reactive pupil and pathologic anisocoria are based on direct observation of the pupil and its photomotor reflex. Therefore, use of the automated pupillary assessment procedures mentioned above could modify these definitions. In a recent study aiming to establish normal ranges for pupil response in paediatric patients using an automated pupillometer, the authors detected that most of the children presented a baseline pupillary asymmetry which did not exceed 0.5mm.49 Use of automated devices would also permit the inclusion of other pupillary examination parameters that cannot be measured using direct observation and which are listed below.
Pupillary latency time, expressed in milliseconds, is defined as the time between exposing the pupil to a light stimulus and the onset of iris constriction.
Pupil constriction velocity refers to the decrease in pupil diameter in millimetres as a function of the elapsed time required for the pupil to reach its minimum diameter from its maximum diameter.
Amplitude of pupillary constriction refers to the difference between maximum pupil diameter before light stimulation and minimum pupil diameter after light stimulation. Pupil dilation velocity, also expressed in millimetres per second, refers to the recovery of baseline pupil diameter over time.
Percentage of decrease in pupil diameter is defined as the difference in pupil diameter after light stimulation expressed as a percentage.
It is important to recall that latency time and amplitude of pupillary constriction are conditioned by the parasympathetic nervous system activity, whereas dilation velocity is conditioned by the sympathetic nervous system. Additionally, constriction and dilation velocities also depend on the initial pupil diameter.49
Pupillary examination using a NeurOptics™ ForSite pupillometerAll pupillometry devices developed to date function according to the same basic principles. Such devices include: (a) an integrated intense light source for pupil stimulation; (b) an image capture system with an infrared digital camera capable of obtaining pupil measurements throughout the entire examination process (pupil diameter at rest, during light stimulation, and at the end of the stimulus), without interfering with pupil response because it provides no visible light; and c) a data processor to perform calculations.
Our market contains various brands of pupillometers (Colvard, Procyon, NeurOptics, etc.), most of which are used in the field of ophthalmology. The NeurOptics pupillometer is the only portable pupillometer specifically designed to assess pupils in severely brain-injured patients (among others).
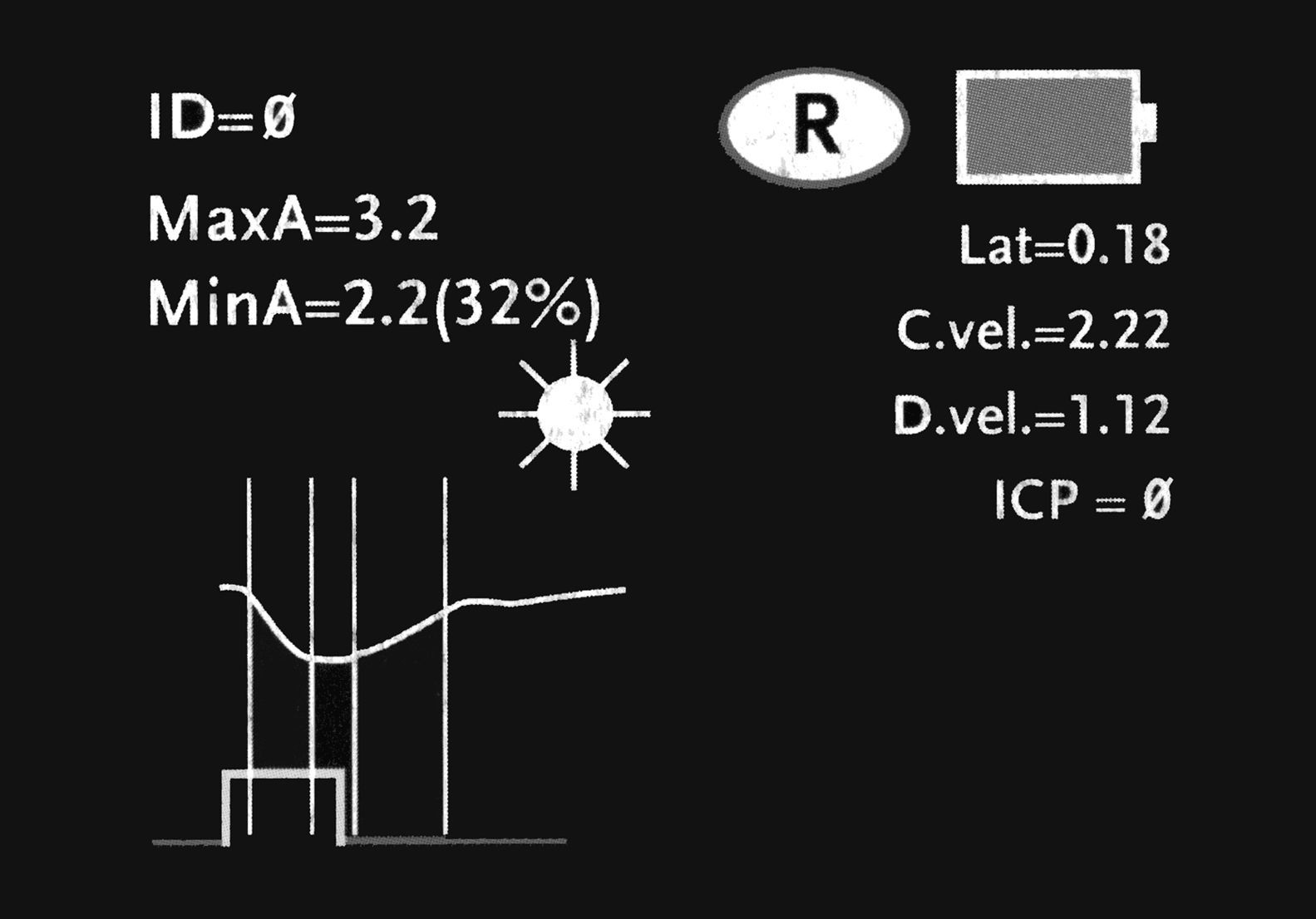
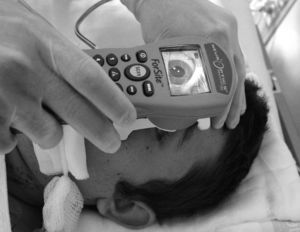
The NeurOptics™ ForSite pupillometer (NeurOptics Inc., Irvine, CA) is a portable device powered by rechargeable battery (Fig. 1). The pupillometer includes: (a) a built-in microprocessor with a colour LCD screen displaying measurements in both graphical and numerical formats; (b) a keyboard to introduce data such as patient ID data, medications, and intracranial pressure (ICP) values; (c) a light source emitting a standardised stimulus; and (d) an image capture system with an infrared digital camera which can obtain data in different lighting conditions, from total darkness to bright light.
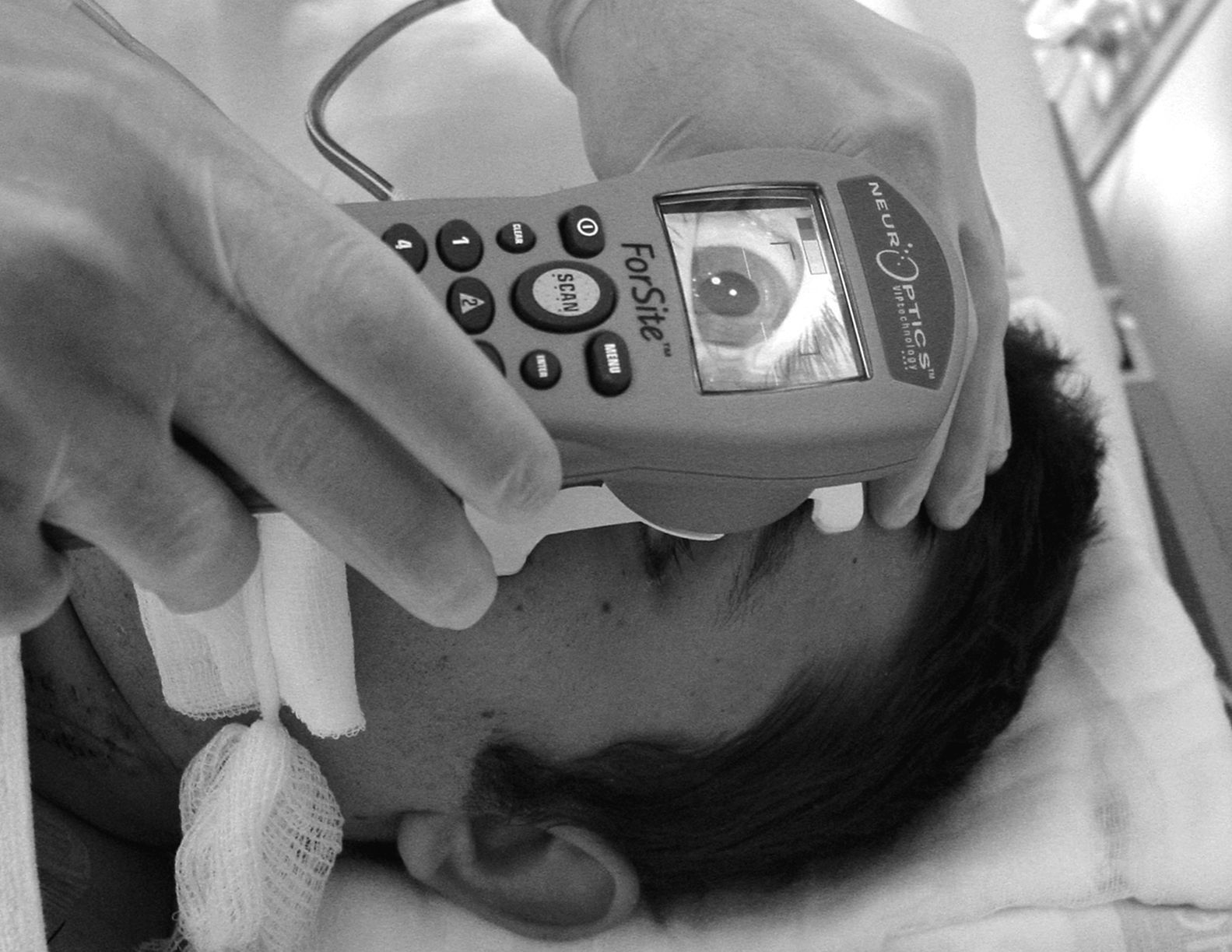
It also includes a removable headrest which allows us to position the pupillometer correctly before the patient's eye during the measurement process.
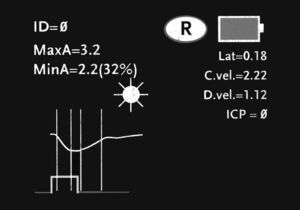
A photomotor reflex examination carried out with this system consists of 3 automated phases. (a) The first is a search for the objective, which lasts 3seconds; this phase starts after we place the pupillometer in front of the patient's eye and press the scan button. During this first phase, the screen shows a video image of the eye, which allows the operator to centre the iris properly. (b) When the device has detected the image correctly, a new phase begins in which images are acquired during 3seconds. Throughout this phase, a fixed-intensity, fixed-duration light stimulus is automatically emitted (0.8seconds). The infrared digital camera simultaneously records pupillary images. Last of all, (c) there is an analysis phase lasting from 8 to 10seconds during which all pupillary images (39 images per second) are analysed sequentially and the screen displays the results in both graphical and numeric formats (Fig. 2). The only point of contact between the device and the patient is a disposable support that creates the distance needed in order to hold the device correctly during the examination (Fig. 3).
The device measures the following variables: diameter of the maximum pupillary aperture in millimetres (baseline or before stimulation); diameter of the minimum pupillary aperture in millimetres (after stimulation); percentage of variation between the above measurements; latency in milliseconds; and constriction velocity/dilation velocity in millimetres per second. These 3 phases are described for the patient we present as a model in our article. All stored data can be sent to a thermal printer by means of an infrared communications port, which allows us to save graphic displays so that they can be analysed subsequently.20
Limitations of the NeurOptics™ ForSite pupillometerThe NeurOptics device is monocular, meaning that it cannot measure consensual response. An afferent pupillary defect, which may be present with optic nerve lesions in patients with HT, could therefore go undetected by this pupillometer.
Moreover, this device cannot be used for assessing patients who have suffered an eye trauma or who have a history of iris surgery or dysmorphia.
Use of pupillometry in severely brain-injured patientsClinical uses of IP have mostly been studied by the field of ophthalmology. Such studies have yielded relevant data for the evaluation of refractive surgery, sleep studies, and assessing the effects of different drugs on the CNS. IP has also been used to objectively measure emotional response in patients with psychiatric disorders and to ascertain autonomic response in patients with diabetic neuropathy. Only a few preliminary studies have been completed which use this non-invasive monitoring tool to assess severely brain-injured patients. However, their results are both interesting and encouraging.20–22
In 1995, Larson et al. described pupillometric analysis in a series of patients whose pupillary reflex was absent according to direct observation. They concluded that IP could reveal the presence of a midbrain impairment that would otherwise have been overlooked in pharmacologically paralysed patients, since motor responses for the remaining brainstem reflexes are abolished under these circumstances.20 The same group subsequently assessed pupillary response in 3 patients with uncal herniation detected by cerebral CT and highlighted 2 key points.21 On the one hand, pupillary response is preserved with use of opioids and, on the other hand, pupillary areflexia may be intermittent with an uncal herniation. This explains some of the interobserver differences that have been recorded. These authors concluded that, compared to brain CT, pupillometry could be a low-cost screening method for continuous evaluation of patients with HT and in early detection of brain herniations.21
Nevertheless, we think that the study by Taylor et al. constitutes the most important contribution to date. These authors took pupillometric measurements in both healthy volunteers and patients with HT and spontaneous subarachnoid haemorrhage. They presented some preliminary observations concerning pupillary responses with different lighting conditions, concomitant use of drugs which could modify pupillary response, and ICP values. Moreover, they compared a pupillary assessment performed by nurses in a patient subgroup with an evaluation using a pupillometer. Some of the results are summarised below.22
- 1.
Pupil constriction velocity: according to these authors, a pupil constriction velocity of less than 0.8mm/s is associated with an increase in ICP. These values are uncommon in healthy subjects. According to their study, values below 0.8mm/s were found in only 1.35% of measurements taken from healthy volunteers. Constriction velocity of less than 0.6mm/s was detected in only 0.32% of the volunteers. However, patients with HT and midline shifts of more than 3mm according to the brain CT showed values below 0.6mm/s on 53% of the measurements when ICP values were higher than 20mmHg. In patients with diffuse cerebral swelling and no midline shift, pupil constriction velocity remained the same until ICP values exceeded 24mmHg.
- 2.
Percentage of decrease in pupil diameter: decreases in pupil diameter of less than 10% are extremely rare in healthy subjects. In the series described by Taylor et al., they were only reported in 0.04% of all measurements. The same is true of patients with HT and ICP values below 20mmHg, in which the mean percentage of decrease exceeded 15%. Nevertheless, in groups of patients with ICP values higher than 20mmHg, decreases in pupil diameter of less than 10% were more common.
- 3.
Latency of pupillary response: latency is directly related to age, and it rarely exceeds 360ms when ICP values are higher than 30mmHg. This parameter therefore has little predictive value for detecting neurological deterioration.
- 4.
Pupillary symmetry: healthy volunteers and patients without intracranial pathology usually present pupillary asymmetry of less than 0.5mm, which leads us to believe that this level of asymmetry may be considered within normal limits. However, patients having an intracranial pathology with focal lesions and mass effect showed pupillary asymmetries of more than 0.5mm in 81% of the measurements when ICP values exceeded 30mmHg.22 In the parallel study carried out by nurses using conventional methods, such asymmetries were only detected in 22% of the cases.22
- 5.
Pupillometry and drugs: according to the study by Taylor et al., pupillary parameters obtained when drugs commonly used in ICUs are administered confirm that use of high doses of barbiturates, which can result in a flatline or burst-suppression pattern on the EEG, have a marked effect on pupillary response. Pathological values for constriction velocities (below 0.6mm/s) and the percentage of decrease in pupil diameter after light stimulation (below 10%) are recorded even when ICP values remain normal. Combined use of morphine and midazolam has only a brief effect on pupillary response.
The study by Taylor et al. allows us to draw several conclusions. The most important point may be that pupillary response is consistently symmetrical in healthy subjects, and that the slightest change in pupillary response should alert doctors to the possibility of a deteriorating condition. A percentage of decrease in pupil diameter of <10% after stimulation may be considered a definite indicator of brainstem or cranial nerve III compression, even when ICP values are normal. In the presence of bilateral diffuse cerebral swelling, altered pupillary responses appear when ICP values exceed 30mmHg. This coincides with prior observations indicating that patients with brainstem and/or cranial nerve III compression present higher ICP values than patients with midline shift.27 The possibility of carrying out a quasi-continuous study of pupillary dynamics and their relationship with processes that lead to brain herniations could help us predict neurological deterioration.8
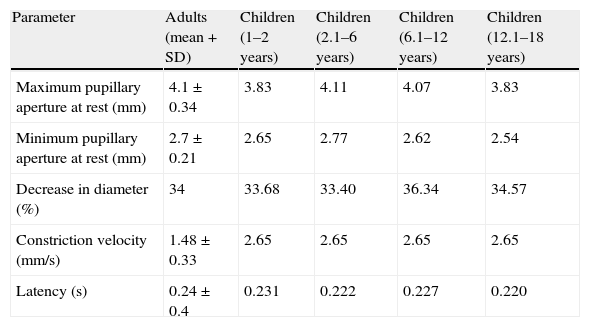
Table 1 shows ‘normal’ values obtained by using IP on 310 healthy volunteers in the study by Taylor et al.22 This table also includes values from the study by Boev et al. of a series of 90 children with no neurological or ophthalmological pathologies.49
Normal pupillary values.
| Parameter | Adults (mean+SD) | Children (1–2 years) | Children (2.1–6 years) | Children (6.1–12 years) | Children (12.1–18 years) |
| Maximum pupillary aperture at rest (mm) | 4.1±0.34 | 3.83 | 4.11 | 4.07 | 3.83 |
| Minimum pupillary aperture at rest (mm) | 2.7±0.21 | 2.65 | 2.77 | 2.62 | 2.54 |
| Decrease in diameter (%) | 34 | 33.68 | 33.40 | 36.34 | 34.57 |
| Constriction velocity (mm/s) | 1.48±0.33 | 2.65 | 2.65 | 2.65 | 2.65 |
| Latency (s) | 0.24±0.4 | 0.231 | 0.222 | 0.227 | 0.220 |
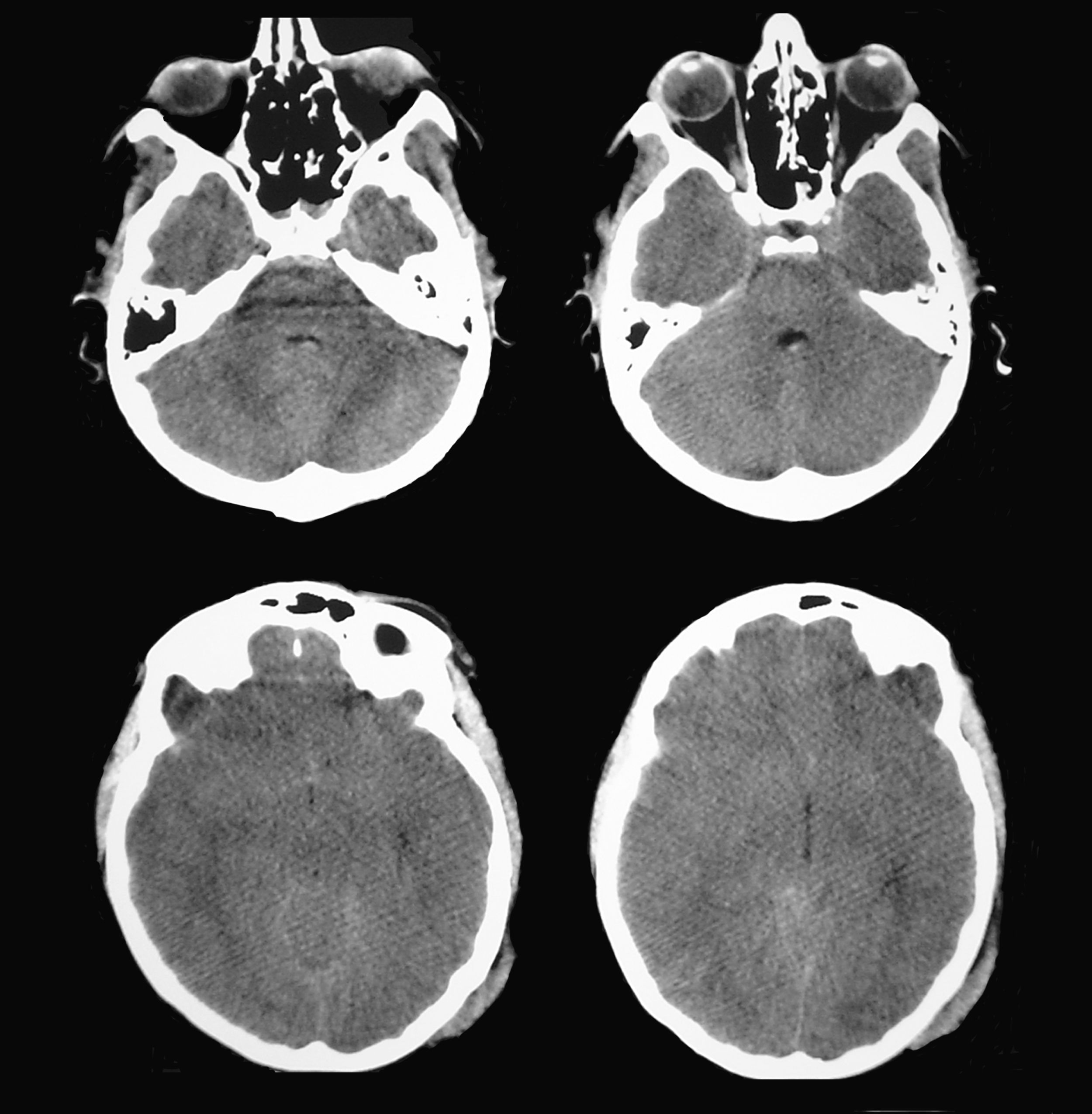
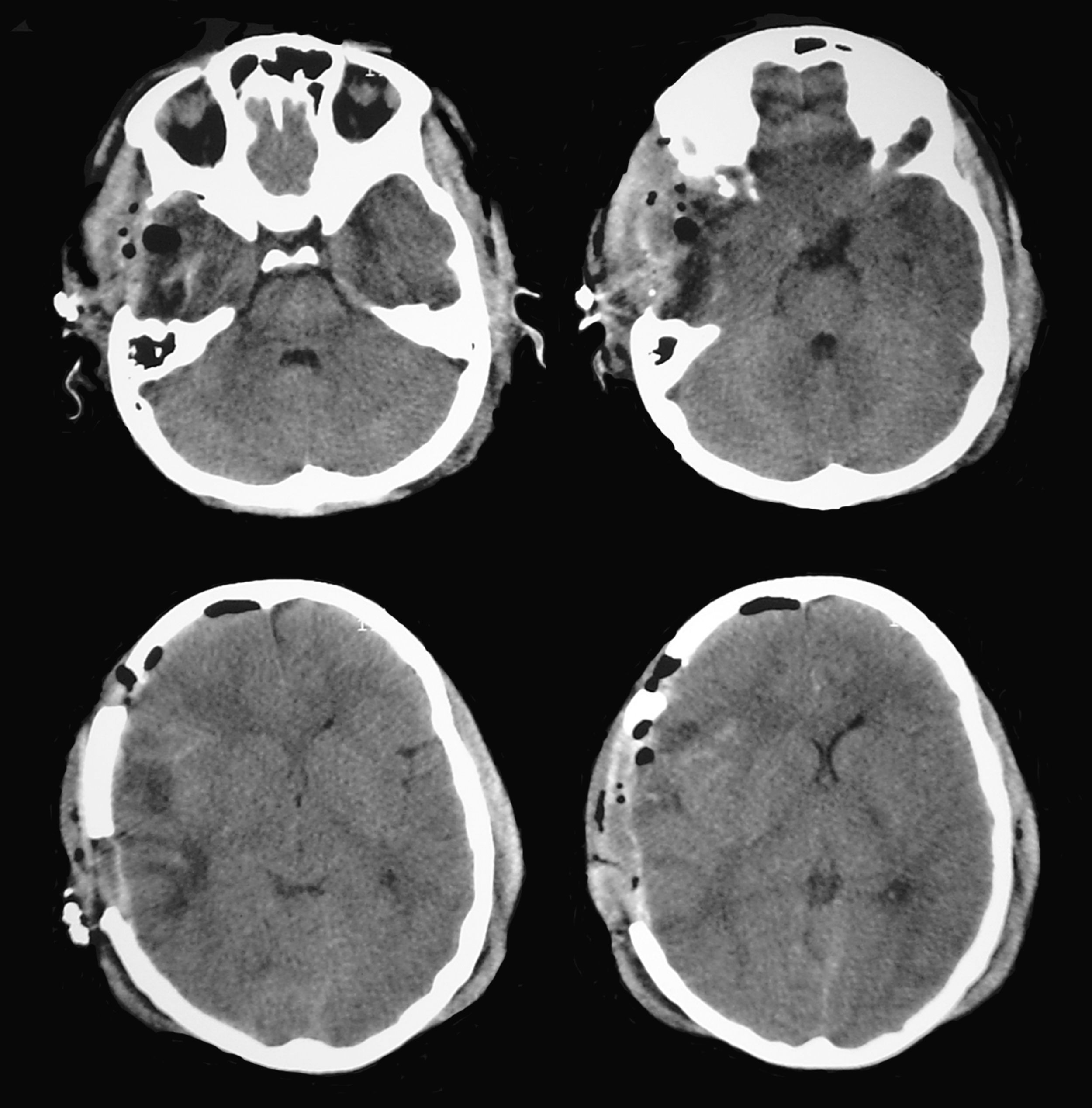
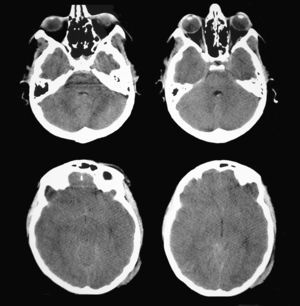
A patient aged 16 years with no history of relevant disease was admitted to the Neurotrauma ICU in our hospital after suffering severe HT (Glasgow Coma Scale score of 8) due to a motorcycle accident. Upon admission, the patient's brain CT showed a right temporal contusion with a volume of 15 cc and no midline shift (Fig. 4). Continuous ICP monitoring showed initial values within the normal range (below 15mmHg), which remained stable during the first 24hours after admission. With ICP values still within the normal range, anisocoria was detected on the second day after admission by direct examination of the pupil. Left pupil size was 4mm and right pupil size was 2.6mm; both were reactive to light. The larger of the 2 pupils was thought to be pathological and the brain CT was repeated.
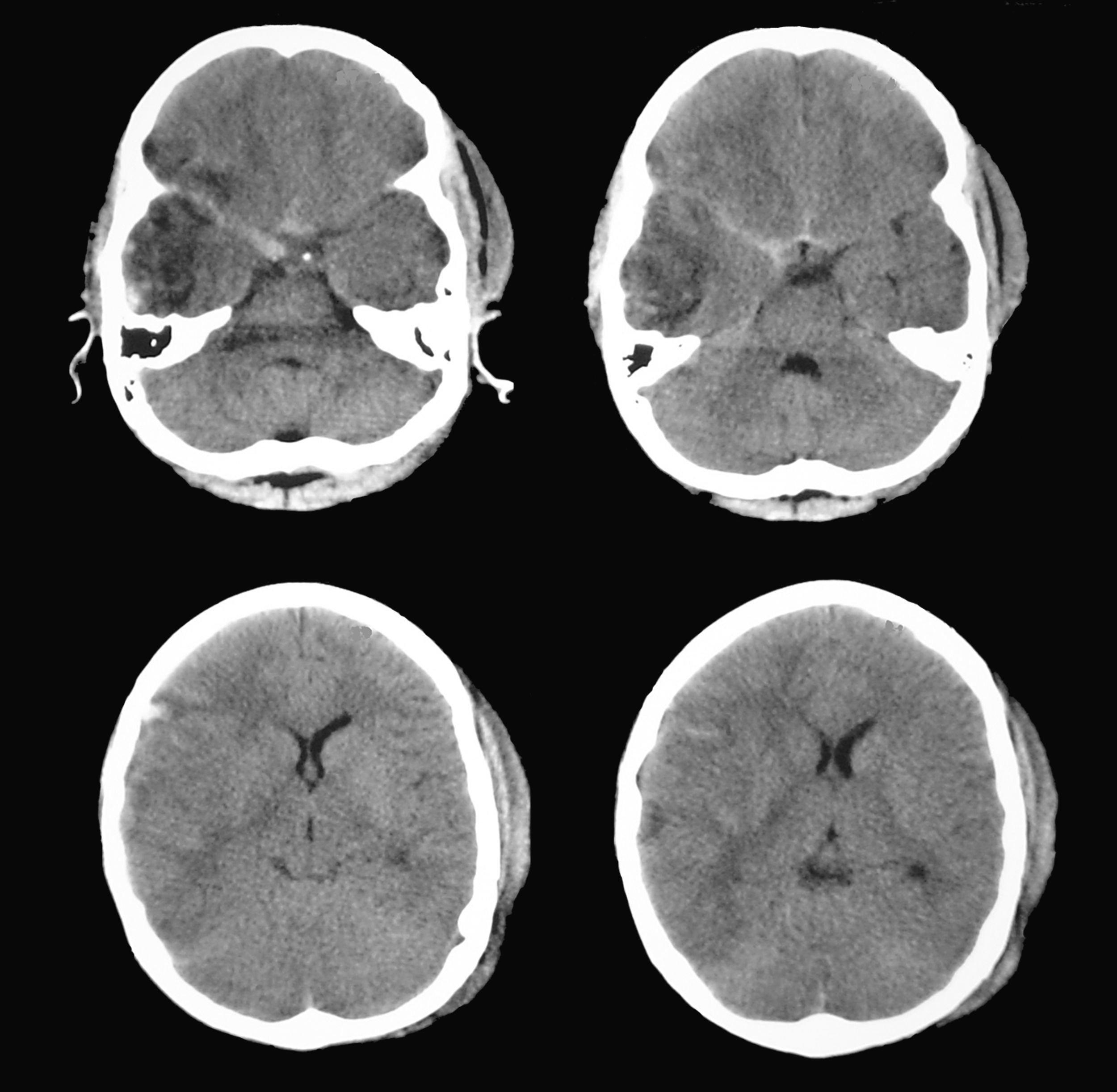
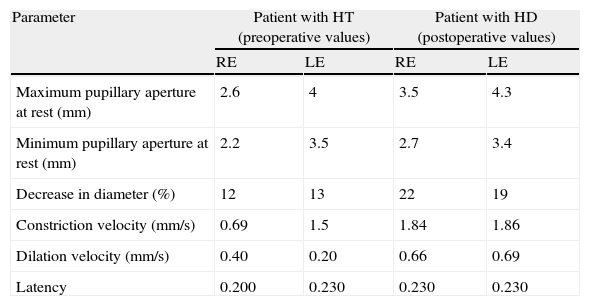
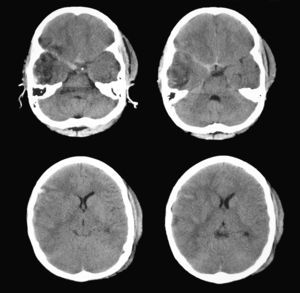
According to this new scan, right temporal contusion appeared more defined, there were no new lesions, and no midline shift was observed. However, there were signs of effacement of the perimesencephalic cisterns ipsilateral to the right temporal lesion, corresponding to the smaller pupil, which until then had been considered normal (Fig. 5). Pupillary reflexes measured by the pupillometer (Table 2) showed obviously abnormal values for right pupil constriction velocity. The values were in ranges considered pathological according to the study by Taylor et al.22
Data obtained using pupillometry in a patient with severe head trauma (HT).
| Parameter | Patient with HT (preoperative values) | Patient with HD (postoperative values) | ||
| RE | LE | RE | LE | |
| Maximum pupillary aperture at rest (mm) | 2.6 | 4 | 3.5 | 4.3 |
| Minimum pupillary aperture at rest (mm) | 2.2 | 3.5 | 2.7 | 3.4 |
| Decrease in diameter (%) | 12 | 13 | 22 | 19 |
| Constriction velocity (mm/s) | 0.69 | 1.5 | 1.84 | 1.86 |
| Dilation velocity (mm/s) | 0.40 | 0.20 | 0.66 | 0.69 |
| Latency | 0.200 | 0.230 | 0.230 | 0.230 |
Brain CT showed a right temporal contusion with mild mass effect and no clear evidence of brainstem compression. Direct pupillary assessment showed a mydriatic but reactive pupil contralateral to the lesion; this was initially identified as a Kernohan's notch phenomenon. Nevertheless, pupillometric data was indicative of compromised function of the pupil ipsilateral to the lesion rather than impairment of the larger pupil. Immediately after surgery (decompressive craniectomy and resection of the anterior temporal pole) pupillary function measurements were found to be within normal ranges. RE: right eye; LE: left eye.
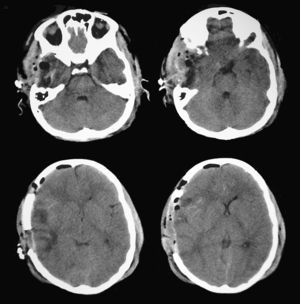
Since findings from the routine brain CT were indicative of an incipient uncal herniation, doctors performed a decompressive craniectomy with partial resection of the area of temporal contusion (Fig. 6). Pupillometric measurements after surgery showed that constriction velocity values for the right pupil had returned to normal and coincided with those for the left pupil. The patient subsequently recovered well with no additional pupillary alterations (Table 2).
ConclusionsThe possibility of performing an early, objective, and nearly continuous assessment of the changes in pupillary reactivity constitutes a new non-invasive monitoring method that could identify predictive factors for neurological impairment and be helpful in bedside monitoring of a patient's neurological state. This method would eliminate unnecessary tests and facilitate early treatment.
IP has significant advantages compared with direct observation of the pupillary response, given that: (a) it standardises intensity of the light stimulus, which eliminates measurement errors; (b) it permits measurement of the response to the light stimulus; (c) it enables measurement of parameters that cannot be assessed clinically such as constriction/dilation velocity and percentage, and response latency, the importance of which is currently unknown; (d) it enables establishment of normal values to allow detection of pathological states; and (e) it lets us record, save, and process data in computerised systems for future comparisons. These possible uses will depend on the publication of new studies to corroborate and expand on findings that have been observed up until now.
Conflicts of interestThe authors have no conflicts of interest to declare.
This review was partly funded by grant PI080480 awarded to Dr. J. Sahuquillo by FIS (Fondo de Investigación Sanitaria). We would like to thank the nursing staff at the Neurotrauma ICU and T.J. Bernard, Marina Maciá, and Iván Adrián Martínez Ricarte for their assistance with our study. The authors would like to thank Medtronic for donating the NeurOptics™ pupillometer to our hospital so that we could complete the study. Regardless of the above, there are no conflicts of interest to declare between the authors and the pupillometer manufacturing and distributing companies.
Please cite this article as: Martínez-Ricarte F, et al. Pupilometría por infrarrojos. Descripción y fundamentos de la técnica y su aplicación en la monitorización no invasiva del paciente neurocrítico. Neurología. 2013;28:41–51.