Hyperthyroidism is rare in paediatric patients.1 The most frequent cause of childhood hyperthyroidism is Graves disease, an autoimmune disorder in which the body produces TSH receptor antibodies (TSI).2 Peak incidence occurs between the ages of 11 and 15.3,4 Symptoms vary greatly, the most common being tachycardia, goitre, weight loss, tremor, palpitations, diarrhoea, and sleep disorders5; neuropsychiatric symptoms are less frequent. We present 2 patients with a diagnosis of Graves disease who were initially referred to the paediatric neurology department due to neuropsychiatric symptoms.
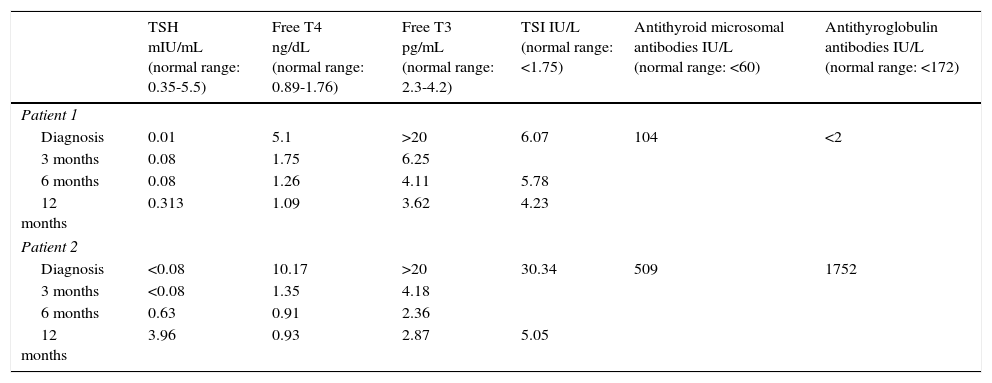
Clinical casesPatient 1Our first patient was an 11-year-old boy who at the age of 5 was diagnosed with attention-deficit/hyperactivity disorder (ADHD). From the age of 6 he had been in treatment with methylphenidate, with partial response. During follow-up, he developed symptoms of impulse control disorder and oppositional defiant disorder (ODD); the psychiatry department prescribed risperidone, which had to be discontinued due to adverse effects. He visited the neurology department due to exacerbation of his behavioural and ADHD symptoms over the previous year; he also had headache, increased appetite, palpitations, emotional lability, and poorer academic performance. Physical examination revealed tachycardia (111bpm), intentional tremor, and motor restlessness. As symptoms progressed slowly and the patient responded poorly to stimulants and neuroleptics, we conducted a thyroid function test, which revealed elevated free T3 and T4 levels, suppressed TSH, and presence of TSI antibodies (Table 1). Our patient was diagnosed with hyperthyroidism and initiated treatment with methimazole and propranolol, with excellent results; tremor and palpitations disappeared, his heart rate normalised, and restlessness, sleep, and academic performance improved.
Thyroid function in our 2 patients. Clinical cases.
| TSH mIU/mL (normal range: 0.35-5.5) | Free T4 ng/dL (normal range: 0.89-1.76) | Free T3 pg/mL (normal range: 2.3-4.2) | TSI IU/L (normal range: <1.75) | Antithyroid microsomal antibodies IU/L (normal range: <60) | Antithyroglobulin antibodies IU/L (normal range: <172) | |
|---|---|---|---|---|---|---|
| Patient 1 | ||||||
| Diagnosis | 0.01 | 5.1 | >20 | 6.07 | 104 | <2 |
| 3 months | 0.08 | 1.75 | 6.25 | |||
| 6 months | 0.08 | 1.26 | 4.11 | 5.78 | ||
| 12 months | 0.313 | 1.09 | 3.62 | 4.23 | ||
| Patient 2 | ||||||
| Diagnosis | <0.08 | 10.17 | >20 | 30.34 | 509 | 1752 |
| 3 months | <0.08 | 1.35 | 4.18 | |||
| 6 months | 0.63 | 0.91 | 2.36 | |||
| 12 months | 3.96 | 0.93 | 2.87 | 5.05 | ||
Our second patient was a 14-year-old girl who visited our centre due to a 12-month history of daily episodes of oppressive frontal headache with no underlying organic cause, generalised anxiety, emotional lability, poorer academic performance, and insomnia. Physical examination revealed tachycardia (137bpm), grade 2 goitre, and postural and resting tremor. A blood test performed to check for hyperthyroidism revealed elevated free T3 and T4 levels, suppressed TSH, and presence of TSI antibodies (Table 1). Treatment with methimazole and propranolol led to progressive symptom resolution and normalisation of thyroid hormone levels (Table 1).
Paediatric patients with hyperthyroidism may display ADHD,3,6,7 behaviour and mood disorders,5 anxiety,8 sleep disorders,9 ODD, learning disorders,10 language impairment,11 and headaches.12 ADHD is one of the main reasons for consultations with the paediatric neurology department; it has a prevalence of 3.4%13 and constitutes the most frequent neuropsychiatric disorder in children with hyperthyroidism, affecting 40% to 80% of that group.3,6,7 Given the high prevalence of ADHD and its strong association with hyperthyroidism, thyroid hormone levels should be determined at the beginning of the diagnostic process and in patients showing poor response to treatment or clinical worsening. Methylphenidate may exacerbate such hyperthyroidism symptoms as tachycardia, headache, insomnia, and emotional lability. In our first case, symptoms could not be attributed to medication since it showed good tolerability initially and required no dose adjustment. The International Classification of Headache Disorders includes headache attributed to hypothyroidism under the heading “Headache attributed to other metabolic or systemic disorders” (A10.7.1).14 In our second patient, chronic headache may be attributed to hyperthyroidism since no other causes were found and symptoms improved after starting treatment with antithyroid drugs. Behaviour and mood disorders have been described in up to 21% of the patients with hyperthyroidism,5 as in the 2 cases presented here (behaviour disorder in the first case and marked emotional lability and generalised anxiety in the second). We should also highlight that our 2 patients had insomnia. Elevated thyroid hormone levels may cause neuropsychiatric alterations or exacerbate neuropsychiatric symptoms already present.7 In our first patient, a previous thyroid hormone measurement had yielded normal results, which suggests that ADHD and behaviour disorders had worsened with hyperthyroidism. On the other hand, our second patient had no history of psychiatric or neurological symptoms; we therefore feel that these were caused by Graves disease. Both patients displayed typical symptoms of hyperthyroidism (tachycardia, tremor), which helped guide the diagnosis. Hyperthyroidism should be included in the differential diagnosis of patients with acute-onset or slow-progressing behaviour disorders, especially when they display tachycardia and tremor. In-depth knowledge of the neuropsychiatric manifestations of this condition is therefore essential.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Losada-del Pozo R, Soto-Insuga V, Martínez González M, Soriano Guillén L. Manifestaciones neuropsiquiátricas de la enfermedad de Graves en la edad pediátrica. Neurología. 2017;32:196–197.
This study was presented as an oral communication at the 9th National Congress of the Spanish Society of Paediatric Neurology, held in Palma de Mallorca on 11–14 June 2014.