The aim of the present study is to analyse the influence that motor and non-motor symptoms have on the quality of life (QoL) of patients with Parkinson's disease (PD), and to study the relationship between the two types of symptoms.
Material and methodsThis cross-sectional study included 103 patients with PD (55 men and 48 women). Quality of life was measured on the PDQ-39 scale. The UPDRS scale (I-IV) was also used, and different items were grouped to analyse the presence of tremor, rigidity, bradykinesia, and axial symptoms. The non-motor symptoms scale (NMSS) was administered to assess non-motor symptoms. We performed correlation analyses between different scales to analyse the influence of motor and non-motor symptoms on QoL.
ResultsCorrelations were observed between the PDQ-39 summary index (PDQ39 SI) and the NMSS (correlation coefficient [cc], 0.56; P<.001), UPDRS III (cc, 0.44; P<.001) and UPDRS IV (cc, 0.37; P<.001) scores. The strongest correlation was between cognitive symptoms and mood. The analysis pointed to a direct relationship between the NMSS score and axial symptoms (cc, 0.384; P<.01), bradykinesia (cc, 0.299; P<.01), and to a lesser extent, rigidity (cc, 0.194; P<.05). No relationship was observed between presence of tremor and the NMSS score.
ConclusionCognitive symptoms and mood exert the most influence on QoL of patients with PD. We found at least two phenotypes; one with predominantly axial symptoms, with significant involvement of non-motor symptoms, and a tremor-associated phenotype in which these symptoms are less prevalent.
El objetivo del presente trabajo es contrastar la influencia que presentan los síntomas motores y no motores en la calidad de vida de los pacientes con enfermedad de Parkinson (EP), y observar la asociación entre ambos tipos de síntomas.
Material y métodosEstudio transversal que incluye 103 pacientes con EP (55 hombres y 48 mujeres). La calidad de vida fue estudiada con la escala 39-Item Parkinson's Disease Questionnaire (PDQ-39). También se administró la escala Unified Parkinson's Disease Rating Scale (UPDRS I-IV), agrupando diferentes ítems para analizar la presencia de temblor, rigidez, bradicinesia y síntomas axiales para definir los subgrupos clínicos. Para valorar los síntomas no motores, administramos la non-motor symptoms scale (NMSS). Se hicieron estudios de correlación entre las diferentes escalas para ver la influencia sobre la calidad de vida de síntomas motores y no motores.
ResultadosSe observaron correlaciones entre las puntuaciones en el PDQ-39 Summary Index (PDQ-39_SI) y la NMSS (cc: 0,56; p<0,001), UPDRS III (cc: 0,44; p<0,001) y con la UPDRS IV; (cc: 0,37; p<0,001). La mayor relación correspondía a los síntomas cognitivos y del estado de ánimo. Existe relación directa entre la puntuación en la NMSS y los síntomas axiales (cc: 0,384; p<0,01); bradicinesia (cc: 0,299; p<0,01) y en menor medida rigidez (cc: 0,194; p<0,05). No se observó ninguna relación entre la presencia de temblor y la puntuación en la NMSS.
ConclusiónHay un mayor peso de los síntomas cognitivos y del estado de ánimo sobre la calidad de vida de los pacientes con EP. Hay al menos 2 fenotipos claramente diferenciados, uno con predominancia de síntomas axiales donde hay una gran afectación de síntomas no motores y un fenotipo tremórico con una significativa menor presencia de los mismos.
Parkinson's disease (PD) is a chronic neurodegenerative disease for which no curative treatment is currently available.1 Despite this lack, multiple studies2–9 have proved that different therapies may increase quality of life for patients with PD. With this in mind, understanding how each symptom impacts the quality of life of these patients is extremely important.10–13
In the past years, much emphasis has been placed on the presence of such non-motor symptoms as sleep, cognitive, and mood disorders.10–14 At present, sleep disorders, mainly fragmented sleep and nocturia, and mood disorders, especially depression, are considered the most frequent and the most disruptive symptoms for PD patients.10,12,13,15 More specifically, instability, gait disorders, and motor impairment in the form of fluctuations are the motor symptoms that affect quality of life the most.11
It is important to assess the presence of motor and non-motor symptoms to assign each patient to a specific clinical subgroup and to evaluate the impact of these symptoms, whether individually or combined, on quality of life. Preliminary studies have described cognitive impairment as being more frequent in phenotypes with more axial symptoms (speech and facial expression anomalies, axial rigidity, postural reflex and posture dysfunction, bradykinesia, difficulty rising from a chair, gait disorders, etc.).16,17 The degree of asymmetry of motor symptoms, and their distribution, may also have an impact on the presence of certain non-motor symptoms.18
The main purposes of this study are to compare the influence of motor and non-motor symptoms on quality of life and to evaluate the distribution and association of both types of symptoms with a view to establishing different clinical profiles.
Patients and methodsWe conducted a cross-sectional study of 103 outpatients (55 men and 48 women) diagnosed with PD according to the UK Parkinson's Disease Society Brain Bank criteria.19 Patients were assessed consecutively by 3 neurologists (JCGE, BT, and KB) from the movement disorders unit at Hospital Universitario de Cruces. The assessments were conducted in our consultation rooms from 16.30 to 18.00 between February 2010 and July 2012. Mean age was 66.49±9.96 years and mean disease duration, 7.93±11.6 years. The mean Hoehn & Yahr stage was 2.2±0.61 (Table 1). Before the interview, participants were given a document which explained the purposes of the study and guaranteed data confidentiality. All patients signed informed consent forms approved by the ethics committee at our hospital before their data were entered into the database. We excluded those patients suspected of having atypical parkinsonism or parkinsonism of any other aetiology, as well as any patients unable to complete the clinical questionnaires (for example, patients with severe language impairment or dementia). Participants had to be at least 18 years old with no upper age limit.
Mean, standard deviation (SD), and range for the variables sex, age, disease duration in years, and Hoehn & Yahr (H&Y) stage in our sample of patients with PD.
| Variable | n (%) or mean±SD | Range |
|---|---|---|
| Sex | 55 men (53.4%) | – |
| Age | 66.50±9.96 | 40.28-86.69 |
| Disease duration | 6.93±5.40 | 0.26-26.43 |
| H&Y stage | 2.29±0.61 | 1-4 |
We recorded the patients’ demographical data, including date of birth, date and age at diagnosis, years elapsed from disease onset to interview, sex, and treatment with dopaminergic drugs.
Quality of life was assessed using the Spanish version of the 39-item Parkinson's Disease Questionnaire (PDQ-39).20 The PDQ-39 summary index (PDQ-39 SI) ranges from 0 to 100, with higher scores indicating poorer function. We analysed each of the 8 dimensions of the scale separately (mobility, activities of daily living, emotional well-being, stigma, social support, cognitions, communication, and bodily discomfort). Patients were also rated on the Unified Parkinson's Disease Rating Scale (UPDRS).21 This scale assesses mental status, activities of daily living, motor function, and complications of dopaminergic treatment. Scores on part I of the UPDRS range from 0 to 16, from 0 to 52 on part II, from 0 to 108 on part III, and from 0 to 44 on part IV; higher scores indicate poorer clinical condition. We grouped the following items of the UPDRS-III subscale to study motor symptoms: resting tremor of all 4 limbs (20r+20l+21r+21l), rigidity in all 4 limbs (24r+24l+25r+25l), and bradykinesia in all 4 limbs (26r+26l+27r+27l+28r+28l+29r+29l). We also grouped items on the UPDRS that assessed axial symptoms (18, 19, 23, 30, 31, 32, 33, 34).
To assess non-motor symptoms, we used the Non-Motor Symptoms Scale (NMSS),22 specifically designed for patients with PD and including 30 items grouped into 9 domains that analyse different clinical aspects of PD, except for motor symptoms. These domains are cardiovascular including falls (2 items), sleep/fatigue (4), mood/cognition (6), perceptual problems/hallucinations (3), attention/memory (3), gastrointestinal tract (3), urinary (3), sexual function (2), and miscellaneous (4). Each item is scored based on severity (0-3) and frequency (0-4).
Statistical analysisWe calculated the mean and standard deviation for quantitative variables and percentages for qualitative variables. The Kolmogorov–Smirnov test was used to determine whether the sample followed a normal distribution and Levene's test to assess the homogeneity of variances. To explore the correlation between the PDQ-39 SI and the remaining variables, we used the Pearson correlation coefficient. A subsequent multiple linear regression analysis identified the variables predicting quality of life in patients with PD, excluding part II of the UPDRS. Statistical analysis was conducted using SPSS statistical software version 12 for Windows (SPSS Inc., Chicago, IL, USA).
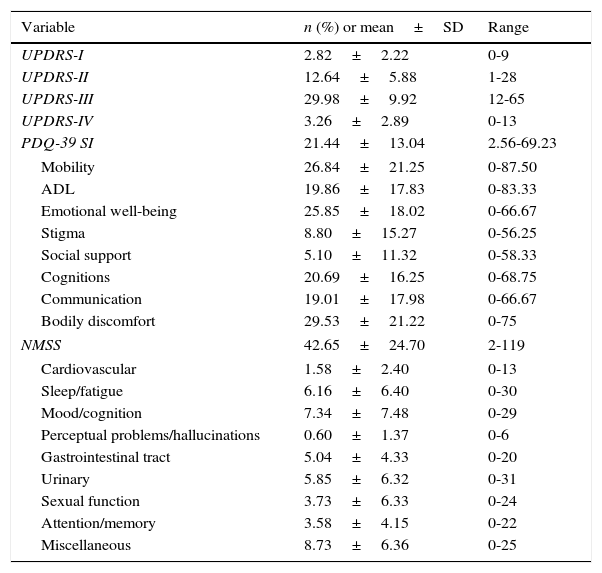
ResultsDescription of the main non-motor symptoms using the Non-Motor Symptoms ScaleThe most frequently reported non-motor symptoms belong to the miscellaneous domain (97 patients, 94.2%), which includes pain, impaired sense of smell, excessive sweating, and weight changes. Sleep (91 patients, 88.3%), urinary (84 patients, 81.6%), gastrointestinal (84 patients, 81.6%), and mood/cognition disorders (83 patients, 80.6%) were also very frequent. Attention deficit (78 patients, 75.7%), cardiovascular symptoms (56 patients, 54.4%), and sexual dysfunction (40 patients, 38.8%) were also common, although less frequent than the symptoms of the domains mentioned previously. The least frequently reported non-motor symptoms were hallucinations and those affecting perception (26 patients, 25.2%). Table 2 displays the scores on each domain of the NMSS and the PDQ-39, as well as the scores on each subscale of the UPDRS.
Mean, standard deviation (SD), and range for the scores on the different scales used in our sample of patients with PD.
| Variable | n (%) or mean±SD | Range |
|---|---|---|
| UPDRS-I | 2.82±2.22 | 0-9 |
| UPDRS-II | 12.64±5.88 | 1-28 |
| UPDRS-III | 29.98±9.92 | 12-65 |
| UPDRS-IV | 3.26±2.89 | 0-13 |
| PDQ-39 SI | 21.44±13.04 | 2.56-69.23 |
| Mobility | 26.84±21.25 | 0-87.50 |
| ADL | 19.86±17.83 | 0-83.33 |
| Emotional well-being | 25.85±18.02 | 0-66.67 |
| Stigma | 8.80±15.27 | 0-56.25 |
| Social support | 5.10±11.32 | 0-58.33 |
| Cognitions | 20.69±16.25 | 0-68.75 |
| Communication | 19.01±17.98 | 0-66.67 |
| Bodily discomfort | 29.53±21.22 | 0-75 |
| NMSS | 42.65±24.70 | 2-119 |
| Cardiovascular | 1.58±2.40 | 0-13 |
| Sleep/fatigue | 6.16±6.40 | 0-30 |
| Mood/cognition | 7.34±7.48 | 0-29 |
| Perceptual problems/hallucinations | 0.60±1.37 | 0-6 |
| Gastrointestinal tract | 5.04±4.33 | 0-20 |
| Urinary | 5.85±6.32 | 0-31 |
| Sexual function | 3.73±6.33 | 0-24 |
| Attention/memory | 3.58±4.15 | 0-22 |
| Miscellaneous | 8.73±6.36 | 0-25 |
ADL: activities of daily living; NMSS: Non-Motor Symptoms Scale; PDQ-39 SI: 39-item Parkinson's Disease Questionnaire summary index; UPDRS: Unified Parkinson's Disease Rating Scale.
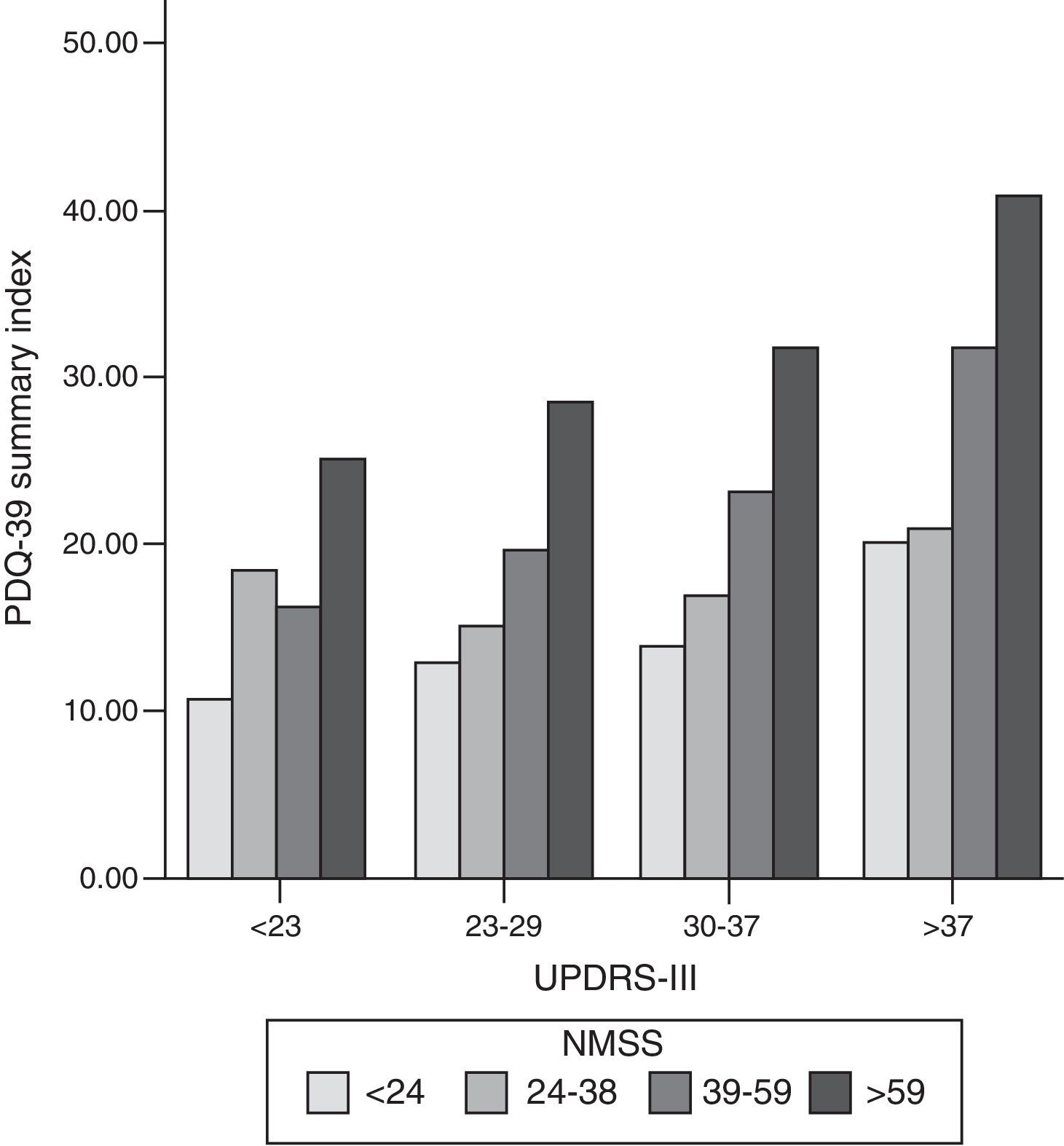
We found a strong correlation between scores on the PDQ-39 SI and the scores on the scales measuring non-motor symptoms (NMSS; cc: 0.56; P<.001), motor symptoms (UPDRS-III; cc: 0.44; P<.001), and motor complications of dopaminergic drugs (UPDRS-IV; cc: 0.37; P<.001) (Fig. 1). Within the NMSS, the domains showing the strongest correlations with the PDQ-39 SI were mood/cognition (cc: 0.54; P<.001) and sleep/fatigue (cc: 0.44; P<.001). The domains showing a weaker correlation with PDQ-39 scores are miscellaneous (cc: 0.17; not significant), urinary (cc: 0.24; P=.017), sexual function (cc: 0.24; P=.017), and attention (cc: 0.24; P=.015).
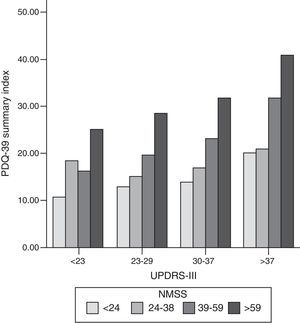
Bar graph representing the association between motor symptoms (UPDRS-III), non-motor symptoms (NMSS), and quality of life (PDQ-39 SI). Patients were classified into quartiles based on their UPDRS-III and NMSS scores. The proportion of PDQ-39 scores is maintained in each motor quartile (UPDRS-III), with a slight increase in quality of life scores as motor function worsens. NMSS: Non-Motor Symptoms Scale; PDQ-39: 39-item Parkinson's Disease Questionnaire; UPDRS: Unified Parkinson's Disease Rating Scale.
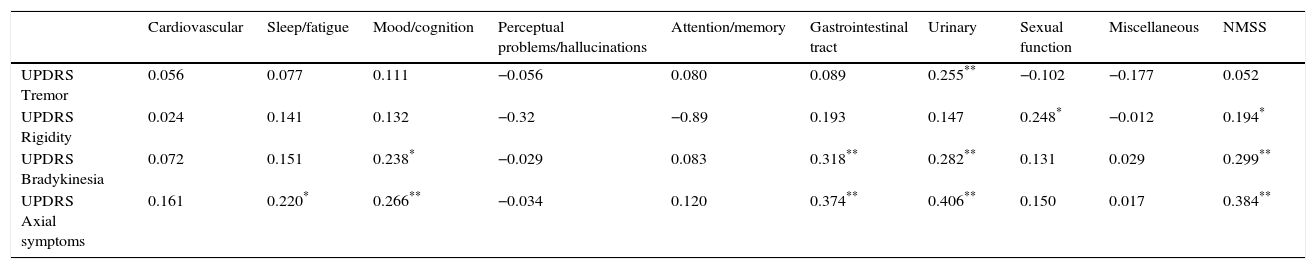
We arranged the items on the UPDRS-III subscale into groups reflecting rigidity, tremor, bradykinesia, and axial symptoms, and observed a positive correlation between NMSS scores and axial symptoms (cc: 0.384; P<.01), bradykinesia (cc: 0.299; P<.01), and to a lesser extent, rigidity (cc: 0.194; P<.05). No correlations were observed between non-motor symptoms as measured on the NMSS and presence of tremor. Bradykinesia and axial symptoms were significantly correlated with the gastrointestinal, urinary, and mood/cognition domains of the NMSS (Table 3). Rigidity and tremor were only very weakly correlated with the different NMSS domains.
Correlation matrix between total NMSS scores and scores on each NMSS domain, and scores for each motor symptom: tremor, bradykinesia, rigidity, and axial symptoms (UPDRS-III).
| Cardiovascular | Sleep/fatigue | Mood/cognition | Perceptual problems/hallucinations | Attention/memory | Gastrointestinal tract | Urinary | Sexual function | Miscellaneous | NMSS | |
|---|---|---|---|---|---|---|---|---|---|---|
| UPDRS Tremor | 0.056 | 0.077 | 0.111 | −0.056 | 0.080 | 0.089 | 0.255** | −0.102 | −0.177 | 0.052 |
| UPDRS Rigidity | 0.024 | 0.141 | 0.132 | −0.32 | −0.89 | 0.193 | 0.147 | 0.248* | −0.012 | 0.194* |
| UPDRS Bradykinesia | 0.072 | 0.151 | 0.238* | −0.029 | 0.083 | 0.318** | 0.282** | 0.131 | 0.029 | 0.299** |
| UPDRS Axial symptoms | 0.161 | 0.220* | 0.266** | −0.034 | 0.120 | 0.374** | 0.406** | 0.150 | 0.017 | 0.384** |
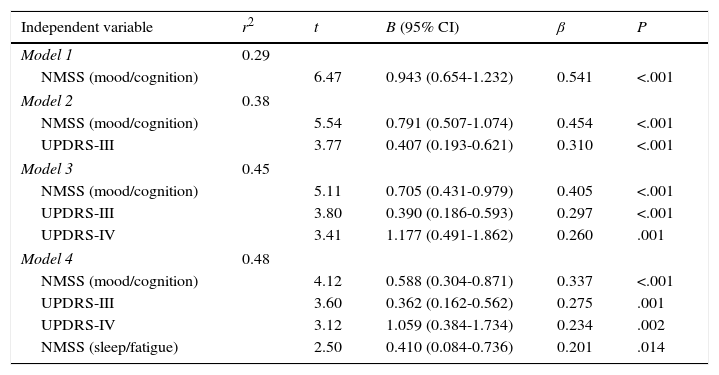
Variables were introduced into the linear regression model, breaking down total NMSS scores into scores for the different domains and using PDQ-39 SI scores as the dependent variable. Four variables were found to represent 48.2% of the total PDQ-39 SI score (F=22.555; P<.001): in order of importance, these variables were the mood/cognition domain of NMSS, UPDRS-II, UPDRS-IV, and the sleep/fatigue domain of the NMSS. Residues, which were independently analysed, were found to follow a normal distribution (Table 4).
Multiple regression analysis of the impact of different motor and non-motor symptoms on quality of life in our sample of patients with PD. B (95% CI): unstandardised regression coefficient; β (95% CI): standardised regression coefficient; UPDRS: Unified Parkinson's Disease Rating Scale; NMSS: Non-Motor Symptoms Scale.
| Independent variable | r2 | t | B (95% CI) | β | P |
|---|---|---|---|---|---|
| Model 1 | 0.29 | ||||
| NMSS (mood/cognition) | 6.47 | 0.943 (0.654-1.232) | 0.541 | <.001 | |
| Model 2 | 0.38 | ||||
| NMSS (mood/cognition) | 5.54 | 0.791 (0.507-1.074) | 0.454 | <.001 | |
| UPDRS-III | 3.77 | 0.407 (0.193-0.621) | 0.310 | <.001 | |
| Model 3 | 0.45 | ||||
| NMSS (mood/cognition) | 5.11 | 0.705 (0.431-0.979) | 0.405 | <.001 | |
| UPDRS-III | 3.80 | 0.390 (0.186-0.593) | 0.297 | <.001 | |
| UPDRS-IV | 3.41 | 1.177 (0.491-1.862) | 0.260 | .001 | |
| Model 4 | 0.48 | ||||
| NMSS (mood/cognition) | 4.12 | 0.588 (0.304-0.871) | 0.337 | <.001 | |
| UPDRS-III | 3.60 | 0.362 (0.162-0.562) | 0.275 | .001 | |
| UPDRS-IV | 3.12 | 1.059 (0.384-1.734) | 0.234 | .002 | |
| NMSS (sleep/fatigue) | 2.50 | 0.410 (0.084-0.736) | 0.201 | .014 | |
In our series, sleep/fatigue and mood/cognition disorders were found in 88.3% and 80.6% of the patients, constituting the non-motor symptoms with the greatest impact on quality of life. This conclusion has already been presented in previous studies published by our research group.10,15,23 The novel aspect of the present study is that it analyses the data as a whole, using scales such as the NMSS specifically addressing non-motor symptoms22 and comparing the relative weights of non-motor symptoms and motor symptoms as assessed with parts III and IV of the UPDRS. According to our data, mood and cognitive symptoms have the greatest impact on quality of life in patients with PD, even surpassing motor symptoms.10,12,24 Of the latter, axial symptoms (instability, gait disorders, motor fluctuations) have the most negative effect on quality of life of these patients.11
Regarding non-motor symptoms, apathy is very frequent (16%-48%),25 has a large impact on quality of life in patients with PD,26 and is associated with axial symptoms.27
Our results show a clinical subgroup of patients with axial symptoms (instability, gait disorders) who also display non-motor symptoms including apathy, mood disorders, and autonomic dysfunctions such as alterations of the gastrointestinal and urinary tracts. Rigidity and tremor are weakly correlated with the presence of non-motor symptoms. Patients with gait instability and difficulty walking show more severe motor and cognitive impairment than patients without those symptoms.16,28,29 Progression of both types of symptoms (cognitive and gait/axial) does not seem to depend on the function of dopaminergic pathways (as demonstrated by the lack of response to dopaminergic drugs) but rather on the neurodegeneration of nuclei and cholinergic structures such as the pedunculopontine nucleus30,31 in the case of gait disorders, and the nucleus basalis of Meynert and the cerebral cortex in the case of cognitive impairment.32,33 It has also been suggested that deterioration profiles may change as disease progresses: whereas deterioration seems to follow a more linear trend in patients with axial symptoms, those patients with symptoms associated with dopaminergic deficits (bradykinesia, rigidity) show a more exponential trend, with more marked deterioration during the early years of the disease.34 We also found a clear association between autonomic dysfunctions (for example, urinary and gastrointestinal tract alterations) and presence of axial symptoms.35 Although our results fail to show an association between cardiovascular and axial symptoms, this relationship has been reported in previous studies.36 Patients with clinical symptoms of tremor tend to have fewer non-motor symptoms; the correlation between NMSS scores and items on the UPDRS-III assessing tremor is not significant. This patient subgroup shows less affectation of the autonomic nervous system, as demonstrated by cardiac MIBG scintigraphy37; less cognitive impairment16; and a lower frequency of sleep disorders such as REM sleep behaviour disorder. These findings suggest that the target for neurodegeneration is different in these patients.
The main limitation of our study is that we did not include patients at advanced stages of the disease since they tend to show dementia or severely impaired speech and may therefore be unable to complete the questionnaire. Consequently, our results do not reflect symptom distribution in patients with advanced PD.
In conclusion, our study takes a global approach and uses specific scales for evaluating non-motor symptoms quantitatively. One of the main findings of our study is that quality of life in patients with PD is highly influenced by the presence of cognitive and mood disorders, followed by sleep disorders. Motor symptoms, while important, affect quality of life to a lesser extent. Another major finding was that there are at least 2 clearly defined clinical subgroups: patients with predominantly axial symptoms who also experience such non-motor symptoms as cognitive dysfunction, autonomic nervous system impairment, or sleep disorders; and patients with tremor, who are significantly less likely to experience those symptoms.
It is important to make this distinction when evaluating the effectiveness of new drugs. These 2 subgroups may display less affectation of the dopaminergic pathways than do patients with classic PD, in which bradykinesia is the predominant symptom.
FundingThis study received no funding of any kind.
Conflict of interestThe authors have no conflict of interest to declare.
Please cite this article as: Berganzo K, Tijero B, González-Eizaguirre A, Somme J, Lezcano E, Gabilondo I, et al. Síntomas no motores y motores en la enfermedad de Parkinson y su relación con la calidad de vida y los distintos subgrupos clínicos. Neurología. 2016;31:585–591.
This study was presented in poster format at the 65th Annual Meeting of the Spanish Society of Neurology, Barcelona 2013.