Over the past decades, neurocysticercosis (NCC) has become the most frequent parasitic infection of the central nervous system (CNS) in our setting, as a result of immigration from developing countries.1,2
Neurological manifestations depend on the location of Taenia solium larvae and on the immune response they trigger in the host. That response depends on the evolutionary stage of the larva, and it will be minimal during the vesicular stage, reach its peak during the colloidal stage, and decrease as it mineralises during the granular and calcified stages. Co-existence of cysts in different stages is not infrequent.1 Neurocysticercosis can affect the brain, the spinal cord, or both parts of the CNS.
When cysts are located in the brain parenchyma, their main clinical manifestations are epileptic seizures (more than 70% of the cases),1,3 although they can present with any kind of focal neurological signs. Cysts located in the subarachnoid space or in the ventricular system may lead to meningitis, arachnoiditis or hydrocephalus due to CSF-flow obstruction in the subarachnoid space or ventricles.3 Cysts in these 2 locations elicit more severe clinical features and sometimes require surgery.
Although there are several published case series of NCC presenting with signs of acute meningitis,4 this presentation is not listed among the most common.1 CSF analysis usually reveals moderate mononuclear pleocytosis and elevated CSF protein levels; and low CSF glucose levels are detected in 12%-18% of the cases.5 The presence of eosinophils in CSF is characteristic, although infrequent (15% at onset). Complementary diagnostic procedures include ELISA for CSF and western blot for blood.1 Most published cases of NCC presenting with meningitis included neuroimaging scans showing viable cysts, hydrocephalus, leptomeningeal enhancement or disorders other than calcifications.1,5,6
The spinal cord is rarely affected even where the infection is endemic, and reported incidence is 1%-3% of all cases of NCC.2,6 Cysticercosis is classified as extradural, subdural, subarachnoid or intramedullary according to its location. Intramedullary NCC (less than 20%) and extradural NCC are rare,7 whereas extramedullary subarachnoid NCC is the most frequent type. Cysticerci migrate from the brain to the spinal subarachnoid space through CSF; intracranial subarachnoid NCC will also be present in most cases. The most frequent manifestations are radiculopathies.1,6 Isolated spinal involvement is very rare.8,9
We present the case of a 29-year-old Bolivian woman with no relevant history who presented at the emergency department with progressive headache of 72hours’ duration, fever, and nausea. Her health was good otherwise and findings from the neurological examination were normal, with no signs of meningeal irritation.
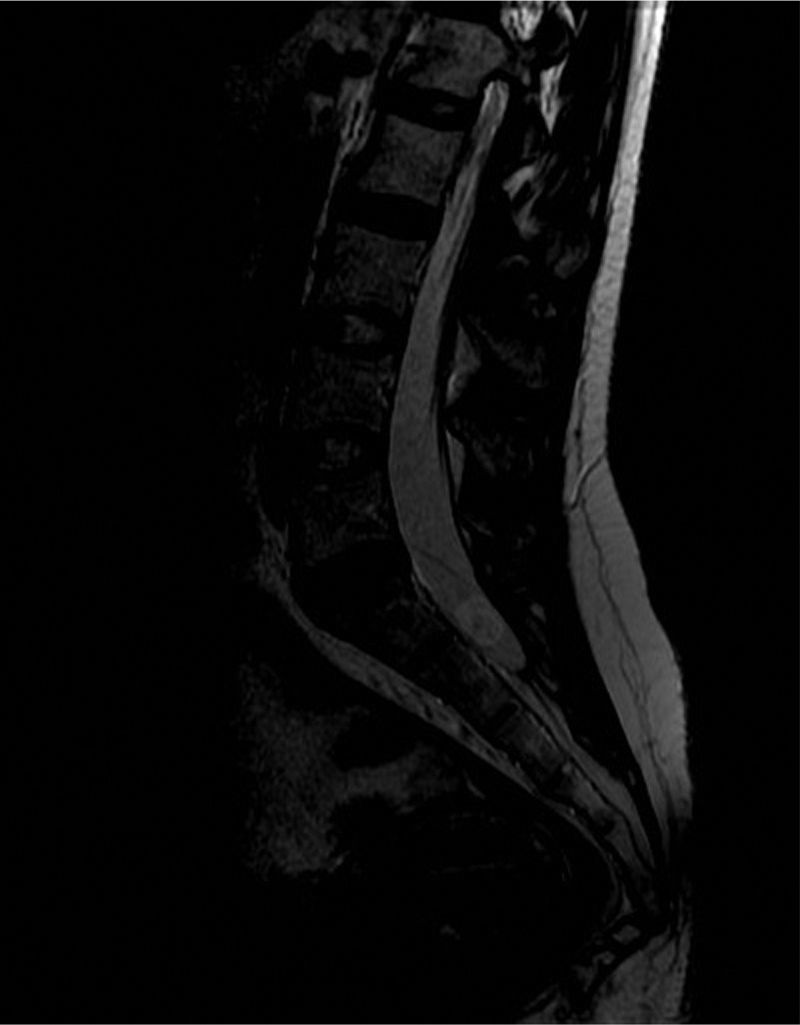
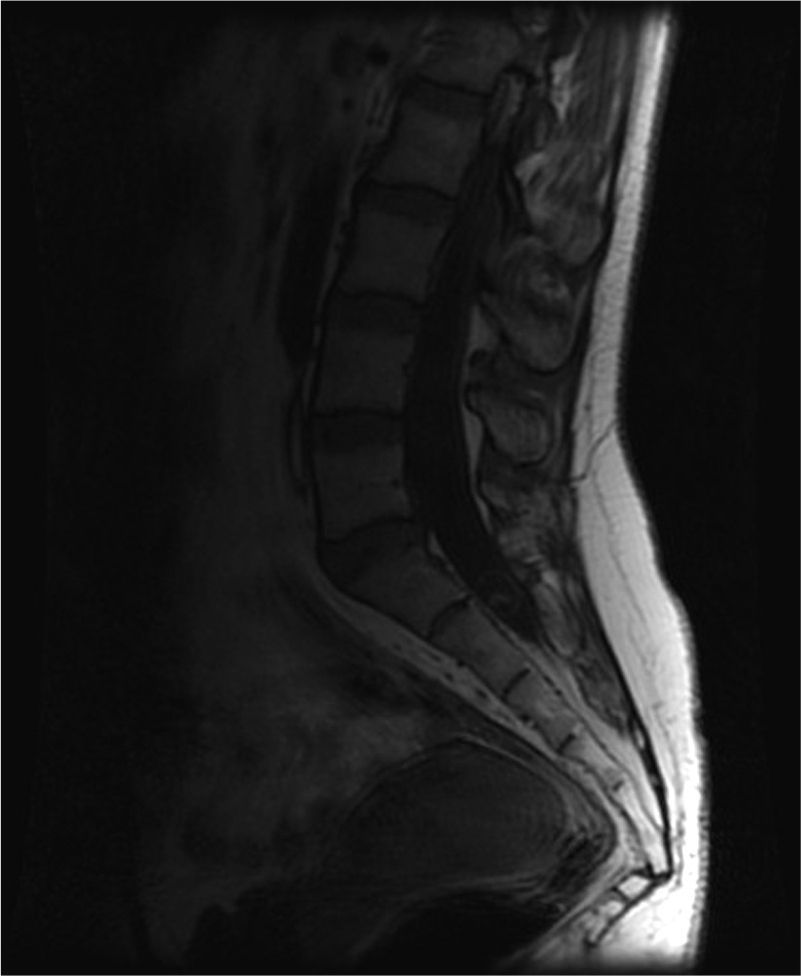
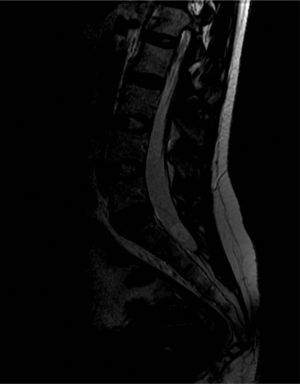
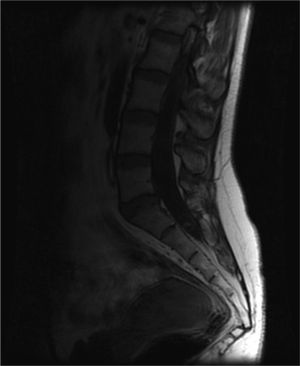
A cranial CT scan showed multiple scattered cortical-subcortical calcifications measuring up to 2.5mm and consistent with NCC in the calcified stage. A lumbar puncture (LP) delivered clear CSF with normal pressure, containing 265 white blood cells (76% mononuclear), 73mg/dL proteins and CSF glucose level of 45mg/dL (50% of glycaemic level). Doctors considered viral (non-herpetic) lymphocytic meningitis as the first aetiological possibility. The patient was admitted to hospital and symptomatic treatment was administered. Signs and symptoms varied during the first 2 weeks of treatment, so LP was performed two more times. Results from CSF analysis showed improvements in pleocytosis and CSF protein level. However, CSF glucose dropped to less than 20% of glycaemia. Doctors screened for different aetiologies for meningitis with low CSF glucose (carcinomatosis, subarachnoid haemorrhage, sarcoidosis, dermoid cysts, bacterial meningitis, meningeal tuberculosis, fungal meningitis and viral meningitis [choriomeningitis, varicella-zoster virus, cytomegalovirus, herpes simplex virus 1 and 2, human immunodeficiency virus, parotitis]). Results from all studies were negative. Brain MRI revealed calcified cysts consistent with NCC, as the CT scan had shown previously. During the third week, the patient reported acute radicular pain in the right S1 territory so doctors requested a spinal MRI. The image showed a rounded lesion at the S1-S2 level, 16mm in diameter, intradural, extramedullary, and hyperintense in T2. Findings were consistent with a cysticercus cyst in the vesicular stage (Figs. 1 and 2).
T2-weighted sagittal spinal MRI of the lumbosacral region. Image shows a rounded lesion 16mm in diameter in the distal region of the intradural spinal canal at the level of the S1 area, hyperintense in T2 with lower signal intensity in the centre. It is consistent with cysticercus cyst in the vesicular colloidal stage. There are no other signs of intra- or extramedullary lesions in other areas of the spinal column.
The patient was treated with analgesics that reduced her pain, and cysticidal treatment was proposed. She improved gradually and the last LP performed 1 month after the onset showed glycorrhachia at 50% of the blood glucose level.
Clinical features and composition of CSF initially seemed to point to viral meningitis. However, the discrepancies between CSF parameters in successive lumbar punctures (improvement in pleocytosis and CSF protein levels, in contrast with lower CSF glucose levels) suggested other possible aetiologies. Tests conducted to rule out other causes of low CSF glucose yielded negative findings and the only related discovery was the viable radicular cyst.
The first question that arises is whether the condition was cysticercal meningitis only or the result of 2 simultaneous conditions: viral meningitis and spinal cysticercosis.
The negative results from tests for different aetiological agents, along with changes in clinical condition and the CSF analysis provide support for the first theory. Although some viral meningitis types can present with low CSF glucose (e.g. lymphocytic choriomeningitis), the level would not typically remain low for such a long time and it would improve along with CSF parameters. However, aseptic meningitis may also present with sustained low CSF glucose in cases of meningitis caused by NCC. In addition, spinal NCC coexists with brain NCC in most cases;10 therefore, finding primary spinal NCC (which is quite rare) in conjunction with meningitis with low CSF glucose testing negative for other aetiological agents is not likely to be a coincidence. However, the improbable theory of coincidental co-existence of these entities could be supported by the fact that active cysts are detected in most cases in which NCC presents with meningitis, and there were no active cysts in the brain in this case.5
The next question to consider is whether the observed inflammatory reaction was caused by the only viable cyst that was identified, or if it could be attributed to the presence of other cysts. Although the literature describes some cases of isolated cervical cysts with meningeal onset,9 it seems unlikely that the cyst in question, located in the sacrum and not appearing to elicit major surrounding inflammation, would be the only cause of meningitis. We suspect that other cysts in the brain may have contributed to the inflammatory reaction. On the one hand, calcified cysts have been proven not to be inert; during the mineralisation process, antigen exposure occurs, and this can maintain inflammatory response.11 On the other hand, and bearing in mind that coexistence of cysts in different stages is not infrequent,1 we suspect that there may be a ruptured viable brain cyst that was not detected by neuroimaging scans and which triggered the meningeal reaction.
In conclusion, we believe that NCC should be included in differential diagnosis for meningitis with low CSF glucose, especially in patients from areas where NCC is endemic. Spinal imaging is needed if there are signs and symptoms at this level or if tests detect sustained low CSF glucose without viable cysts in the brain.
Please cite this article as: Abarrategui Yagüe B, García García ME, Orviz García A, Casas Limón J. Meningitis linfocitaria y neurocisticercosis espinal. A propósito de un caso y revisión de la literatura. Neurología. 2014;29:574–576.