Giant cell tumours of the tendon sheath (GCTTS) are a rare type of synovial neoplasm, frequently involving the large joints of the lower limbs. These tumours can present in localised or diffuse forms; both types consist of a proliferation of mononuclear synovial cells mixed with multinucleated giant cells, foam cells, siderophages, and inflammatory cells.1 Localised GCTTSs, usually appearing in young adults (age 30-50 years), are more common than the diffuse form, and constitute the most frequently observed neoplasm of the hand. Although these tumours are benign from a histopathological viewpoint, diffuse GCTTSs are locally aggressive; local recurrence occurs at a rate of 15%-24%.1–4 This tumour has been described in the joints of the appendicular skeleton; cases involving the spinal column are extremely rare. Spinal involvement usually affects posterior elements, such as the joints (facets), laminae, and pedicles. Clinically, the condition usually presents with local or radicular pain and chronic myelopathy. We present an exceptional case of acute compression of the spinal cord secondary to a GCTTS in the upper thoracic region and review previously described cases from the literature.
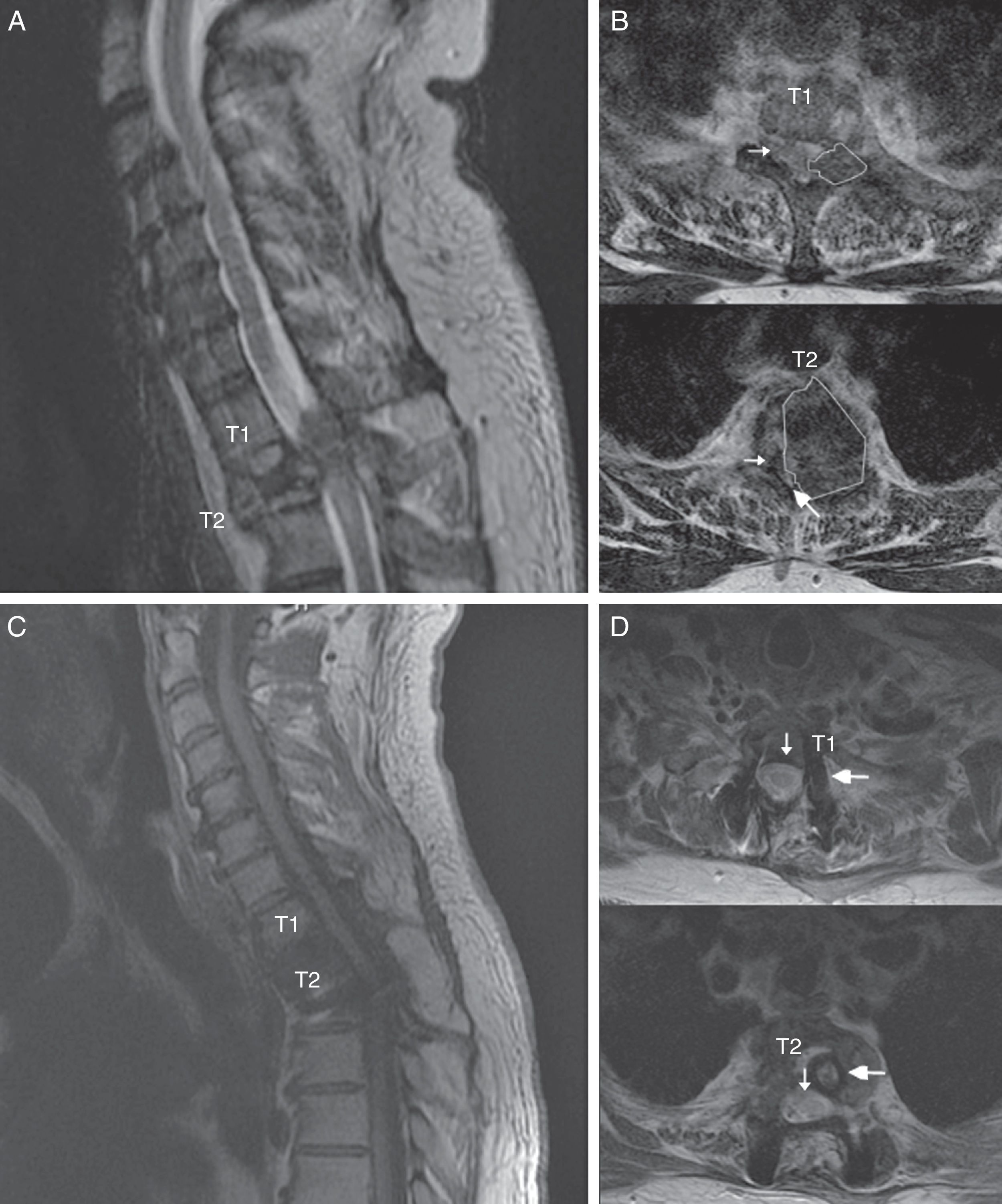
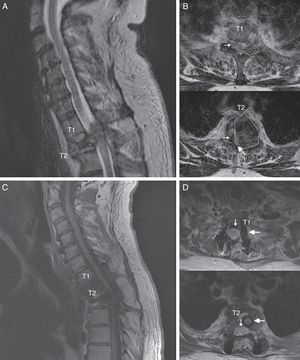
The patient was a 55-year-old man with no relevant medical history. He attended our department following sudden onset of chest pain and weakness in the lower limbs. The neurological examination revealed paraplegia (T10 sensory level), hyperreflexia in the lower limbs, bilateral extensor plantar reflex, and loss of sphincter control. A magnetic resonance imaging (MRI) scan revealed a lesion to vertebra T2, affecting the vertebral body, laminae, pedicles, and facet joints, and displaying no contrast uptake; invasion of the epidural space; and a pathological fracture associated with myelopathy (Fig. 1). An emergency T1-T2 laminectomy was performed; evidence was observed of destruction of the laminae, facet joint, and pedicles. We also observed a dense, dark-coloured, partially delimited mass in the epidural space; this was partially resected.
Spinal MR images of the patient. (A) Sagittal section of the cervicodorsal spine, showing the tumour invading vertebra T2. (B) Axial sections of the T1 and T2 vertebrae, showing the tumour (outlines), the significant rightward displacement of the spinal cord (small arrow), and the reduced subarachnoid space (large arrow), demonstrating the severe compression of the spinal cord. (C) MRI of the cervicodorsal spine showing the release of the spinal canal where it was previously affected by the tumour. (D) Axial sections of the T1 and T2 vertebrae showing the release of the spinal canal (small arrow) and the complete resection of the lesion and placement of material for fixation and stabilisation (large arrow).
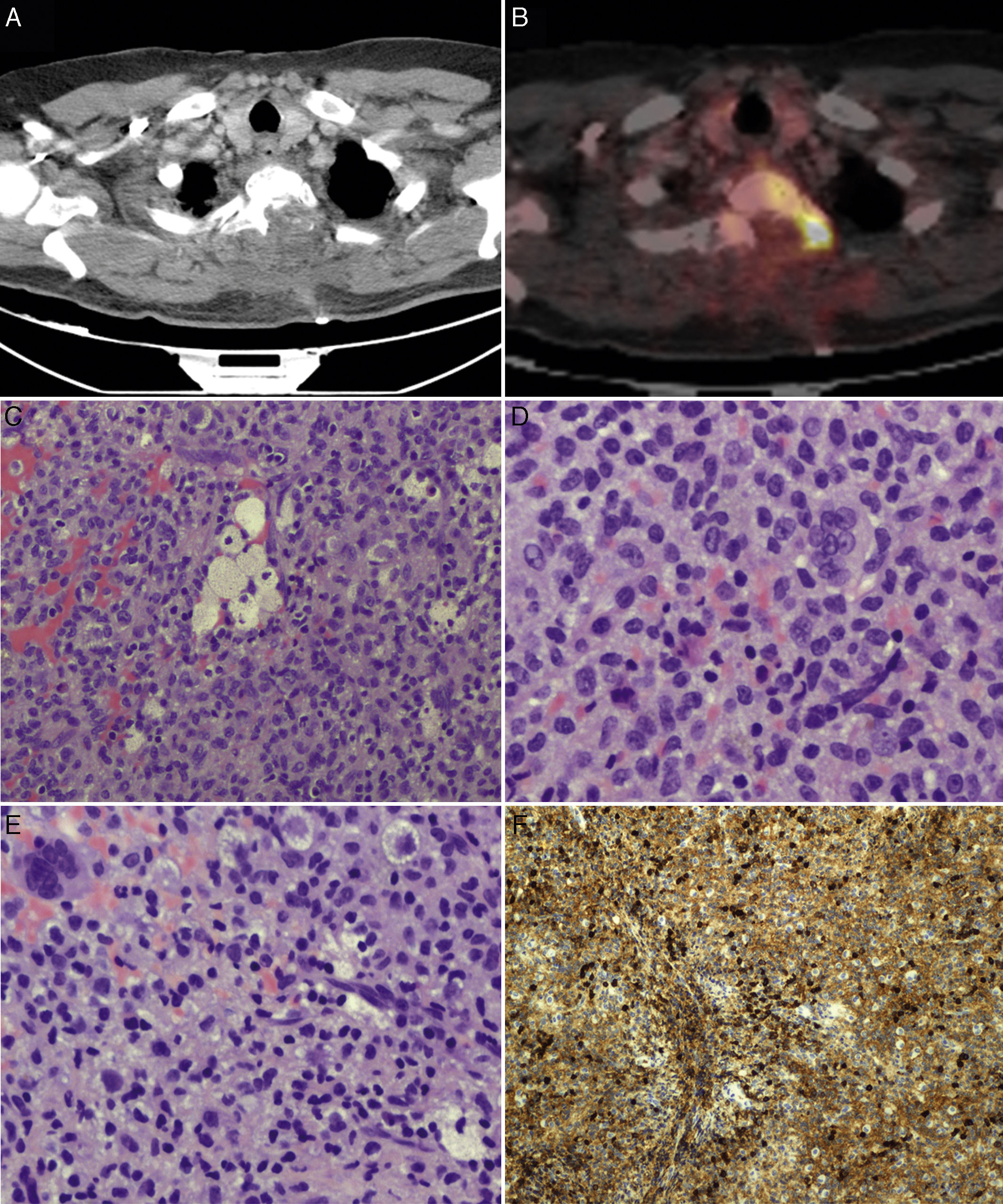
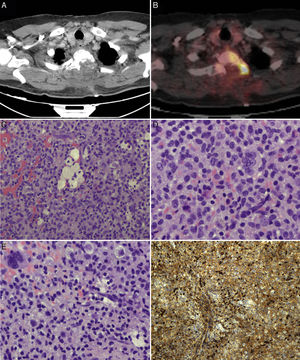
We suspected metastasis and performed a positron emission tomography (PET) scan. The tumour remnants in vertebra T2 showed high fluorodeoxyglucose (FDG) uptake, with a maximum standardised uptake value (SUVmax) of 18.6 (values greater than 2.5 are considered malignant in bone tissue). No lesions were detected in other organs. Finally, anatomical pathology results were consistent with diffuse GCTTS (Fig. 2). In a second surgical procedure, we fully resected the tumour and performed a corpectomy through a transpedicular costotransverse approach. A polyether ether ketone cylinder was placed in the T2 space and affixed to vertebrae T1 and T3 with transpedicular screws. The patient gradually recovered sensitivity, showing 2/5 strength in the right leg and 1/5 in the left (according to the modified British Medical Research Council scale for muscle strength) on postoperative day 5. Sphincter control was restored. At 24 months, the patient was able to walk with a cane and independently perform the activities of daily living; there was no sign of tumour recurrence (Fig. 1).
PET scans of the T2 vertebra (A and B), showing high levels of FDG uptake, with a SUVmax of 18.6 (B). Anatomical pathology revealed a neoplasm with a diffuse growth pattern, made up of round or oval mononuclear cells and abundant eosinophilic cytoplasm (C and E: haematoxylin and eosin staining; magnification 40× and 100×, respectively). These are accompanied by foam cells, siderophages, and multinucleated giant cells. Mitotic rate was low (D: haematoxylin and eosin staining; 400×), and there were localised areas of necrosis and lymphocytic infiltrate. Immunohistochemical analysis revealed CD68 positivity (F).
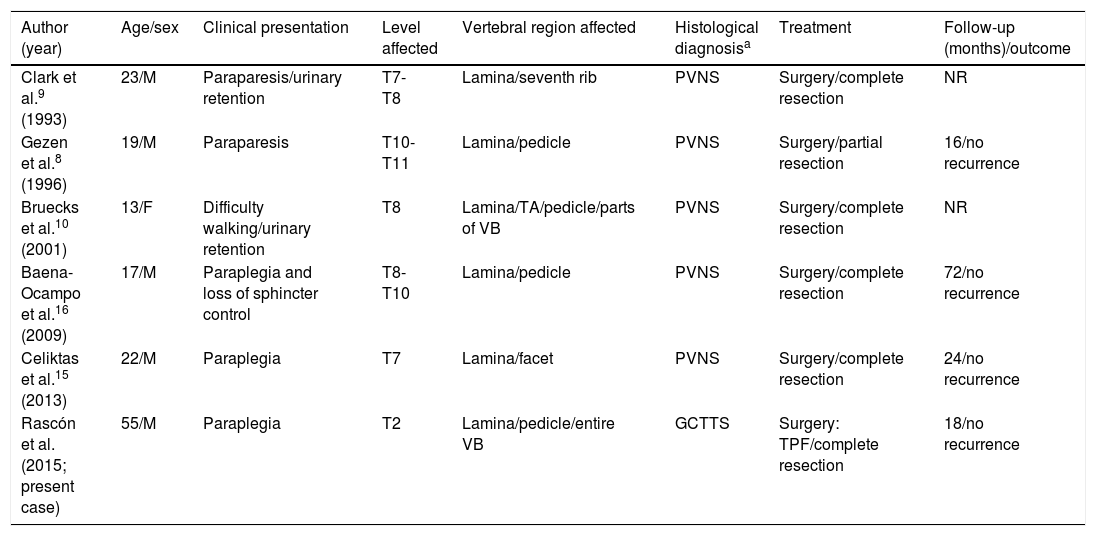
GCTTSs are rare synovial neoplasms which are histopathologically similar to pigmented villonodular synovitis (PVNS).1 Diffuse GCTTSs are more locally aggressive, less delimited, more frequent in young adults, and usually involve the large joints of the lower limbs (the knees in 80% of cases).1 Series of rare cases in which these tumours affect the spinal column show a mean age of onset of approximately 35 years; the condition is slightly more frequent in women.5 Spinal GCTTSs usually cause subacute or chronic symptoms including pain, radiculopathy, or subacute/chronic myelopathy. They typically appear in the cervical or lumbar spine, affecting posterior elements, and may erode the bone, often leading to multi-level involvement.5–7 Acute compression of the spinal cord is very rare.5,6,8 In our literature review of 65 cases of GCTTS or PVNS affecting the spinal column, chronic myelopathy was frequently associated with the tumour, in some cases leading to established paraplegia; there was only one case of acute compression of the spinal cord, following a traffic accident.5,6,8,9 Some authors suggest that previous trauma may have a destabilising effect, exacerbating or triggering symptoms when a neoplastic lesion is present in the spinal column.5,7 Our patient also presented severe involvement of the vertebral body, which led to a pathological fracture causing loss of vertebral height. This type of clinical/radiological finding is extremely rare: other reported cases of subacute myelopathy do not describe significant involvement of the vertebral body or previous trauma that may destabilise the affected location (Table 1).6,7,10,11
Review of cases of GCTTS/PVNSa with acute/subacute compression of the spinal cord as the initial manifestation.
| Author (year) | Age/sex | Clinical presentation | Level affected | Vertebral region affected | Histological diagnosisa | Treatment | Follow-up (months)/outcome |
|---|---|---|---|---|---|---|---|
| Clark et al.9 (1993) | 23/M | Paraparesis/urinary retention | T7-T8 | Lamina/seventh rib | PVNS | Surgery/complete resection | NR |
| Gezen et al.8 (1996) | 19/M | Paraparesis | T10-T11 | Lamina/pedicle | PVNS | Surgery/partial resection | 16/no recurrence |
| Bruecks et al.10 (2001) | 13/F | Difficulty walking/urinary retention | T8 | Lamina/TA/pedicle/parts of VB | PVNS | Surgery/complete resection | NR |
| Baena-Ocampo et al.16 (2009) | 17/M | Paraplegia and loss of sphincter control | T8-T10 | Lamina/pedicle | PVNS | Surgery/complete resection | 72/no recurrence |
| Celiktas et al.15 (2013) | 22/M | Paraplegia | T7 | Lamina/facet | PVNS | Surgery/complete resection | 24/no recurrence |
| Rascón et al. (2015; present case) | 55/M | Paraplegia | T2 | Lamina/pedicle/entire VB | GCTTS | Surgery: TPF/complete resection | 18/no recurrence |
F: female; GCTTS: giant cell tumour of the tendon sheath; M: male; NR: not reported; PVNS: pigmented villonodular synovitis; TA: transverse apophysis; TPF: transpedicular fixation; VB: vertebral body.
PVNS and GCTTSs are synovial neoplasms which coincide in terms of physiopathology, histological imaging, treatment, and prognosis. The 2 entities are differentiated by growth pattern: PVNS follows a “villonodular” or intra-articular growth pattern, whereas GCTTSs follow an extra-articular pattern and present as soft tissue masses.5 Both are included in our review, in line with the literature.
It is very difficult to differentiate GCTTSs from primary or secondary bone tumours in radiological studies. In MRI scans, GCTTSs tend to appear as isointense lesions with respect to the muscle on T1-weighted sequences and display heterogeneous signal intensity on T2-weighted sequences; the exact origin of the lesion is difficult to determine.5–7 Differential diagnosis includes far more common lesions, such as multiple myeloma, metastasis, or lymphoma, which frequently cause multiple lesions. However, primary bone tumours often cause isolated lesions, as is the case with GCTTSs.12 Our patient presented a single lesion affecting the entire vertebra. It was therefore impossible, from a topographical perspective, to perform a clear differential diagnosis prior to surgery. From an epidemiological perspective, however, the clinical profiles of patients with vertebral lesions are usually more informative in diagnosis.12 In this case, the patient's relatively young age and single vertebral lesion implied a minimal chance of the lesion being metastatic or lymphoproliferative. Cases have been described where PET scans showed FDG uptake with a SUVmax below 8; our patient's PET scan showed very high FDG uptake, with a SUVmax of 18.6. This is the first case of GCTTS with such high values for FDG uptake; we believe these results are due to overexpression of the glucose transporter 1 (GLUT1) protein in the macrophages and giant cells in these tumours.12,13 PET imaging may be useful alongside MRI as part of the radiological follow-up of patients.
The condition is managed surgically, through complete resection of the tumour, wherever possible, and surgical reintervention in the event of recurrence (15% following macroscopically complete extraction); death of the patient due to the tumour is rare.5,7 Gezen et al.8 report a case in which partial resection and spinal decompression were performed. Although there was no evidence of recurrence after 16 months of follow-up, these authors recommend complete resection wherever this is possible; where this is not the case, surgery should be complemented with radiotherapy. With the addition of radiotherapy (mean dose 39.8Gy) in cases of partial resection of infiltrative/diffuse tumours, 94% local control was achieved after a mean follow-up period of 94 months.14 However, on account of the rareness of the disease, there are no clinical trials confirming the usefulness of radiotherapy in treating these tumours. Other authors have reported cases of GCTTSs where complete resection was possible because the tumour was well delimited from the adjacent tissues and was exophytic, emerging from the posterior elements of the vertebra towards the spinal canal. In these cases, clinical presentation is most often subacute; involvement of the vertebral body or associated fractures were not observed in any of the cases in these studies.9,15,16 Symptoms were acute in our patient due to the presence of a pathological fracture secondary to invasion of the tumour into the entire vertebral body. We were able to promptly perform the complete resection of the tumour, and the patient's long-term prognosis was good.
GCTTSs can present as acute compression of the spinal cord. This is treated by complete resection with wide margins; this appears to be the main prognostic factor, particularly for diffuse tumours.1,6 Radiotherapy is a useful tool for controlling tumour remnants or inoperable recurrences.2,4,14 Recent studies have suggested using colony-stimulating factor 1 receptor inhibitors, with positive results.17 Given the possibility of recurrence (15%), we recommend close follow-up of patients for 2 years with annual MRI or PET/CT scans.5,6
We are grateful to Ingrid Carranza for her assistance with imaging.
Please cite this article as: Rascón-Ramírez FJ, Avecillas-Chasín JM, Bautista Balbás LA, Kita SD. Un tumo espinal benigno con comportamiento maligno: presentación clínica extremadamente rara. Neurología. 2018;33:269–273.