Malignant hemispheric infarction (MHI) is a specific and devastating type of ischaemic stroke. It usually affects all or part of the territory of the middle cerebral artery although its effects may extend to other territories as well. Its clinical outcome is frequently catastrophic when only conventional medical treatment is applied.
ObjectiveThe purpose of this review is to analyse the available scientific evidence on the treatment of this entity.
DevelopmentMHI is associated with high morbidity and mortality. Its clinical characteristics are early neurological deterioration and severe hemispheric syndrome. Its hallmark is the development of space-occupying cerebral oedema between day 1 and day 3 after symptom onset. The mass effect causes displacement, distortion, and herniation of brain structures even when intracranial hypertension is initially absent. Until recently, MHI was thought to be fatal and untreatable because mortality rates with conventional medical treatment could exceed 80%. In this unfavourable context, decompressive hemicraniectomy (DHC) has re-emerged as a therapeutic alternative for selected cases, with reported decreases in mortality ranging between 15% and 40%.
ConclusionsIn recent years, several randomised clinical trials have demonstrated the benefit of DHC in patients with MHI. This treatment reduces mortality in addition to improving functional outcomes.
El infarto hemisférico maligno (IHM) constituye un tipo específico y devastador de ictus isquémico. Usualmente afecta el territorio completo de la arteria cerebral media, aunque a veces involucra además otros territorios, presentando evolución clínica frecuentemente catastrófica, cuando solo se aplica tratamiento médico convencional.
ObjetivoEl propósito de esta revisión es analizar la evidencia científica disponible sobre el tratamiento de esta entidad.
DesarrolloEl IHM tiene una morbimortalidad elevada. Clínicamente se caracteriza por deterioro neurológico temprano y síndrome hemisférico severo. Su sello distintivo es el desarrollo de edema cerebral ocupante de espacio, entre el primer y tercer día del inicio de los síntomas. El efecto de masa provoca desplazamientos, distorsiones y herniaciones de las estructuras encefálicas, aún en ausencia inicial de hipertensión endocraneal. Hasta hace pocos años, el IHM era considerado una entidad fatal e intratable, ya que la mortalidad asociada al tratamiento convencional podía superar el 80%. En este contexto desfavorable, la hemicraniectomía descompresiva ha resurgido como una alternativa terapéutica eficaz en casos seleccionados, reportándose un descenso de la mortalidad entre un 15%-40%.
ConclusionesEn los últimos años diversos estudios clínicos aleatorizados han demostrado el beneficio de la hemicraniectomía descompresiva en los pacientes con IHM, la cual no solo ha disminuido la mortalidad sino que también ha mejorado los resultados funcionales.
Cerebrovascular disease (CVD) is one of the most frequent causes of death and functional disability worldwide.1 An epidemiological study conducted in Catalonia which analysed both types of CVD (ischaemic and haemorrhagic) revealed annual incidence rates of 218 and 127 per 100000 population in men and women, respectively. The crude mortality rate at 28 days after stroke was 36%; 62.5% of these patients died out of hospital.2
The clinical consequences of ischaemic brain lesions depend on their extension and the eloquence of the involved parenchyma.3 Ischaemic injuries range from clinically silent lesions to life-threatening infarctions; as a result, the associated morbidity and mortality varies greatly.3–5 The in-hospital mortality rate for patients with middle cerebral artery (MCA) infarctions is 17%,6 while a specific subtype, generically termed ‘malignant’, has shown a mortality rate of up to 80%.7 Today, thanks to a multidisciplinary approach to MCA infarction and the advances in critical care, neuromonitoring, neuroimaging, and surgery, mortality has decreased dramatically, with rates between 25% and 40%.5,7,8
The concept of ‘malignant MCA territory infarction’, coined by Hacke et al.9 in 1996, refers to a specific type of ischaemic stroke which usually affects the entire MCA territory and may also extend to other vascular territories. This type of infarction produces a mass-effect secondary to oedema, mainly cytotoxic, and has a fatal clinical outcome in the majority of cases.10 It is most commonly caused by an embolic or thrombotic occlusion of the distal internal carotid artery or the main branch of the MCA (M1 segment). These occlusions are rarely recanalised, either spontaneously or after intravenous administration of tissue plasminogen activator.5,7–9
Although malignant MCA infarctions account for fewer than 10% of all supratentorial ischaemic strokes,5,7–9 their huge impact on mortality and quality of life has led researchers to look for new therapeutic strategies. In the last decade, several research groups have shown excellent results in experimental models of ischaemia using moderate hypothermia10; these results, however, are yet to be confirmed in a clinical setting.11–15
On the other hand, recent randomised studies have underlined the benefits of decompressive hemicraniectomy in terms of survival and functional outcome.16–19
The purpose of this review article is to critically analyse the options available for treating malignant hemispheric infarction (MHI).
Pathophysiology of MHIThe pathophysiological substrate of MHI is cerebral oedema, which usually presents between the first and third days after symptom onset.20 MHI exerts a mass effect which compresses, distorts, and herniates brain structures, resulting in neurological deterioration that may lead to death.5,7–9 From a pathophysiological viewpoint, severe decreases in cerebral blood flow to the ischaemic territory compromise normal functioning of the Na+-K+-ATPase pump in cell membranes, leading to the intracellular accumulation of sodium and water and resulting in cytotoxic oedema.7,21,22 At this early stage, no intravascular fluids are involved in the increase in brain volume. In addition, ischaemia increases permeability of the blood—brain barrier, thus enabling extracellular fluid accumulation, which increases the volume of the affected tissues (vasogenic oedema).7,21,22 Consequently, patients with MHI present both cytotoxic and vasogenic oedema. Tissue pressure increases as the volume of the brain parenchyma expands, compromising the normal function of the cerebral vasculature and altering brain metabolism and self-regulation. This creates a vicious circle that perpetuates and aggravates ischaemic damage.7,21,22
In some cases, endothelial damage allows macromolecules and red blood cells into the extracellular space, leading to haemorrhagic transformation of ischaemic brain tissue.7,21,22
‘Malignant’ MCA infarctions: definition and diagnostic criteriaHacke coined the term ‘malignant’ to describe large MCA territory infarctions due to the high mortality rate (nearly 80%) of this type of stroke even when optimal intensive treatment is used.9 Despite the more than 15 years that have passed, we still lack a consensual, validated, and universally accepted definition. MHI is clinically characterised by progressive deterioration of the level of consciousness, severe neurological impairment, and imaging findings indicating ischaemic changes affecting >50% of the MCA territory.5,7–9,22,23 These parameters were used as inclusion criteria in several randomised clinical trials evaluating the utility of DHC.16,17,19 In these clinical trials, a diagnosis of MHI was established based on the clinical examination (scores on the Glasgow Coma Scale and/or the NIH Stroke Scale) and an assessment of the extension of ischaemia in neuroimaging (CT and/or MRI).16,17,19
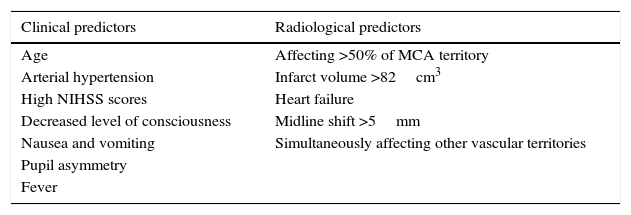
How to predict progression to malignant MCA infarction?Clinical assessment and neuroimaging tests are essential in cases of massive MCA infarction. Both parameters together provide the data necessary for early decision-making. The most relevant predictive factors are listed below (Table 1).
Middle cerebral artery infarction: clinical and radiological predictors of malignant progression.
| Clinical predictors | Radiological predictors |
|---|---|
| Age | Affecting >50% of MCA territory |
| Arterial hypertension | Infarct volume >82cm3 |
| High NIHSS scores | Heart failure |
| Decreased level of consciousness | Midline shift >5mm |
| Nausea and vomiting | Simultaneously affecting other vascular territories |
| Pupil asymmetry | |
| Fever |
Patients’ histories are relevant. A history of heart failure, arterial hypertension, ischaemic heart disease, and atrial fibrillation should be considered prognostic.8,24–26
The most predictive demographic factors in the clinical assessment are older age and, to a lesser extent, female sex.8,27 In the initial neurological evaluation, scores on the NIHSS or the Glasgow Coma Scale are closely correlated with in-hospital clinical progression, vital prognosis, and short- and long-term functional outcome.25 The risk of early deterioration of the level of consciousness and mortality is especially high in patients scoring ≥20 (left hemisphere involvement) or ≥15 (right hemisphere involvement) on the NIHSS within the first 6hours after symptom onset.8,25 Typical symptoms of complete MCA infarction include conjugate gaze deviation, severe sensory and motor impairment, global aphasia in patients with infarction of the dominant hemisphere, and inattention in the case of involvement of the right hemisphere. Severe lower limb weakness is suggestive of infarction of the subcortical structures supplied by the MCA (internal capsule) or simultaneous involvement of the territory of the anterior cerebral artery. Occlusion of the internal carotid artery with extensive frontal ischaemia should be suspected when patients present early impairment of the level of consciousness, whose deterioration may fluctuate.28–31 Nausea, vomiting, or hyperthermia within the first 24hours after symptom onset are warning signs of malignant oedema.7,24,25
Several biochemical markers with the ability to predict malignant progression have been described; these include hyperglycaemia,32 leukocytosis (>10000 leukocytes/mm3),24 cellular-fibronectin, matrix metalloproteinase-9,33 and protein S100B.34
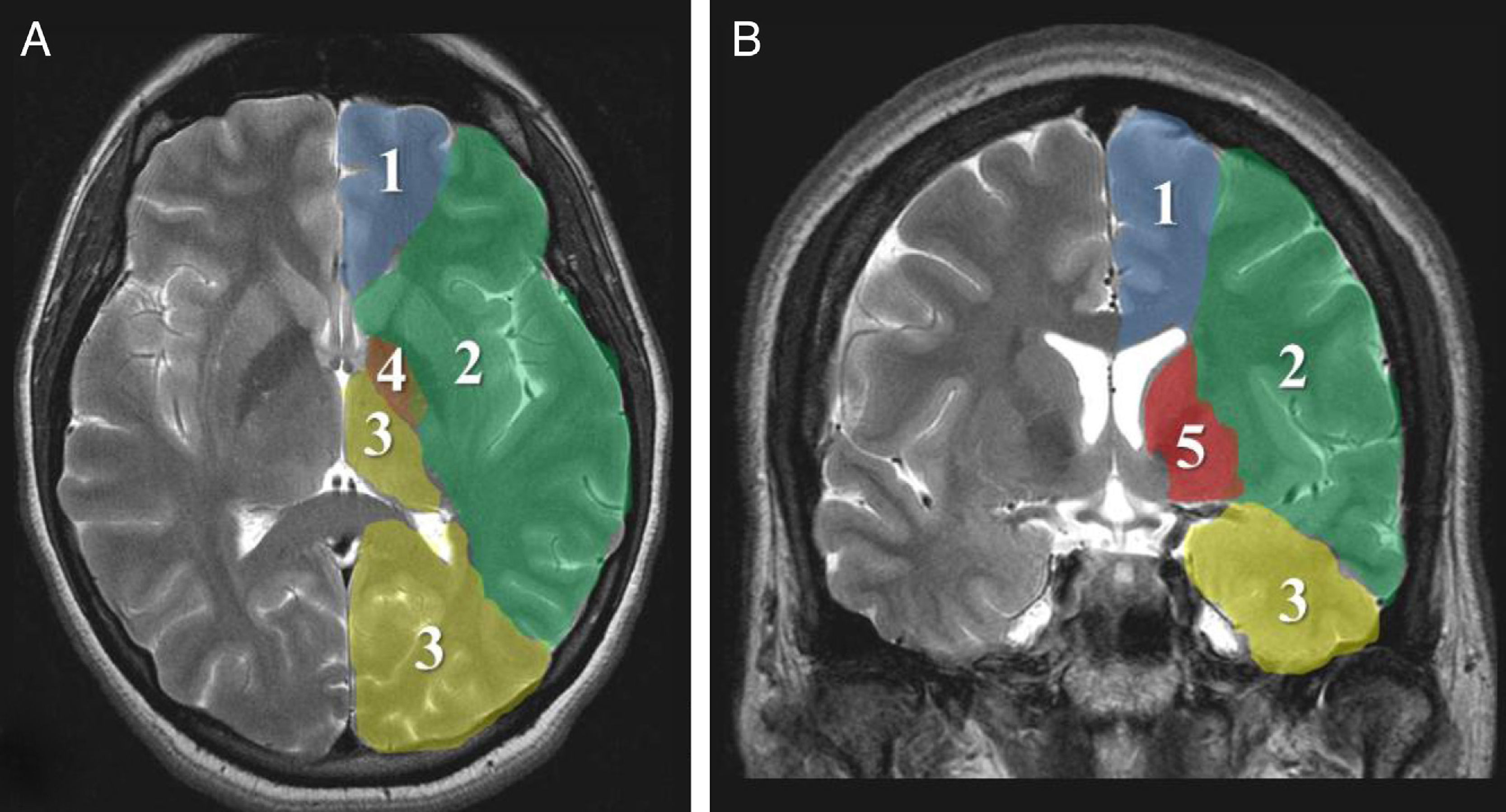
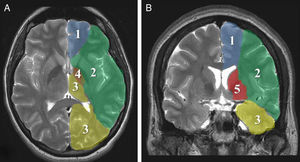
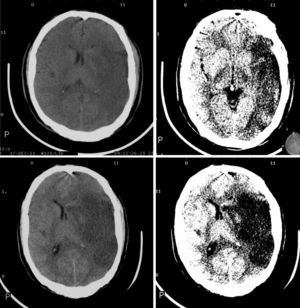
Imaging parametersThe ‘malignant’ progression of ischaemic stroke is related to extensive involvement of the MCA territory, including deep tissues irrigated by lenticulostriate arteries (Fig. 1). Simultaneous occlusion of other vascular territories (anterior and posterior cerebral arteries, anterior choroidal artery) triples the risk of malignant oedema.26
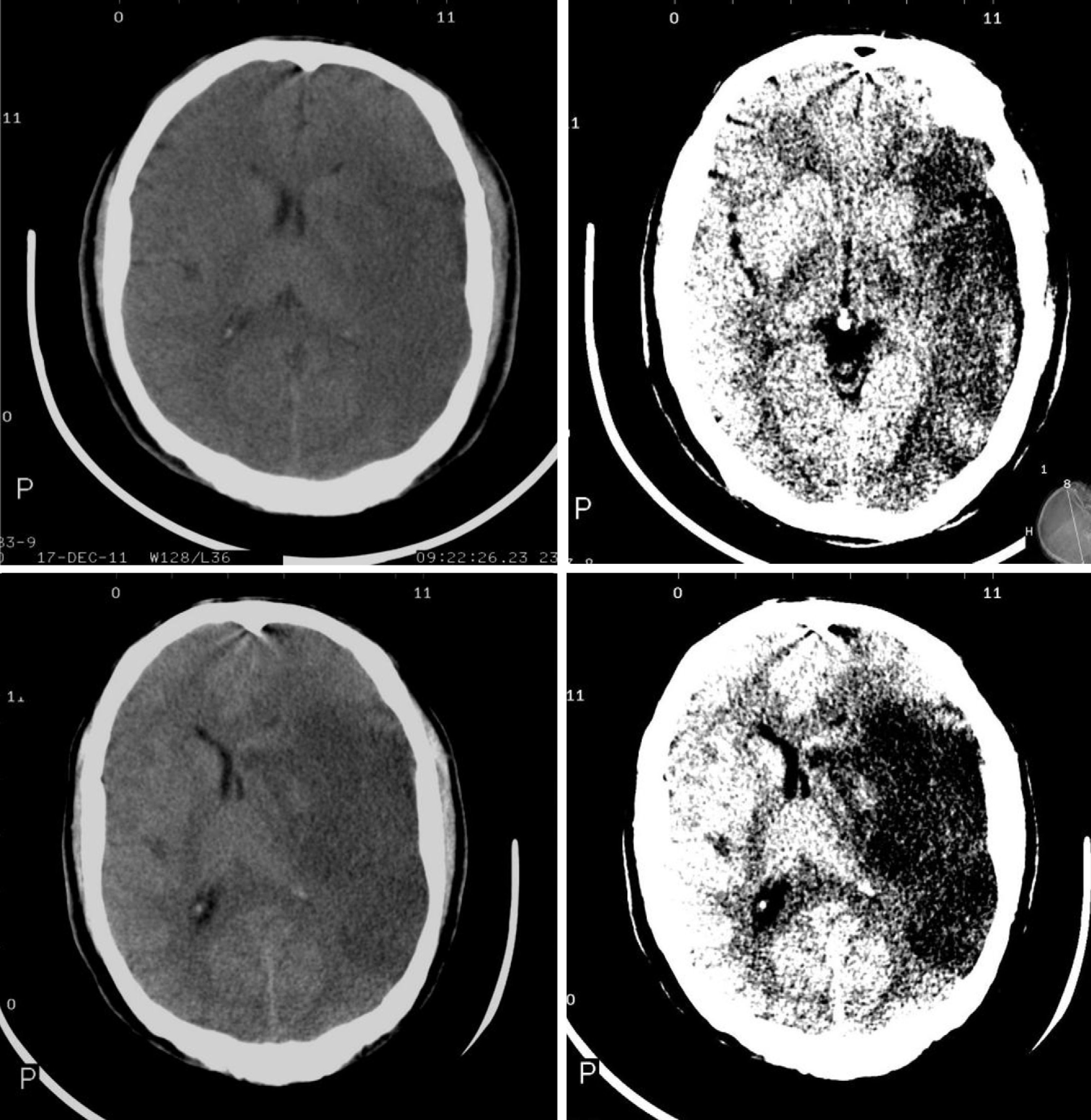
The CT findings with the highest predictive value for malignant progression are: involvement of more than two-thirds of the MCA territory, simultaneous involvement of other vascular territories (anterior and posterior cerebral arteries), and midline shift (Fig. 2).7,8,26,27,35
Although non-contrast CT is the most frequently used technique in the initial assessment of acute stroke,36 the increasing availability of MRI scans has improved our knowledge of the pathophysiology of this entity. DWI sequences (DWI-ADC) are much more sensitive to hyperacute ischaemic changes secondary to failure of the Na+-K+-ATPase pump.37–39 Calculating the volume of brain tissue showing restricted diffusion helps predict an eventual deterioration of the level of consciousness and the probability of brain herniation.37–39 In a study by Oppenheim et al.,40 ischaemic tissue volume <145cm3 was shown to predict deterioration of the level of consciousness in nearly 100% of patients. Recent studies using lower cut-off points (82-89cm3) have reported an only slightly lower sensitivity (85%) and a specificity reaching almost 95% even when the study was conducted within the first 2 to 6hours after symptom onset.37,41
From a physiological viewpoint, tolerance to increases in brain tissue volume secondary to oedema is greater in patients with a large subarachnoid space. Older patients with brain atrophy tolerate volume increases better than younger patients; in these, the main cause of death due to MCA infarction is brain herniation within the first week after stroke onset.27,29 Changes in brain tissue volume have been thoroughly studied in patients with stroke. In the case of patients with occlusion of the internal carotid artery or the main branch of the MCA, CSF volume, and more specifically, the ratio of brain blood volume to CSF volume at the time of admission may predict malignant progression. In a recent study, a ratio lower than 0.92 was found to be a predictive factor of clinical deterioration, with a sensitivity of 96.2% and a specificity of 96.2%.42
Other types of imaging studies used in these patients are perfusion CT/MRI, PET, and SPECT. However, these diagnostic tools are mainly limited to research.5,43
NeuromonitoringIntracranial pressure (ICP) is not a good predictor of neurological deterioration or progression to malignant infarction since it increases in only around 25% of cases.44,45 Recent studies on multimodal monitoring have provided new data. A tissue oxygen pressure <10.5mm Hg predicts malignant progression with a sensitivity of 94% and a specificity of 100%.8,43 Microdialysis shows that the levels of glutamate, glycerol, and lactate increase in the peri-infarct region.8,43 These and such other techniques as continuous electroencephalogram and detection of cortical spreading depression are still being developed.8
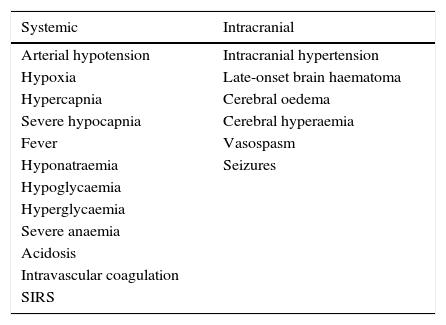
Neurointensive treatmentPhysiological neuroprotectionMedical treatment for MHI, as in other cases of acute brain damage, must aim to prevent secondary neurological deterioration, referring to those systemic or intracranial conditions than may worsen the primary lesion (Table 2).3,36,46
Secondary neurologic deterioration that may worsen the primary ischaemic lesion.
| Systemic | Intracranial |
|---|---|
| Arterial hypotension | Intracranial hypertension |
| Hypoxia | Late-onset brain haematoma |
| Hypercapnia | Cerebral oedema |
| Severe hypocapnia | Cerebral hyperaemia |
| Fever | Vasospasm |
| Hyponatraemia | Seizures |
| Hypoglycaemia | |
| Hyperglycaemia | |
| Severe anaemia | |
| Acidosis | |
| Intravascular coagulation | |
| SIRS |
SIRS: systemic inflammatory response syndrome.
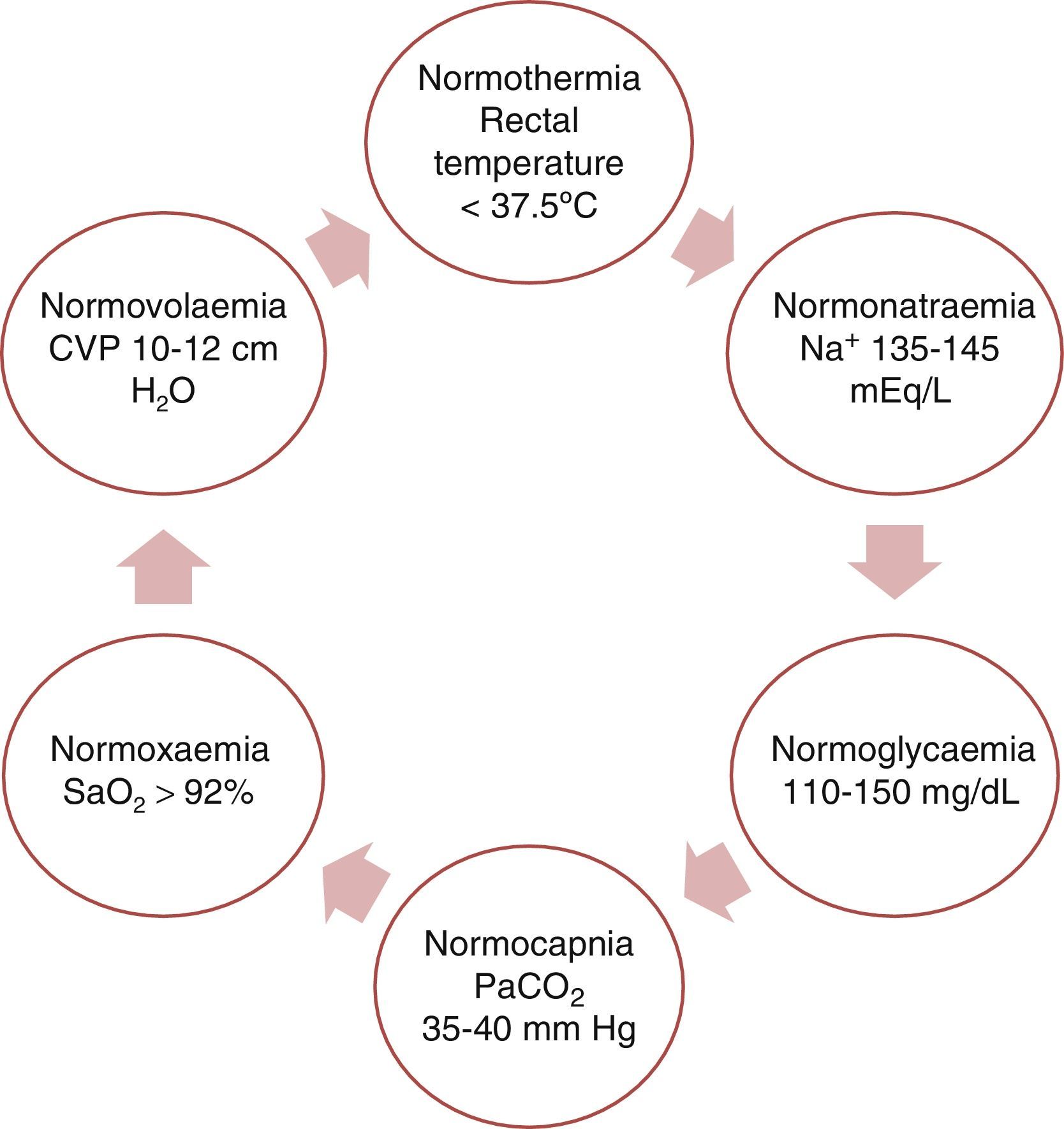
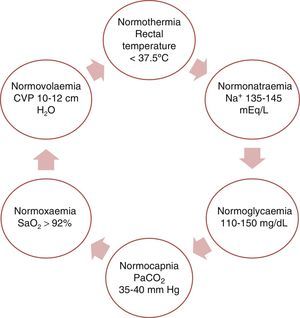
This purpose can be fulfilled by achieving physiological balance (the ‘6N rule’ as shown in Fig. 3).
Regarding arterial blood pressure, recommendations are identical to those for patients with ischaemic stroke.3,36,46 During the postoperative period of DHC, systolic blood pressure should be maintained at 140 to 160mmHg to minimise the risk of bleeding.36
Likewise, we should not forget other more general measures that are applied to critical care patients, including nutritional support, prevention of gastrointestinal bleeding and venous thromboembolism, etc.3,36,46
Recanalisation of occluded vesselsIntravenous thrombolysis within a narrow time window (until 4.5h from symptom onset) is the only treatment with class I evidence available today47; the ECASS III trial did not include any patients scoring >25 on the NIHSS.48 Early restoration of the circulation may decrease the size of the infarction and the area of oedema, which may in turn prevent malignant progression, at least theoretically.47,48 Recanalisation rates after administration of tissue plasminogen activator are low for occlusions of such large vessels as the internal carotid artery or the main branch of the MCA.49 Intra-arterial thrombolysis and endovascular embolectomy may achieve higher recanalisation rates, although these procedures have not yet been studied in randomised controlled clinical trials.49
Antioedema therapyClinicians managing patients with MHI should apply the basic principles of critical neurological care: avoid administration of hypotonic fluids (dextrose 5%, lactated Ringer solution) and hypoventilation, since hypernatraemia and hypercapnia exacerbate cerebral oedema.3,50 The patient's head should be placed in a neutral position (with the neck neither flexed nor extended); there is controversy on whether it is preferable to keep it slightly raised or in a flat position. Elevating the patient's head 30° helps draining the blood and CSF, and it also reduces the possibility of microaspiration of gastric contents.2,48 Clinical trials on patients with MHI suggest that both cerebral perfusion pressure and mean blood flow velocities in transcranial Doppler scans are greater with the head in a horizontal position.51,52
The purpose of the present review article is not to provide a detailed analysis of the available options for treating cerebral oedema secondary to ischaemia. However, all the therapeutic approaches available today share a number of characteristics that should be considered before choosing a treatment3,50:
- •
Most of them need the cerebrovascular physiology to be preserved (self-regulation, reactivity to CO2, intact permeability of the blood–brain barrier).
- •
Therapeutic effects are normally transient and limited.
- •
They may have the opposite effect.
- •
Discontinuation may have a rebound effect.
- •
They may cause severe and even fatal adverse effects.
- •
There is no solid scientific evidence on the use of these treatments according to the principles of evidence-based medicine.53,54
We should also mention some ideas specifically linked to management of MHI.5,6,50,55–58 Hyperventilation should only be used in case of emergency, such as when clinical signs of herniation are present, and should be used moderately (PaCO2 30-48mmHg) for short intervals to prevent the aggravation of ischaemia due to excessive vasoconstriction.5,8,49,55–58 The following osmotic agents may be used: mannitol, glycerol, and hypertonic saline solutions. The latter have the advantage that they require a smaller infusion volume and are recommended for patients with renal damage, since mannitol should not be used in these patients.5,7,8,49,55–58 Although there are few studies comparing hypertonic saline solutions and mannitol, both treatment options may be similarly effective if they are administered at equimolar doses.5,7,8,49,55–58 Hypertonic saline solutions at a concentration above 3% should be administered with a central venous catheter and closely monitored in patients with congestive heart failure.5,7,8,49,55–58 A barbiturate-induced coma should only be used in highly refractory patients; however, it should not be considered a therapeutic option due to the high rate of severe complications.5,7,8,49,55–58 Corticosteroids are not a treatment option for ischaemic oedema.5,7,8,56–58
Although the neuroprotective effects of hypothermia are well known, few studies have addressed its use in patients with MHI. To date, the randomised clinical trials comparing moderate hypothermia (33°C for 24h) with standard medical treatment for acute ischaemic stroke (though not limited to patients with a malignant course) are early-phase clinical trials; therefore, firm conclusions cannot be drawn from their data.59,60 However, hypothermia should only be used in clinical trials due to the high rate of patients presenting pneumonia as an adverse effect of the treatment60 and the lack of functional improvements.59,60
Hypothermia was compared with DHC in a cohort of patients with large MCA infarction.61 In this study, 36 patients were treated according to the affected hemisphere: while patients with infarction of the dominant hemisphere were treated with moderate hypothermia, those with infarction affecting the non-dominant hemisphere underwent DHC. No differences were found between groups in terms of age, neurological state (NIHSS scores), or infarct volume. Mortality was significantly higher in the group treated with hypothermia (47% vs 12%) mainly due to refractory intracranial hypertension.61
Hypothermia combined with DHC has been suggested as a therapeutic option since these 2 treatments have completely different mechanisms of action and may therefore have a synergistic effect.62 In a study including a small series of 25 patients undergoing DHC, mild hypothermia was subsequently induced in 12 patients (35°C).62 Although no differences were found in mortality between groups, functional outcome was better at 6 months (Barthel Index and NIHSS) in the group treated with hypothermia.62
In conclusion, large-scale carefully designed studies are necessary to further determine the effectiveness of hypothermia. The DEPTH-SOS clinical trial is currently underway.63
Should we monitor ICP?Early incidence of intracranial hypertension in patients with MHI barely reaches 25%, and in most cases, clinical deterioration occurs in the absence of high ICP.44 Poca et al. correlated the findings of intraparenchymatous ICP monitoring with pupillary abnormalities and neuroimaging findings.45 ICP values remained normal in 63% of the patients with a midline shift >5mm (6.7±2mm) and ischaemic tissue volume ≥241.3±83cm3; these values were also normal in 2 patients with anisocoria.45
In a study by Schwab et al.,64 treatment for intracranial hypertension used conventional measures and followed a standardised protocol with ICP monitoring. The pharmacological treatment was ineffective; in addition, clinical signs of herniation preceded the increase in ICP, which is not a good predictor for deterioration in these patients.64
ICP monitoring may be valuable in certain circumstances65: (1) when clinicians decide on pharmacological treatment; in these cases, ICP values may guide treatment and help assess response to treatment (hyperventilation, osmotherapy, etc.); (2) when a clinical examination cannot be performed due to anaesthesia or sedoanalgesia during mechanical ventilation; (3) after surgery: sudden changes in ICP may indicate haemorrhage secondary to decompression, accumulation of extra-axial blood, or worsening of mass-effect due to hemicraniectomy with unsatisfactory results.65
Other monitoring techniques such as tissue oxygen pressure may provide valuable information for making decisions based on the pathophysiology of the disease.5 CT and duplex sonography are useful tools for monitoring midline shifts and should be used for monitoring progression in these patients.66
Surgical decompression: clinical effectivenessDHC is a surgical technique aimed at increasing the potential volume of the cranial cavity, allowing the oedematous brain to expand eccentrically, which in turn prevents compression of the brainstem, reduces ICP, and increases cerebral blood flow and tissue oxygenation.
Clinical studies assessing DHC have consistently shown beneficial short- and medium-term effects. However, there is controversy regarding functional outcomes.
Three European multicentre randomised controlled studies analysed the effects of DHC in patients with MHI; 2 of them were terminated prematurely due to the low recruitment rate and the significant differences in mortality rates, with the surgical group showing significantly decreased rates.17,19
The DECIMAL study included 38 patients undergoing surgery within the first 30hours after symptom onset. This study reported a mortality rate of 52.8% at one year after stroke,19 whereas the DESTINY study, which only included 32 patients, showed a 41% absolute reduction in mortality.17
The results of the HAMLET trial were published in 2009; this trial randomised 64 patients within 96hours after symptom onset.16 Although no differences in functional status were found between the groups, the patients undergoing DHC within 48hours after symptom onset displayed greater improvements. A pooled analysis of the HAMLET, DESTINY, and DECIMAL trials18 showed that 75% of the patients undergoing DHC scored ≤4 on the mRS whereas the percentage in the pharmacological group was 24%. Functional improvements may be explained by the fact that DHC prevents further compromise of microcirculation in the penumbra due to regional mass-effect. However, further studies should be conducted to determine the mechanisms allowing neurological recovery in surgery patients.67–71
Age is the main predictor of functional outcome after DHC.67–69 A systematic review comparing clinical results between elderly patients and patients younger than 60 found significant differences in mortality (51.3% vs 20.8%) and a much higher disability rate in older patients (81.8% vs 33.1%).67 In a recent prospective randomised study published in China and including 47 patients of up to 80 years old, more than half of the patients undergoing DHC (n=29) were older than 60 (61-78). The results showed marked benefits in the surgery group in terms of mortality and functional outcome. Results were similar for the subgroup of patients older than 60.72 The DESTINY II trial is currently underway. The purpose of this trial is to determine the utility of DHC specifically in patients aged 60 to 75.73
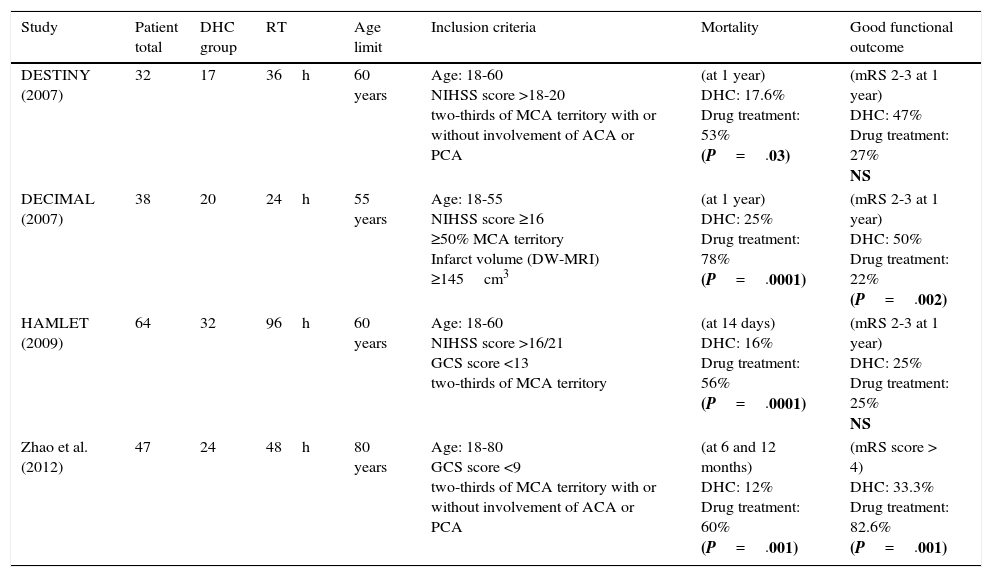
Table 3 compares the main characteristics of the randomised studies assessing DHC for MHI.
Characteristics of the published randomised trials analysing DHC in patients with MHI.
| Study | Patient total | DHC group | RT | Age limit | Inclusion criteria | Mortality | Good functional outcome |
|---|---|---|---|---|---|---|---|
| DESTINY (2007) | 32 | 17 | 36h | 60 years | Age: 18-60 NIHSS score >18-20 two-thirds of MCA territory with or without involvement of ACA or PCA | (at 1 year) DHC: 17.6% Drug treatment: 53% (P=.03) | (mRS 2-3 at 1 year) DHC: 47% Drug treatment: 27% NS |
| DECIMAL (2007) | 38 | 20 | 24h | 55 years | Age: 18-55 NIHSS score ≥16 ≥50% MCA territory Infarct volume (DW-MRI) ≥145cm3 | (at 1 year) DHC: 25% Drug treatment: 78% (P=.0001) | (mRS 2-3 at 1 year) DHC: 50% Drug treatment: 22% (P=.002) |
| HAMLET (2009) | 64 | 32 | 96h | 60 years | Age: 18-60 NIHSS score >16/21 GCS score <13 two-thirds of MCA territory | (at 14 days) DHC: 16% Drug treatment: 56% (P=.0001) | (mRS 2-3 at 1 year) DHC: 25% Drug treatment: 25% NS |
| Zhao et al. (2012) | 47 | 24 | 48h | 80 years | Age: 18-80 GCS score <9 two-thirds of MCA territory with or without involvement of ACA or PCA | (at 6 and 12 months) DHC: 12% Drug treatment: 60% (P=.001) | (mRS score > 4) DHC: 33.3% Drug treatment: 82.6% (P=.001) |
ACA: anterior cerebral artery; MCA: middle cerebral artery; PCA: posterior cerebral artery; GCS: Glasgow Coma Scale; h: hours; DHC: decompressive hemicraniectomy; MHI: malignant hemispheric infarction; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; MRI: magnetic resonance imaging; RT: randomisation time.
Although DHC is recommended by the European Stroke Organisation, it is very rarely used in clinical settings. According to a study, only 10% of patients undergo surgery.74 This may reflect the belief that surgery results in neurological deficit and poor quality of life, although randomised clinical trials conducted to date prove the opposite.70,75 Several studies have randomly divided patients’ functional prognosis into good (mRS score ≤3) or poor (mRS score ≥4) with a view to performing dichotomous analyses. However, and despite limited functional independence, patients scoring 4 on the mRS have been shown to be able to function in a social environment with the support of their relatives.70,75,76
Regarding quality of life, several studies have found a positive relationship between DHC and mobility, household management, body care, and independence for activities of daily living.77–81 Most patients with MHI have sequelae that prevent them from leading a normal life, but quality of life is greater in patients who underwent DHC.16,77 This can be seen in the proportion of patients who would give retrospective consent, that is, patients who would again consent to surgery given the experienced preoperative clinical symptoms and their functional outcomes.77–81 Hofmeijer et al. found that, although 22 of 38 survivors scored 4 or 5 on the mRS, 21 were satisfied with the treatment.16
In light of these factors and before considering surgery, patients should receive sufficient and adequate information reflecting current evidence to ensure they understand the benefits of this treatment and the possibility of needing special care for life.
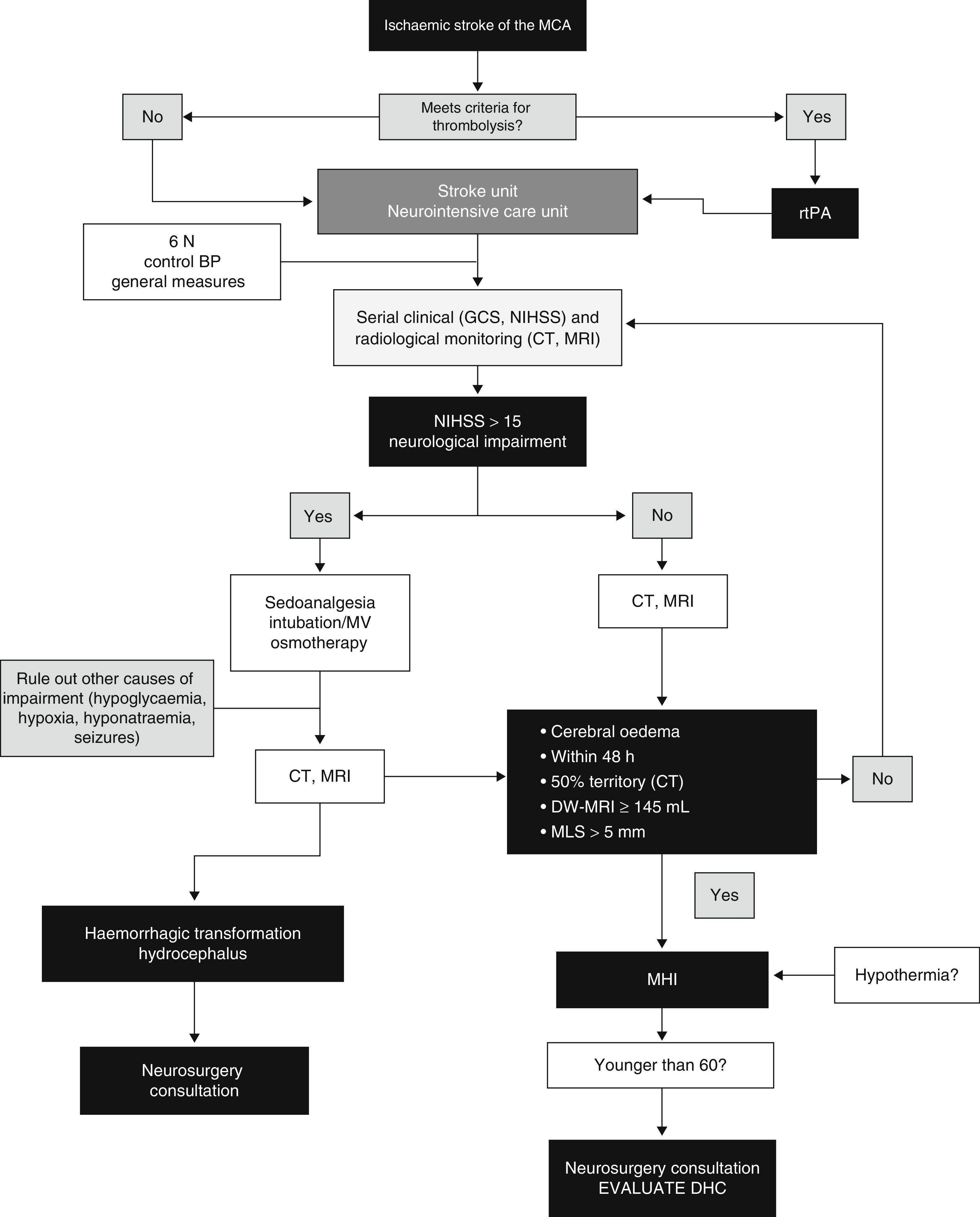
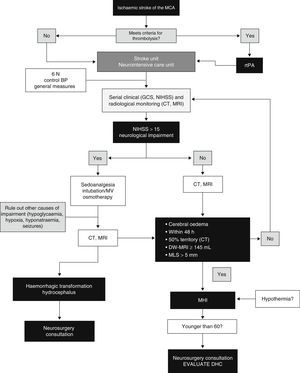
ConclusionsThe main feature of MHI is cerebral oedema with mass-effect; the volume of oedema in neuroimaging is the factor showing the highest predictive value. Clinical deterioration is caused by mass-effect with lateral herniation. This finding is rarely associated with intracranial hypertension; therefore, monitoring ICP is not a reliable method for detecting herniation. DHC decreases mortality rates significantly and improves functional results in some cases when compared to conservative treatment. However, we should highlight that most survivors are left with moderate to severe disabilities. Self-perceived quality of life in patients surviving DHC should be studied in more detail. Several studies evaluating different aspects of DHC are currently underway. These are aimed at determining the ideal moment for surgery, analysing its indications and contraindications, establishing optimal age groups, and assessing the impact of complications. Likewise, multimodal monitoring and DHC combined with hypothermia show promising results and require further research. A multidisciplinary team is the cornerstone of the treatment for MHI. Fig. 4 provides an example of a management algorithm for this entity.
Suggested management algorithm for decision-making in MHI. MCA: middle cerebral artery; MV: mechanical ventilation; MLS: midline shift; GCS: Glasgow Coma Scale; DHC: decompressive hemicraniectomy; MHI: malignant hemispheric infarction; NIHSS: National Institutes of Health Stroke Scale; MRI: magnetic resonance imaging; rtPA: recombinant tissue plasminogen activator; BP: blood pressure; CT: computed tomography.
The authors have no conflicts of interest to declare.
Please cite this article as: Godoy D, Piñero G, Cruz-Flores S, Alcalá Cerra G, Rabinstein A. Infarto hemisférico maligno de la arteria cerebral media. Consideraciones diagnósticas y opciones terapéuticas. Neurología. 2016;31:332–343.