Migraine has become an important vascular risk factor during the past few years, along with the presence of white matter and clinically silent ischemic lesions. Whether these findings contribute to the migraine becoming chronic has been a source of debate. People with chronic migraine also have a less favorable metabolic profile.
An exhaustive review of the literature has been made in order to try to clarify the relationship between migraine and vascular risk factors.
DevelopmentMigraine, particularly with aura and in women <45 years-old, is associated with an increased risk of cerebral infarction. This risk increases if the patient smokes or uses oral contraceptives. Migraine can also be a direct cause of a stroke, although it is an infrequent complication. Migraine with aura is associated with a risk factor of 12 of having subclinical infarctions in posterior fossa circulation.
ConclusionsSince migraine is an independent vascular risk factor, better control of migraine attacks, as well as other possible concomitant vascular risk factors, should decrease the likelihood of a stroke. Overall, the real risk of infarction is low, with 3.8 new cases per 100,000 women and year.
En los últimos años ha cobrado relevancia la migraña como factor de riesgo vascular así como la presencia de lesiones inespecíficas de sustancia blanca y lesiones isquémicas clínicamente silentes. Se ha intentado relacionar la presencia de estos hallazgos en la neuroimagen con la cronificación de la migraña. A esto hay que añadir la detección de un peor perfil metabólico en pacientes migrañosos.
Con el fin de aclarar la relación entre la migraña y las alteraciones vasculares cerebrales, se ha realizado una exhaustiva revisión de la literatura.
DesarrolloMúltiples estudios han demostrado una asociación significativa entre la migraña, especialmente la migraña con aura (MCA), y el riesgo de infarto cerebral, sobre todo en mujeres < 45 años. El riesgo de ictus aumenta en presencia de otros factores asociados: más de 3 veces con hábito tabáquico y más de 4 veces con el consumo de anticonceptivos orales (ACO). La migraña puede causar directamente un infarto isquémico, aunque es infrecuente. La MCA tiene un riesgo 12 veces superior de infartos subclínicos en fosa posterior.
ConclusionesComo la migraña es un factor de riesgo vascular independiente, se presupone que un mejor control de la misma, así como de otros factores de riesgo vascular asociados, disminuirán la incidencia de ictus. Se aconseja un abandono del hábito tabáquico y suprimir el uso de ACO, sobre todo en mujeres con MCA. A pesar de todo, el riesgo absoluto de infarto es bajo y se traduce aproximadamente en 3,8 casos adicionales por cada 100.000 mujeres al año.
Migraine is one of the head complaints arousing the most interest in the scientific community. In recent years, there has been great interest in its relationship with ischemic events: migraine as a vascular risk factor, the presence of clinically silent strokes in patients with migraine, and stroke as a direct complication of migraine with aura (MA). This new approach to previously existing and known problems has reopened the debate on the role of blood vessels in the pathophysiology of migraine. To complicate matters further, population studies have shown that patients with migraine, particularly MA, present a higher incidence of dyslipidemia, smoking, arterial hypertension and myocardial ischemia than populations without migraine.1–3
The aim of this study was to review the relationship between migraine, stroke and migraine as a vascular risk factor.
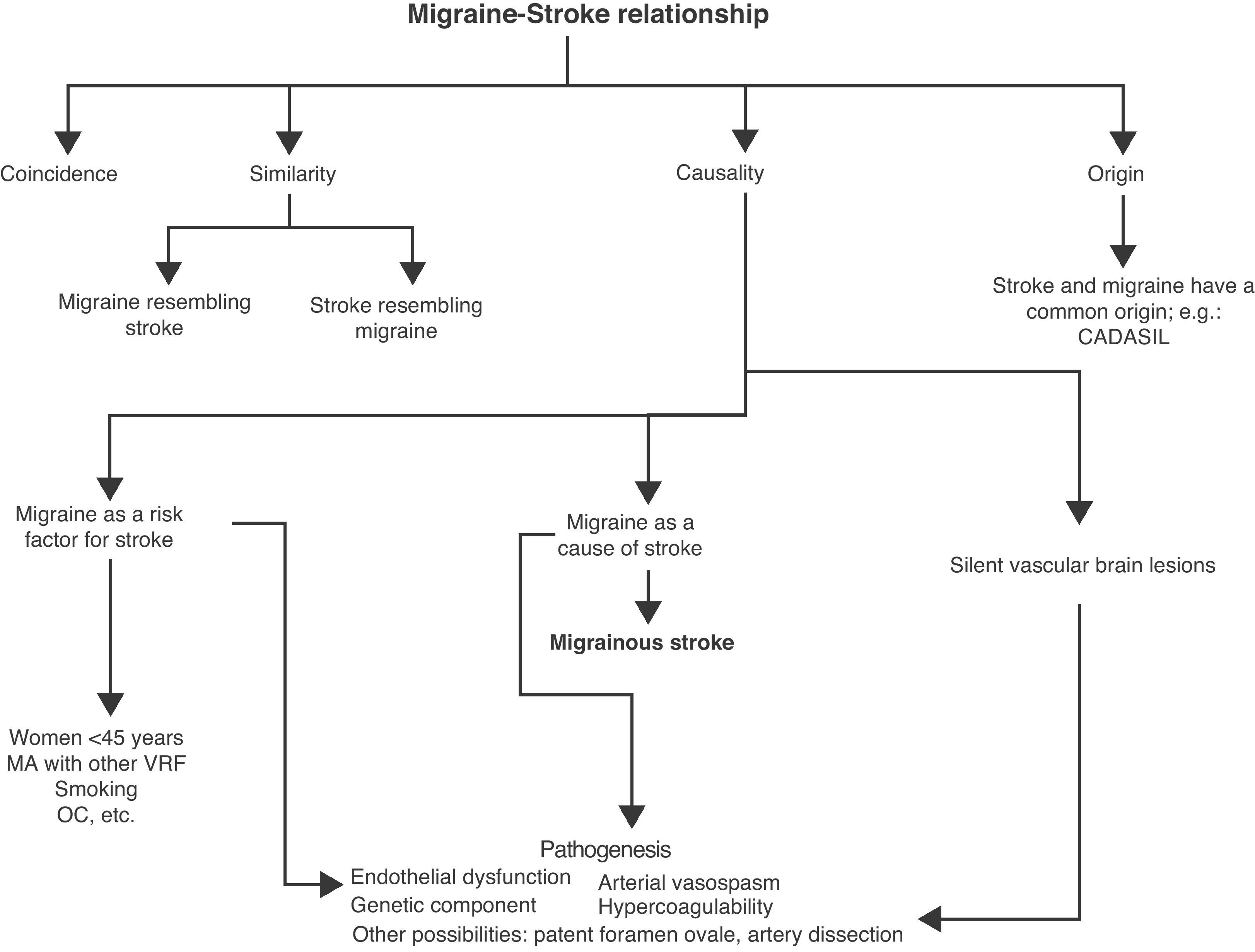
Migraine and stroke: relationship of comorbidityAlthough there are various clinical and pathogenic differences between migraine and stroke, there is a complex and bidirectional relationship between the 2 factors. It is one that, generically, has been described or defined as “comorbid”.4 The following may be confounding factors in this relationship (Fig. 1):
- 1.
Relationship of coincidence. The 2 conditions may coexist without a necessary causal relation. For example, a stroke can appear in a migraine patient.
- 2.
Relationship of similarity.
- –
Migraine resembling stroke. Different types of migraine resemble cerebrovascular syndromes, in particular some forms of MA, especially when the aura is prolonged. This is the case of hemiplegic migraine, basilar migraine and persistent aura without stroke.5
- –
Stroke resembling migraine. Stroke can appear with pain resembling migraine. This is more frequent in strokes of the posterior cerebral artery (42%) and exceptional in lacunar strokes.6,7
- –
- 3.
Relationship of origin. Migraine and stroke are manifestations of the same underlying process. There is a common cause (usually genetic) justifying the onset of migraine (especially MA) and stroke in the same patient. This can occur in cases of arteriovenous malformation, leptomeningeal angiomatosis (Sturge–Weber syndrome), hereditary hemorrhagic telangiectasia, CADASIL, MELAS or in hereditary cerebroretinal vasculopathy syndromes.8,9
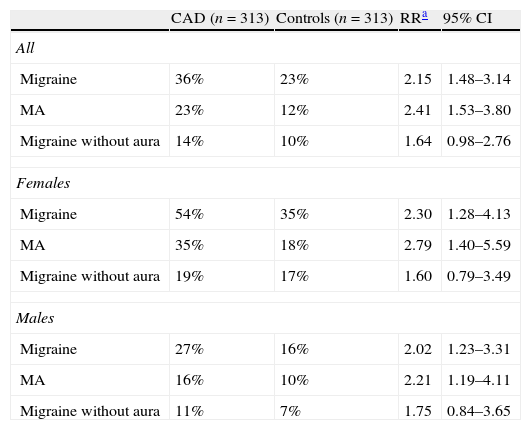
In addition, migraine patients have an increased risk of suffering cervical artery dissections (CAD) (adjusted RR 3.6; 95% CI, 1.5–8.6), especially of the vertebral artery.10–13 A recent study conducted on 313 patients with CAD and 313 controls confirmed the association between migraine and CAD, and was especially significant for MA and in women (Table 1).13 The lifetime prevalence of migraine in patients with CAD was 36%. Up to 51% of patients with CAD and migraine experienced an improvement independent of the territory affected or the presence of stroke.13
Prevalence of migraine in patients with cervical artery dissection vs. controls.
| CAD (n=313) | Controls (n=313) | RRa | 95% CI | |
| All | ||||
| Migraine | 36% | 23% | 2.15 | 1.48–3.14 |
| MA | 23% | 12% | 2.41 | 1.53–3.80 |
| Migraine without aura | 14% | 10% | 1.64 | 0.98–2.76 |
| Females | ||||
| Migraine | 54% | 35% | 2.30 | 1.28–4.13 |
| MA | 35% | 18% | 2.79 | 1.40–5.59 |
| Migraine without aura | 19% | 17% | 1.60 | 0.79–3.49 |
| Males | ||||
| Migraine | 27% | 16% | 2.02 | 1.23–3.31 |
| MA | 16% | 10% | 2.21 | 1.19–4.11 |
| Migraine without aura | 11% | 7% | 1.75 | 0.84–3.65 |
CAD: cervical artery dissection; MA: migraine with aura; RR: relative risk.
The most intriguing part about the relationship between migraine and stroke is the possibility that migraine is an independent risk factor for stroke, or that it may be the cause of stroke.
If migraine is a vascular risk factor, stroke occurs independently on time with respect to a typical migraine.
If instead stroke occurs as a complication of migraine, then it should develop during the course of a migraine (Table 2).14 From the pathogenic point of view, the term “migraine-induced stroke” would be preferable.
Diagnostic criteria for migrainous stroke (1.5.4) (IHS).a
| A. The current attack is triggered in a patient with a diagnosis of “migraine with aura” and is similar to previous attacks except for that 1 or more aura symptoms persist for more than 60min |
| B. Neuroimaging demonstrates an ischemic stroke in a relevant area |
| C. It cannot be attributed to any other disorder |
This distinction is missing in the vast majority of studies conducted in patients with stroke and a history of migraine.
Migraine as a risk factor for strokeMultiple case–control studies have shown a significant association between migraine and the risk of stroke, especially in women <45 years.1,5,15–25
Considering this subgroup (women <45 years), the risk is especially high for MA (relative risk between 1.3 and 14.8) in relation to migraine without aura (RR between 0.8 and 3).15,17,18,21–23,26–28 This risk persists even when the influence of other vascular risk factors is excluded (RR adjusted for MA between 1.5 and 6.2 and for migraine without aura between 0.8 and 3).5,17,18,22,24,26,28
Some studies have also shown a positive, although not significant, association in males,1 while others have not.19,27 There are far fewer studies including patients >45 years. In this sense, the results have also been varied.11,17,18,25 In any case, this increase in the adjusted relative risk for age >45 years (1.5 times the risk for all types of stroke and 1.7 for ischemic stroke) is lower than in patients aged <45 years. This difference may be due to a higher prevalence of other risk factors for stroke in the population aged >45 years.17,18
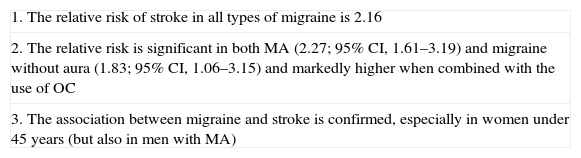
Although migraine is an independent risk factor for stroke, the risk of stroke increases in the presence of other associated factors. It increases more than 3 times in migraine patients who smoke and more than 4 times in migraine patients who use oral contraceptives (OC)26,28; the higher the dose of estrogen contained in birth control pills, the higher the risk of stroke.26 The risk is even greater if both factors coincide28 or if more vascular risk factors are added. Despite these relatively high relative risk figures, absolute risk remains low and roughly translates into 3.8 additional cases per 100,000 women per year.18 Recently, a series of findings have suggested that neither OC nor smoking, both evaluated independently, substantially increase the probability of stroke in women with MA.22 Still, the combination of both has resulted in a tenfold increase in the risk of ischemic stroke compared with women without migraine who do not smoke or use OC.22Table 3 summarizes the findings of a recent metaanalysis.29
Conclusions on the risk of ischemic stroke among migraine patients.a
| 1. The relative risk of stroke in all types of migraine is 2.16 |
| 2. The relative risk is significant in both MA (2.27; 95% CI, 1.61–3.19) and migraine without aura (1.83; 95% CI, 1.06–3.15) and markedly higher when combined with the use of OC |
| 3. The association between migraine and stroke is confirmed, especially in women under 45 years (but also in men with MA) |
MA: migraine with aura.
A prospective study of records from primary care centers (n=3502) determined that 3.7% of ischemic stroke patients suffered active migraine (at least 1 migraine in the previous month). Considering the subgroup of subjects aged <45 years with stroke, 15.8% suffered active migraine compared to 2.1% of subjects aged ≥45 years. Therefore, it follows that the frequency of migraine attacks and the recent onset of migraine syndrome, and not just the diagnosis of migraine, are factors that influence the risk of stroke. In contrast, the intensity of migraine was not a determining factor.22
In relation to the different subtypes of ischemic stroke, the proportion of lacunar strokes and strokes of undetermined origin (not of atherosclerotic or cardioembolic strokes) was higher among women with MA than among subjects without migraine.22
If we consider studies from hospital records, we can observe that migraine is the cause of 0.8% of all ischemic strokes registered, as well as between 5%30 and 20%31,32 of strokes in patients younger than 50 years.
Migraine, particularly MA, also seems to be a risk factor for strokes in the coronary bed. There is an increased adjusted relative risk of developing any vascular event, which is 2 times higher in women with MA with respect to those without migraine. This increase was not observed for migraine without aura.33 This increased diathesis for general vascular pathology also affected males.33
Migrainous strokeMigraine may directly cause ischemic stroke,14 although this is a very rare complication.
Only 1 study has evaluated the incidence of migrainous stroke (MS) strictly following the definition of the IHS.34 This study found that the incidence of MS was 0.5% of all strokes in all age groups, but rose to 10–14% when considering only stroke in individuals younger than 45 years. Although there are cases described of stroke that occurred during an attack of migraine without aura,35–37 IHC-II confirmed MS as a complication of only MA.
The classical phenotype of a subject with MS is that of a woman aged <45 years with MA, often displaying other associated vascular risk factors (smoking and consumption of OC) and particularly affecting the posterior region.35 Stroke size is moderate or small (<3cm in maximum diameter) and generally with a good functional recovery.
Migraine and subclinical vascular brain lesionsWhite matter (WM) lesions of uncertain significance are a common finding in cranial MRI scans of migraine patients. Small-sized hyperintensities are observed (on T2-weighted sequences and FLAIR), especially in the periventricular and deep WM. In very rare instances, detection of these small lesions has led to the diagnosis of an underlying disease (CADASIL, MELAS, Fabry disease, adrenoleukodystrophy, inflammatory, infectious or toxic-metabolic pathologies, etc.).
Up to 10% of individuals in the general population in their fourth decade of life and up to 80% in their eighth decade present such lesions.38 Their prevalence and number increase when cardiovascular risk factors are present (diabetes, arterial hypertension, hypercholesterolemia, consumption of tobacco) or if there is cardio-cerebrovascular disease and dementia.39
The results on the prevalence of WM lesions in migraine patients differ according to their location (deep vs. periventricular). The presence of subclinical lesions in the deep WM is 4 times more prevalent in women with migraine (both MA and migraine without aura) than in those without migraine. Furthermore, the risk of these lesions appears to be independent of age and the presence or absence of associated vascular risk factors.40,41 On the other hand, there is a significant association in women suffering >1migraine/month.41,42 There have been no differences in the prevalence of these lesions in males. However, in periventricular WM lesions, no association with the presence of migraine attacks (with and without aura) or their frequency has been found in males or females.41
An epidemiological study on brain abnormalities in migraine also supports the observation that 8.1% of patients with MA and 2.2% of patients with migraine without aura (vs. 0.7% controls [P=.05]) present subclinical brain lesions similar to strokes, even in the absence of a clinical history of stroke.41,42 These small lesions are often multiple and preferentially located in the posterior vascular territory, especially in the cerebellum.41,43 This association is especially significant in women, among whom the prevalence of cerebellar stroke is 23% in MA patients vs. 14.5% in women without migraines (relative risk 1.9; 95% CI, 1.4–2.6).42 In the case of males with MA, the prevalence was 19% vs. 21% of controls.42 Once again, the highest risk was observed in patients with MA who had ≥1attacks/month.42,43 The presence of these lesions is not necessarily associated with the presence of lesions in the deep WM.
Overall, patients with MA had a 12 times higher risk of developing these lesions. The presence or absence of traditional vascular risk factors and specific migraine medication with a vasoconstrictor effect did not alter that association.41,43 This suggests that, when present, such lesions do not have an arteriosclerotic origin or reflect small-vessel disease. The most likely aetiological mechanism is a combination of hypoperfusion (which may be related to the migraine) and embolism.41
PathogenesisThe mechanisms by which migraine becomes a vascular risk factor and leads to the development of MS are not entirely known, possibly because they are multiple and complex. They are summarized below.
Migraine as a risk factor for ischemic strokeThe hypotheses that follow are contemplated and complementary with each other.44
Vascular endothelial dysfunctionMigraine can modify the homeostatic properties of the endothelium, favoring a prothrombotic, pro-inflammatory and proliferative condition that predisposes towards atherogenesis and the development of thrombotic events other than migraine.45,46 Endothelial dysfunction is the result of (a) a reduction in the bioavailability of vasodilator substances (nitric oxide, for example) and an increase in contractile factors derived from the endothelium, causing an alteration in vascular reactivity (including the microvasculature), and (b) oxidative stress, which in turn promotes inflammatory processes. Oxidative stress markers are increased in migraine patients.47
It is thought that prothrombotic, pro-inflammatory and other vasoactive peptides are released during migraine attacks, and that these could damage the vascular endothelium. On the other hand, cortical depression (CD) involves an alteration in the permeability of the BBB through the activation of metalloproteinases.48 Their activation causes a direct cellular damage that, along with the release of vasoactive neuropeptides during migraine attacks, may stimulate inflammatory responses inside and outside of the brain.49
It has been postulated that migraine patients, particularly those with MA, have a decreased endothelial regeneration capacity.50 In this sense they have been observed to have decreased levels of endothelial progenitor cells (measured by flow cytometry), with a decrease in their migratory capacity and increased senescence markers. These progenitor cells are derived from bone marrow, circulate in the peripheral blood and are able to proliferate and differentiate into endothelial cells and play a role in angiogenesis after ischemia.51,52 Although it is not known if a reduction in endothelial progenitor cells represents a primary abnormality in migraine or is a consequence of repeated migraine attacks, their alteration acts may possibly act as a factor that mediates the increased vascular risk.
Migraine is associated with an increased prevalence of vascular risk factorsSubjects with migraine and especially with MA have a more adverse profile with respect to vascular risk factors than controls (worse lipid profiles, arterial hypertension) and are more likely to smoke, consume OC and present a family history of acute myocardial infarction or stroke in early age.1 Moreover, the frequency and severity of migraines have also been associated with a higher body mass index.53 However, it seems unlikely that this relationship between migraine and stroke is due to the known pathogenic effect of traditional vascular risk factors, since there is no reduction in the risk of stroke when the adjusted relative risk is calculated.54
Migraine has been associated with an increase in prothrombotic factors, such as prothrombin, Leiden factor V and von Willebrand factor, whose role in the pathogenesis of vascular events is not properly understood.44
Arterial vasospasmThe possibility of the existence of vasospasm in migraine has been suggested based on 2 facts: (a) the ARIC study found that migraine was associated with Rose angina (caused by vasospasm), but not with coronary artery disease,55 and (b) there have reports of non-arteriosclerotic vasospasm of coronary arteries in migraine patients without cardiac symptoms.56
This hypothesis is based on an increased risk attributable to the use of different drugs for the treatment of migraines, such as triptans and ergot agents. Studies on the clinical and pharmacological cardiovascular safety of triptans do not support a direct association between these drugs and vascular events.57,58 On the other hand, data regarding the association between migraine and stroke precede the use of triptans. Since MA and migraine without aura are treated similarly, this hypothesis does not seem very plausible.59
Neither has it been possible to demonstrate that the rational use of ergot agents increases vascular risk, although this may be increased in situations of abuse of these compounds.44
Common genetic component (monogenic or polygenic)A common genetic component (monogenic or polygenic) may explain the finding of both migraine and ischemic stroke in the same patient.
- –
Monogenic forms. As already mentioned, ischemic stroke and migraine are 2 manifestations of some monogenic disorders such as CADASIL, MELAS, cerebroretinal vasculopathy and hereditary endotheliopathy with retinopathy, nephropathy and stroke. These diseases are evidence of the coexistence of stroke and migraine within a single syndrome characterized by a particular phenotype, and may be due to chronic hereditary disorders of the walls of small-calibre vessels.44
Familial hemiplegic migraine (FHM) is the only known monogenic cause of migraine to date, with 3 identified causative genes. Less than 5% of patients with FHM present stroke.60
- –
Polygenic forms. Many specific genetic variants have been implicated in susceptibility to migraine on the one hand and to ischemic stroke on the other.44 Migraine and ischemic stroke are probably phenotypic manifestations of polygenic disorders reflecting the effect of some genetic loci that modulate different pathophysiological processes and the result of the combination of hundreds of genetic variants. To mention just one example among them, MA has been linked with a specific genotype associated with methylenetetrahydrofolate reductase, which can lead to hyperhomocysteinemia.61
Apart from the hypotheses mentioned, 2 other possible mechanisms could favor an increase in the association between migraine and stroke:
Patent foramen ovaleThe possible relationship between the presence of patent foramen ovale (PFO) in patients with migraine and/or stroke is controversial. Some studies suggest that PFO is associated with an increased risk of ischemic stroke in young adults, and that subjects suffering from MA present this malformation more frequently.62 On the other hand, among subjects suffering from ischemic stroke, MA is twice as prevalent among those who have associated PFO than among those who do not.63 In contrast, other, more recent findings indicate no association between MA and ischemic stroke in women with PFO compared to those without it.22 There are no sufficient data to substantiate the hypothesis that PFO closure leads to a reduction in migraine frequency (and hence in ischemic stroke).64
Cervical artery dissectionThere has been speculation about a possible, genetically mediated, constitutional predisposition towards developing CAD. In these cases, an increased vascular fragility would predispose to its development under the influence of environmental factors such as infections or minor trauma. There may be a common arteriopathy in CAD and migraine, or simply the concomitant existence of a genetic susceptibility.13
Autonomic dysfunctionA higher prevalence of syncope among patients with migraine than among the general population (46% vs. 31%) has recently been found.65,66 However, the relationship of syncope with severity or the type of migraine could not be established. There are currently some studies searching for a relationship between autonomic system dysfunction and the presence of WM lesions and subclinical stroke.
Brain iron depositsStudies conducted with MRI in patients with migraine have shown an accumulation of iron in the periaqueductal grey matter, putamen, globus pallidus and red nucleus.66,67 No differences were observed between MA and migraine without aura. A positive correlation exists between the evolution of migraine and an increased accumulation of iron. All these have led to the postulate that iron accumulation is the result of a repetitive activation of the pain system, leading to its malfunction and contributing to its possible chronicity. However, it is also possible that the accumulation of iron is simply a marker of the activation of the nociceptive system.
Migrainous strokeCortical spreading depressionThe exact pathogenetic mechanism of MS is not known and there is speculation that it could be related to regional oligemia accompanying CD. A neuronal depolarization which progresses slowly through the visual cortex, CD is accompanied by a transient hyperaemia followed by a more prolonged oligemia. During normal migraine auras, the level of perfusion does not reach the ischemic threshold and, in any case, it is the consequence of a reduced neuronal metabolism.68 It is possible that during MS the decrease in regional cerebral blood flow exceeds the energy needs of neurons, leading to the development of ischemia. Alternatively, it could be associated with other conditions which favor ischemia.8
HypercoagulabilityThe release of calcitonin gene-related peptide as a consequence of trigeminovascular system activation during a migraine attack stimulates cerebral endothelial cells, platelets and mast cells, which release platelet activating factor.44 This factor is involved in tissue ischemia through several mechanisms: it induces platelet activation and aggregation and stimulates the release of von Willebrand factor; this factor, acting on glycoprotein IIb/IIIa platelet receptor, has a crucial effect on fibrinogen binding and primary homeostasis. This leads to speculation that, during the course of a migraine attack, a prothrombotic process could become activated in those vascular beds with vasoconstriction secondary to a propagated CD wave.44 Conversely, cerebral ischemia induced by a hypercoagulability state may trigger the propagated CD wave, thus causing a symptomatic migraine.
ConclusionsMigraine and stroke are associated with a higher frequency than expected when observing the prevalence of both diseases among the general population. This relationship may be one of coincidence, similarity (migraine resembling stroke or stroke resembling migraine), origin (migraine and stroke as manifestations of the same underlying process) and causality (migraine may act as an additional factor risk for stroke or may be the direct cause of stroke, as in MS).
Several studies have established this causal relationship between migraine and stroke. These studies have shown that the RR of stroke is increased in women younger than 45 years who suffer MA (adjusted relative risk between 1.5 and 6.2 according to different studies) and this risk increases even further in the presence of other associated risk factors such as smoking and OC consumption. Despite these increased RR data, the absolute risk remains low and only the association with other concomitant factors produces a significant increase. This increase in RR has not been demonstrated clearly in other age groups or in males.
In line with these findings, it has been observed in recent years that migraine is not only a risk factor for ischemic cerebrovascular pathology, but also for thrombotic vascular events in other arterial beds (“marker of ischemic vascular disorder”); this is true for both males and females.
MS is assumed to be directly caused by the pathophysiological phenomena underlying MA and explains 0.5% of stroke cases in all age groups. It also explains 10% if we consider only strokes in patients younger than 45 years.
Patients with migraine (especially MA) have also been reported to have a higher risk of developing silent ischemic lesions in the deep WM, as well as small lesions at the level of the posterior circulation (especially in the cerebellum), which are probably silent strokes.
It is assumed that better control of migraine, as well as of other, associated vascular risk factors, will decrease the incidence of stroke in these patients. Discontinuation of smoking and of OC is suggested, especially in those women with MA who present other, additional vascular risk factors. If, despite everything, OC are consumed, the advice is to use those products with lower doses of estrogen or else those containing only progestin. The IHS recommends that migraine patients using OC should stop smoking and undergo regular reviews to control other, additional vascular risk factors that they may have or that may arise over time.69
Once an MS has taken place, secondary prevention measures should be applied, as in stroke cases with other aetiologies, as long as no studies providing other data are available. Aspirin or clopidogrel should be used according to the cardiovascular risk, concomitant comorbidities and adjuvant drugs for each patient. There are currently no studies that have been specifically designed to evaluate the effect of secondary prevention in patients who have suffered an MS.
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Caminero AB, Sánchez Del Río González M. Migraña como factor de riesgo cerebrovascular. Neurología. 2011;27:103–11.