Mild traumatic brain injury brain injury (mTBI) has traditionally been considered to cause no significant brain damage since symptoms spontaneously remit after a few days. However, this idea is facing increasing scrutiny. The purpose of this study is to demonstrate the presence of early cognitive alterations in a series of patients with mTBI and to link these findings to different markers of brain damage.
MethodsWe conducted a prospective study of a consecutive series of patients with mTBI who were evaluated over a 12-month period. Forty-one (3.7%) of the 1144 included patients had experienced a concussion. Patients underwent a routine clinical evaluation and a brain computed tomography (CT) scan and were also administered a standardised test for post-concussion symptoms within the first 24h of mTBI and also 1-2 weeks later. The second assessment also included a neuropsychological test battery. The results of these studies were compared to those of a control group of 28 healthy volunteers with similar characteristics. Twenty patients underwent a magnetic resonance imaging (MRI) scan.
ResultsVerbal memory and learning were the cognitive functions most affected by mTBI. Seven out of the 20 patients with normal CT findings displayed structural alterations on MR images, which were compatible with diffuse axonal injury in two cases.
ConclusionsResults from this pilot study suggest that early cognitive alterations and structural brain lesions affect a considerable percentage of patients with post-concussion syndrome following mTBI.
Los traumatismos craneoencefálicos leves (TCE-L) han sido tradicionalmente considerados acontecimientos sin repercusiones cerebrales significativas, cuya sintomatología remite espontáneamente en unos días. Sin embargo, estos hechos son cada vez más cuestionados. Este estudio pretende objetivar la existencia de alteraciones cognitivas precoces en una serie de pacientes con TCE-L y relacionar los hallazgos con distintos marcadores de lesión cerebral.
MétodosEstudio prospectivo de una cohorte de pacientes con un TCE-L valorados de forma consecutiva durante 12 meses. De un total de 1.144 pacientes, se seleccionó a 41 (3,7%) que habían presentado una conmoción cerebral. Además de la valoración clínica habitual y de la práctica de una tomografía computarizada (TC) cerebral, los pacientes fueron estudiados mediante un test estandarizado para síntomas posconmocionales en las primeras 24h después del TCE-L y al cabo de 1-2 semanas y, coincidiendo con la segunda valoración, mediante una batería neuropsicológica. Los resultados se compararon con los de un grupo de 28 voluntarios sanos de características parecidas. En 20 pacientes se practicó una resonancia magnética (RM) craneal.
ResultadosEn este análisis exploratorio, la memoria y el aprendizaje verbal fueron las funciones cognitivas más afectadas después del TCE-L. Siete de los 20 pacientes con TC cerebral normal presentaron alteraciones estructurales visibles por RM, que en dos casos fueron compatibles con la presencia de lesión axonal difusa.
ConclusionesLos resultados de este estudio piloto sugieren la presencia de alteraciones cognitivas precoces y lesiones cerebrales estructurales en un porcentaje no despreciable de pacientes que han presentado una conmoción cerebral recuperada después de un TCE-L.
Traumatic brain injury is highly prevalent in both industrialised and developing countries, with an estimated annual incidence of between 150 and 250 cases per 100 000 population.1 In terms of severity, 10% of cases are severe (Glasgow Coma Scale [GCS] score ≤8), 10% are moderate (9-13), and 80% are mild (14 or 15).2
Little attention has historically been dedicated to the consequences of mild traumatic brain injury (mTBI), as it is considered an essentially reversible condition, with no detectable brain injury and few or no residual sequelae. However, recent years have seen the publication of numerous studies questioning this belief. In the hospital context, management protocols for mTBI typically establish that patients with normal CT findings may be discharged, often with no clinical follow-up. However, there is evidence that in as many as 25% of cases with normal CT findings, brain MRI does display alterations.3
In addition to GCS score (14-15), the traditional diagnostic criteria for mTBI are loss of consciousness (lasting <30min) and post-traumatic amnesia (PTA; duration of less than 24h). When any of these conditions is present, patients are considered to have concussion.4 The consequences of mTBI are variable, ranging from the total absence of sequelae to an array of symptoms including headache, dizziness, nausea, gait instability, irritability, memory alterations, or difficulty concentrating. Approximately 30% of patients do not fully recover within 3 months of the trauma5; this is known as post-concussive syndrome.6
Despite advances in techniques for identifying brain injury, most studies acknowledge that some proportion of patients present persistent, incapacitating symptoms after MHT, despite normal neuroimaging findings. This explains why many authors believe that brain injury may not be the only cause of long-term alterations in some patients following mTBI. Residual sequelae in these patients may be influenced by a series of conditioning factors, such as personality traits, existing systemic or mental health disorders, comorbidities (chronic pain, anxiety or depressive disorders, etc.), sociopsychological factors, or the patient's involvement in legal claims.7
In the classic view, the great majority of neuropsychological alterations secondary to moderate or severe head trauma can be explained by the location of the associated brain lesions.8 However, in cases where brain CT scans do not clearly show focal lesions, cognitive dysfunction may be explained by the disconnection of various brain structures due to diffuse axonal injury (DAI).5 This phenomenon may also explain the presence of residual symptoms and cognitive alterations following mTBI. The most frequent neuropsychological sequelae of mTBI include alterations in information processing speed, attention, and memory.9–12
The susceptibility-weighted imaging (SWI) sequences included in modern MRI protocols aid the diagnosis of potential structural lesions in mTBI.13 SWI is extremely sensitive to paramagnetic elements and is particularly useful for identifying microbleeds. Some studies have shown the technique to be up to six times more effective than T2*-weighted sequences in detecting punctiform microbleeds associated with DAI.14
This pilot study aims to evaluate the early presence (<14 days after trauma) of cognitive, affective, and behavioural symptoms in a series of patients with concussion secondary to mTBI and in a group of healthy controls and to explore the potential association between the cognitive deficits observed and the clinical symptoms. We also aimed to determine brain injury severity through MRI analysis of structural lesions in a subgroup of patients.
Patients and methodsPatient and control groupsThe patients included in the study were treated between April 2013 and April 2014 at the neurotraumatology unit of Vall d’Hebron University Hospital’s emergency department. To be included in the study, patients had to meet all the inclusion criteria and none of the exclusion criteria listed below:
- -
Inclusion criteria: (1) age between 18 and 65 years; (2) being a fluent speaker of Catalan or Spanish; (3) having had mTBI with a GCS score of 14-15 in the 24h prior to study inclusion; (4) having experienced concussion, identified by loss of consciousness lasting <30min (verified by a witness), vomiting, seizures, PTA lasting <24h, or intense post-concussive symptoms (Table 1); (5) normal neurological examination findings; and (6) normal brain CT findings.
Table 1.Criteria used to identify concussion in patients attended within the first 24h after mTBI. Study inclusion required that patients display at least one of the following indicators, which were classified dichotomously (yes/no).
Indicator Remarks Loss of consciousness Witness confirmation was required. Must be differentiated from syncopal episodes PTA Evaluation of the detailed account of events occurring immediately before and after the mTBI Seizures Objective post-concussive symptoms Vomiting Severe post-concussive symptoms A 0-4 scale was used to record severity of symptoms: headache, nausea, feeling of instability, hypersensitivity to light, hypersensitivity to sound, disorientation, blurred vision, and dizziness
Symptoms were classified as severe if any scored ≥3 points or if the sum of all symptoms was ≥5 - -
Exclusion criteria: (1) previous head trauma requiring hospital care; (2) history of chronic substance abuse; (3) known psychiatric or neurological condition; (4) chronic systemic disease with potential cognitive effects (renal insufficiency or kidney failure, metabolic syndrome, etc.); and (5) polytrauma with an Injury Severity Scale score above 6.
Neurological examinations and initial analyses of brain CT scans were performed by the on-call neurosurgeon; these are routine procedures in the assessment of mTBI. Brain CT scans obtained in the emergency department were subsequently reassessed by neuroradiologists.
Participants of the control group were companions, and family members of patients admitted to the neurosurgery department. Of the candidates interested in participating, we selected those who met inclusion criteria 1 and 2 and none of the exclusion criteria. Volunteers were also matched to patients for age and level of education. The final control group comprised 28 volunteers (18 men and 10 women) with a median age of 29 (interquartile range [IQR], 21; range, 18-64).
All patients and controls signed informed consent forms approved by Vall d’Hebron University Hospital’s Ethics Committee (PR-AG-47-2013).
Assessment and follow-up proceduresIn addition to the initial clinical assessment, all patients were evaluated with CT scanning and a standardised test for post-concussion symptoms within 24h of the trauma. Thirty-four patients were also evaluated a second time within 2 weeks of the trauma. Brain MRI scans were also performed for a subgroup of 20 patients (14 men and 6 women; median age, 29; IQR, 21; range, 18-64). All controls were assessed once only.
Standardised concussion assessmentPatients were assessed with the Sport Concussion Assessment Tool 2 (SCAT2)15 within 24h of the mTBI during their stay at the emergency department's neurotraumatology unit and subsequently during the neuropsychological examination. SCAT2 is a standardised assessment tool designed to measure the acute effects of concussion incurred during sport. The test records post-concussive symptoms, loss of consciousness (with confirmation from witnesses), and GCS score; it also involves an assessment of balance and coordination and a cognitive evaluation, performed using the Standardized Assessment of Concussion (SAC) tool. The SAC evaluates temporal orientation, immediate and delayed memory, and concentration. The SCAT2 is particularly useful in clinical contexts as it is quick to administer and addresses multiple dimensions.16 The maximum scores for the SAC and the SCAT2 are 30 and 100, respectively.
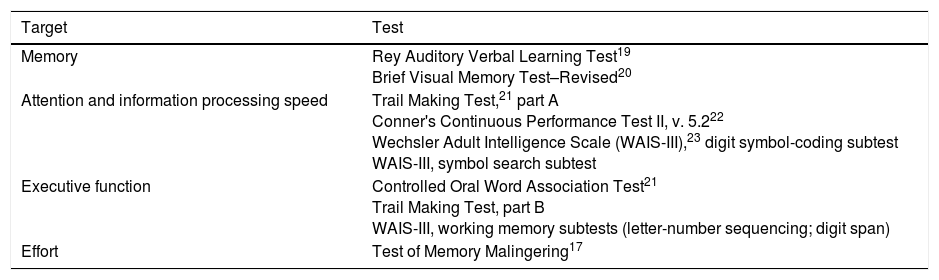
Neuropsychological assessmentAll participants underwent neuropsychological assessments on one occasion; patients were evaluated within the first 2 weeks after the mTBI. Cognitive function was assessed using a neuropsychological battery testing attention, memory, information processing speed, and complex executive functions (Table 2). Tests were selected in line with the recommendations of the National Institute of Neurological Disorders and Stroke.2 The tests selected are included in the Institute's Core Data Elements for studying patients with traumatic brain injury.2 Effort was tested using the Test of Memory Malingering (TOMM)17 in order to detect any feigned symptoms or a lack of collaboration during the examination. Patients also completed the Hospital Anxiety and Depression Scale,18 as anxiety and depression can influence cognitive profiles. The duration of the complete cognitive examination was approximately 120min, including a 5-10-min rest period, where needed. These studies were performed by researchers with specific training in neuropsychology (A.R. and V.C.).
List of tests in the neuropsychological assessment battery.
| Target | Test |
|---|---|
| Memory | Rey Auditory Verbal Learning Test19 Brief Visual Memory Test–Revised20 |
| Attention and information processing speed | Trail Making Test,21 part A Conner's Continuous Performance Test II, v. 5.222 Wechsler Adult Intelligence Scale (WAIS-III),23 digit symbol-coding subtest WAIS-III, symbol search subtest |
| Executive function | Controlled Oral Word Association Test21 Trail Making Test, part B WAIS-III, working memory subtests (letter-number sequencing; digit span) |
| Effort | Test of Memory Malingering17 |
The MRI study was performed using a SIEMENS Magnetom Trio Tim syngo 3T MRI scanner at Hospital Clínic de Barcelona's Centre for Diagnostic Imaging. Images were analysed by a neuroradiology expert (N.B.) who was not a member of the study team. For each patient, we obtained a high-resolution, T1-weighted structural image (3D magnetisation-prepared rapid gradient echo [MP-RAGE]) and T2-weighted FLAIR and gradient echo sequences. Microbleeds were identified using SWI sequences. Sequences were obtained in the same order for all participants. These studies were performed within 14 days of the mTBI.
Statistical analysisAll variables were analysed using version 22 of the SPSS statistics package (Chicago, Illinois, USA). As most variables did not follow a normal distribution, non-parametric tests (the χ2-test and the Mann–Whitney U-test) were used to compare and analyse associations between variables.
In the neuropsychological assessment, in which analysis involves many related variables, the likelihood of type I error is known to increase when multiple statistical comparisons are made. We may therefore consider applying the Benjamini–Hochberg correction24 for the false discovery rate, or using a more conservative significance threshold (P<.01). However, the small sample size causes a considerable reduction in statistical power. We therefore decided on a more liberal statistical approach, assuming a 5% likelihood of error for all results. In order to better describe the magnitude of the differences identified, effect size was calculated with the correlation coefficient r.
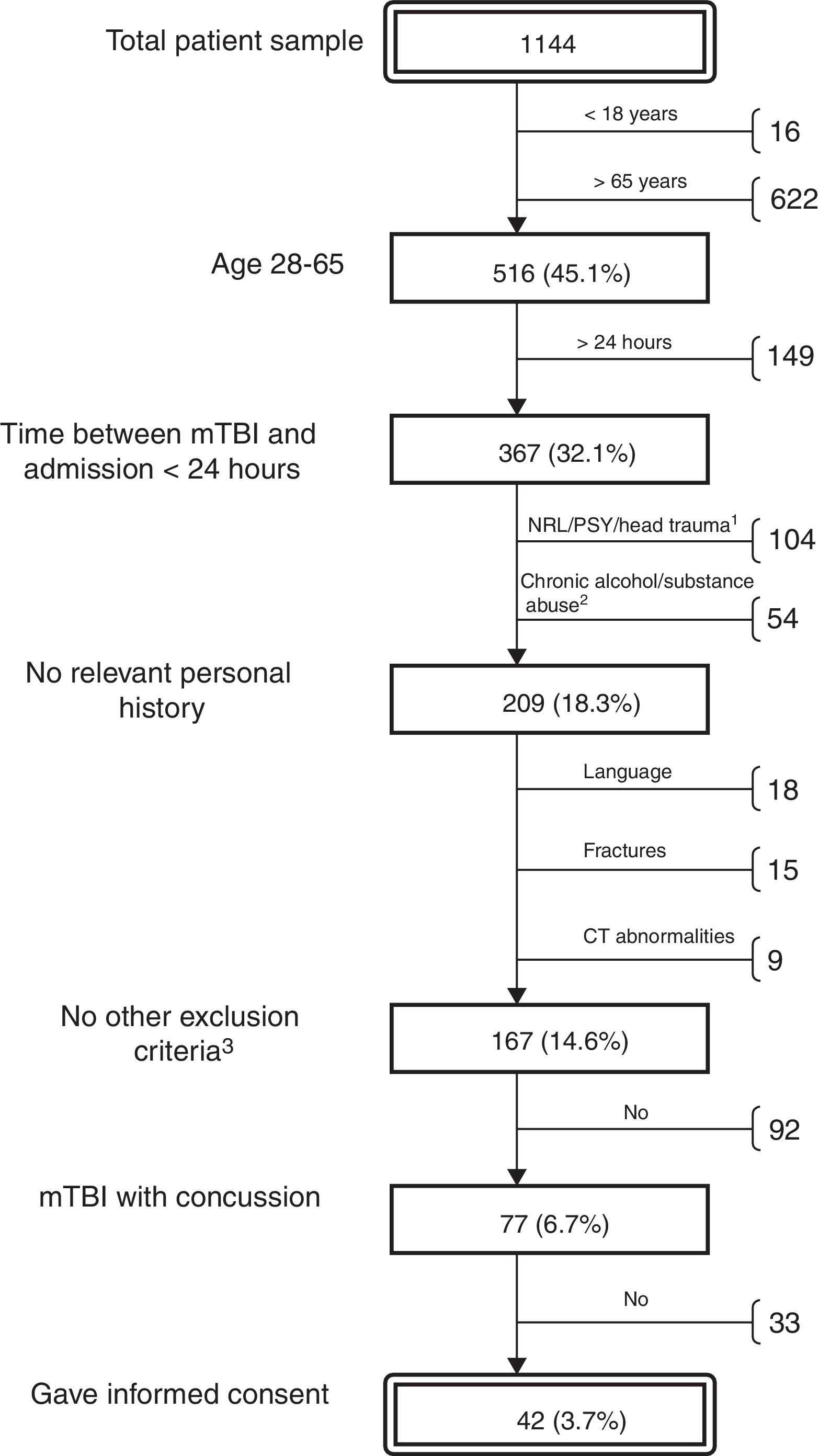
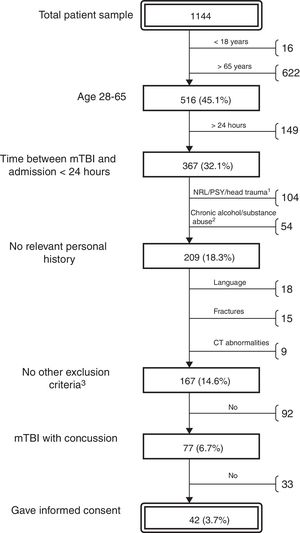
ResultsThe neurosurgery department treated 1144 patients diagnosed with mTBI during the study period. The majority of these patients were elderly or had incurred minor head trauma not associated with loss of consciousness, PTA, or other relevant symptoms. We selected a total of 41 patients (16 women and 25 men; median age, 34; IQR, 24; range, 18-64) (Fig. 1) according to the protocol described above. For all patients, we performed a brief cognitive assessment and recorded post-concussive symptoms (with the SCAT2) the day the trauma occurred; results were compared with those of the control group. As 7 patients (17%) did not attend the follow-up visit, the extensive follow-up cognitive assessment was only performed for the remaining 34 patients (12 women and 22 men; median age, 32.5; IQR, 23; range, 18-64).
Algorithm for the selection of patients with mTBI treated during the study period at the Vall d’Hebron University Hospital emergency department's neurotraumatology unit. Percentages refer to the total number of patients. Reasons for exclusion: previous neurological/psychiatric condition or traumatic brain injury (1); chronic alcohol or substance abuse (2); other (3): insufficient level of Spanish or Catalan, fractures requiring admission to hospital, and pathological brain CT findings.
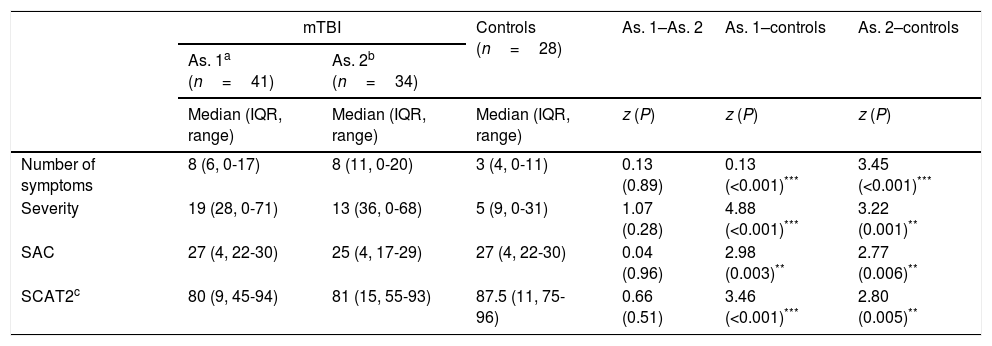
As expected, patients displayed significantly more symptoms and significantly greater symptom severity than controls in the hours following the mTBI (z=–4.44, P<.001, r=0.53, vs z=–4.88, P<.001, r=0.58, respectively) (Table 3). Significant intergroup differences were also observed for overall SCAT2 score (z=3.46, P<.001, r=0.43).
Longitudinal evaluation of SCAT2 scores in patients and controls.
| mTBI | Controls (n=28) | As. 1–As. 2 | As. 1–controls | As. 2–controls | ||
|---|---|---|---|---|---|---|
| As. 1a (n=41) | As. 2b (n=34) | |||||
| Median (IQR, range) | Median (IQR, range) | Median (IQR, range) | z (P) | z (P) | z (P) | |
| Number of symptoms | 8 (6, 0-17) | 8 (11, 0-20) | 3 (4, 0-11) | 0.13 (0.89) | 0.13 (<0.001)*** | 3.45 (<0.001)*** |
| Severity | 19 (28, 0-71) | 13 (36, 0-68) | 5 (9, 0-31) | 1.07 (0.28) | 4.88 (<0.001)*** | 3.22 (0.001)** |
| SAC | 27 (4, 22-30) | 25 (4, 17-29) | 27 (4, 22-30) | 0.04 (0.96) | 2.98 (0.003)** | 2.77 (0.006)** |
| SCAT2c | 80 (9, 45-94) | 81 (15, 55-93) | 87.5 (11, 75-96) | 0.66 (0.51) | 3.46 (<0.001)*** | 2.80 (0.005)** |
SCAT2 score could not be calculated for patients who were unable to perform the balance assessments; this explains the difference in the number of patients assessed in the baseline examination (n=35) and the follow-up examination (n=31).
As.: assessment; IQR: interquartile range; mTBI: mild traumatic brain injury;fAs expected, patients displayed significant SAC: Standardized Assessment of Concussion; SCAT2: Sport Concussion Assessment Tool 2.
In the clinical follow-up examination performed several days after the trauma, there continued to be a significant difference between groups for number and severity of symptoms and overall SCAT2 score (z=–3.45, P<.001, r=0.44; z=3.22, P<.001, r=0.41; and z=2.80, P=.005, r=0.36, respectively). However, Hospital Anxiety and Depression Scale scores showed no significant difference between groups for the anxiety or depression subscales (z=–0.59, P=.55; and z=–0.68, P=.50, respectively).
Neuropsychological assessmentThe 34 patients who attended the follow-up visit underwent an extensive neuropsychological assessment; results were compared to those of the control group. Most patients were assessed within the first week after the mTBI (median, 5 days; range, 2-13) (Table 4). Table 5 displays the most relevant results from the cognitive assessment of the groups studied. As is shown in the table, sample size changed between tests, as patients with mild injuries to their dominant arm did not perform tests requiring manual skills, such as drawing or psychomotor speed tasks.
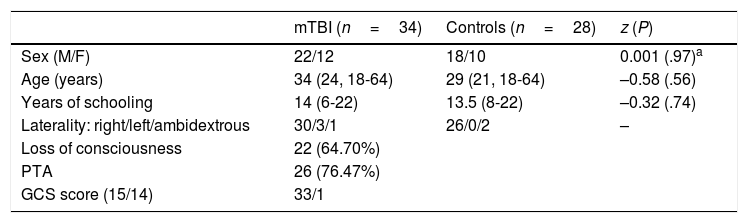
Relevant sociodemographic and clinical variables of the patient and control groups.
| mTBI (n=34) | Controls (n=28) | z (P) | |
|---|---|---|---|
| Sex (M/F) | 22/12 | 18/10 | 0.001 (.97)a |
| Age (years) | 34 (24, 18-64) | 29 (21, 18-64) | –0.58 (.56) |
| Years of schooling | 14 (6-22) | 13.5 (8-22) | –0.32 (.74) |
| Laterality: right/left/ambidextrous | 30/3/1 | 26/0/2 | – |
| Loss of consciousness | 22 (64.70%) | ||
| PTA | 26 (76.47%) | ||
| GCS score (15/14) | 33/1 |
Median, interquartile range, and range are shown in brackets.
GCS: Glasgow Coma Scale; mTBI: mild traumatic brain injury; PTA: post-traumatic amnesia.
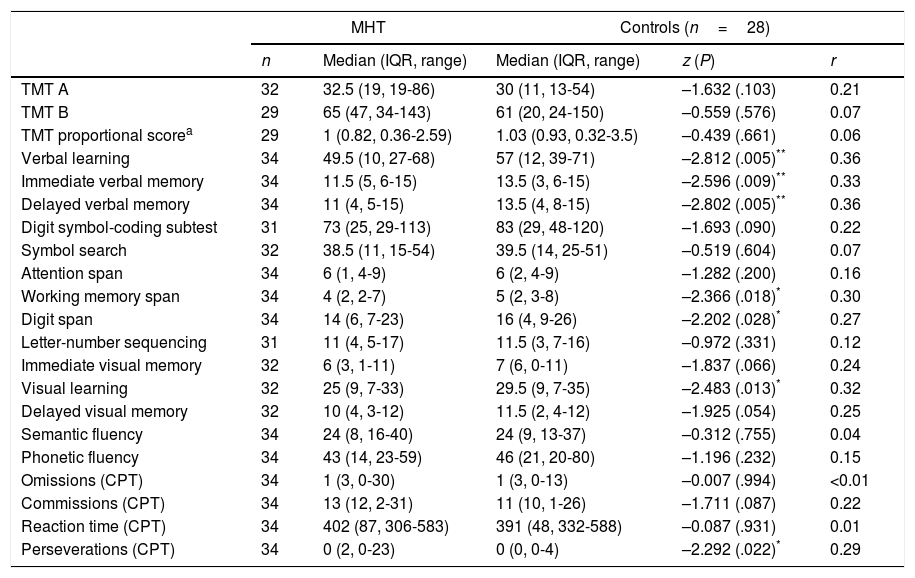
Comparative analysis of neuropsychological test results for patients and controls.
| MHT | Controls (n=28) | ||||
|---|---|---|---|---|---|
| n | Median (IQR, range) | Median (IQR, range) | z (P) | r | |
| TMT A | 32 | 32.5 (19, 19-86) | 30 (11, 13-54) | –1.632 (.103) | 0.21 |
| TMT B | 29 | 65 (47, 34-143) | 61 (20, 24-150) | –0.559 (.576) | 0.07 |
| TMT proportional scorea | 29 | 1 (0.82, 0.36-2.59) | 1.03 (0.93, 0.32-3.5) | –0.439 (.661) | 0.06 |
| Verbal learning | 34 | 49.5 (10, 27-68) | 57 (12, 39-71) | –2.812 (.005)** | 0.36 |
| Immediate verbal memory | 34 | 11.5 (5, 6-15) | 13.5 (3, 6-15) | –2.596 (.009)** | 0.33 |
| Delayed verbal memory | 34 | 11 (4, 5-15) | 13.5 (4, 8-15) | –2.802 (.005)** | 0.36 |
| Digit symbol-coding subtest | 31 | 73 (25, 29-113) | 83 (29, 48-120) | –1.693 (.090) | 0.22 |
| Symbol search | 32 | 38.5 (11, 15-54) | 39.5 (14, 25-51) | –0.519 (.604) | 0.07 |
| Attention span | 34 | 6 (1, 4-9) | 6 (2, 4-9) | –1.282 (.200) | 0.16 |
| Working memory span | 34 | 4 (2, 2-7) | 5 (2, 3-8) | –2.366 (.018)* | 0.30 |
| Digit span | 34 | 14 (6, 7-23) | 16 (4, 9-26) | –2.202 (.028)* | 0.27 |
| Letter-number sequencing | 31 | 11 (4, 5-17) | 11.5 (3, 7-16) | –0.972 (.331) | 0.12 |
| Immediate visual memory | 32 | 6 (3, 1-11) | 7 (6, 0-11) | –1.837 (.066) | 0.24 |
| Visual learning | 32 | 25 (9, 7-33) | 29.5 (9, 7-35) | –2.483 (.013)* | 0.32 |
| Delayed visual memory | 32 | 10 (4, 3-12) | 11.5 (2, 4-12) | –1.925 (.054) | 0.25 |
| Semantic fluency | 34 | 24 (8, 16-40) | 24 (9, 13-37) | –0.312 (.755) | 0.04 |
| Phonetic fluency | 34 | 43 (14, 23-59) | 46 (21, 20-80) | –1.196 (.232) | 0.15 |
| Omissions (CPT) | 34 | 1 (3, 0-30) | 1 (3, 0-13) | –0.007 (.994) | <0.01 |
| Commissions (CPT) | 34 | 13 (12, 2-31) | 11 (10, 1-26) | –1.711 (.087) | 0.22 |
| Reaction time (CPT) | 34 | 402 (87, 306-583) | 391 (48, 332-588) | –0.087 (.931) | 0.01 |
| Perseverations (CPT) | 34 | 0 (2, 0-23) | 0 (0, 0-4) | –2.292 (.022)* | 0.29 |
CPT: Continuous Performance Test; MHT: mild head trauma; TMT: Trail Making Test.
The Rey Auditory Verbal Learning Test showed the most marked difference between groups. Patients’ performance was significantly poorer than that of controls in learning and immediate and delayed verbal memory, with a medium effect size (r>0.3). Scores for visual learning and delayed visual memory were also lower in patients than in controls, although intergroup differences in delayed visual memory scores displayed only a trend toward statistical significance (P=.054). Patients also scored significantly lower than controls for 3 indicators (working memory span, digit span subtest, and number of perseverations in the Continuous Performance Test). These variables shed light on important aspects of attention and executive function (due to working memory and inhibition components) that appear to be affected several weeks after mTBI.
It should be noted that given the high number of variables analysed, any more conservative statistical strategy involving correction for multiple comparisons would require a significance threshold of approximately P<.003. The alterations observed in our cohort had a significance level of .005 or higher.
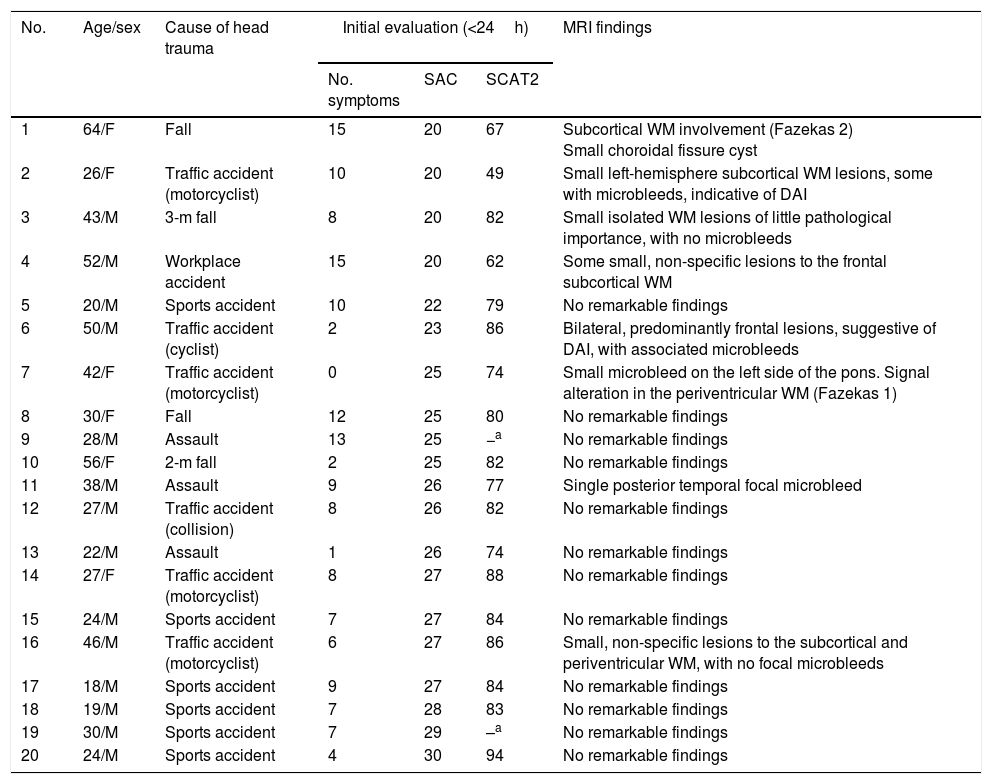
Magnetic resonance imaging findingsBrain MRI studies were performed for a subgroup of 20 patients, selected on the basis of their availability for testing. MRI was performed a median of 6 days after trauma occurred (range, 1-13). Table 6 provides a detailed description of demographic characteristics and neuroradiology findings for this subgroup.
Demographic, clinical, and neuroradiological characteristics of the 20 patients who underwent MRI studies, ordered by SAC score.
| No. | Age/sex | Cause of head trauma | Initial evaluation (<24h) | MRI findings | ||
|---|---|---|---|---|---|---|
| No. symptoms | SAC | SCAT2 | ||||
| 1 | 64/F | Fall | 15 | 20 | 67 | Subcortical WM involvement (Fazekas 2) Small choroidal fissure cyst |
| 2 | 26/F | Traffic accident (motorcyclist) | 10 | 20 | 49 | Small left-hemisphere subcortical WM lesions, some with microbleeds, indicative of DAI |
| 3 | 43/M | 3-m fall | 8 | 20 | 82 | Small isolated WM lesions of little pathological importance, with no microbleeds |
| 4 | 52/M | Workplace accident | 15 | 20 | 62 | Some small, non-specific lesions to the frontal subcortical WM |
| 5 | 20/M | Sports accident | 10 | 22 | 79 | No remarkable findings |
| 6 | 50/M | Traffic accident (cyclist) | 2 | 23 | 86 | Bilateral, predominantly frontal lesions, suggestive of DAI, with associated microbleeds |
| 7 | 42/F | Traffic accident (motorcyclist) | 0 | 25 | 74 | Small microbleed on the left side of the pons. Signal alteration in the periventricular WM (Fazekas 1) |
| 8 | 30/F | Fall | 12 | 25 | 80 | No remarkable findings |
| 9 | 28/M | Assault | 13 | 25 | −a | No remarkable findings |
| 10 | 56/F | 2-m fall | 2 | 25 | 82 | No remarkable findings |
| 11 | 38/M | Assault | 9 | 26 | 77 | Single posterior temporal focal microbleed |
| 12 | 27/M | Traffic accident (collision) | 8 | 26 | 82 | No remarkable findings |
| 13 | 22/M | Assault | 1 | 26 | 74 | No remarkable findings |
| 14 | 27/F | Traffic accident (motorcyclist) | 8 | 27 | 88 | No remarkable findings |
| 15 | 24/M | Sports accident | 7 | 27 | 84 | No remarkable findings |
| 16 | 46/M | Traffic accident (motorcyclist) | 6 | 27 | 86 | Small, non-specific lesions to the subcortical and periventricular WM, with no focal microbleeds |
| 17 | 18/M | Sports accident | 9 | 27 | 84 | No remarkable findings |
| 18 | 19/M | Sports accident | 7 | 28 | 83 | No remarkable findings |
| 19 | 30/M | Sports accident | 7 | 29 | –a | No remarkable findings |
| 20 | 24/M | Sports accident | 4 | 30 | 94 | No remarkable findings |
DAI: diffuse axonal injury; MRI: magnetic resonance imaging; SAC: Standardized Assessment of Concussion; SCAT2: Sport Concussion Assessment Tool 2; WM: white matter.
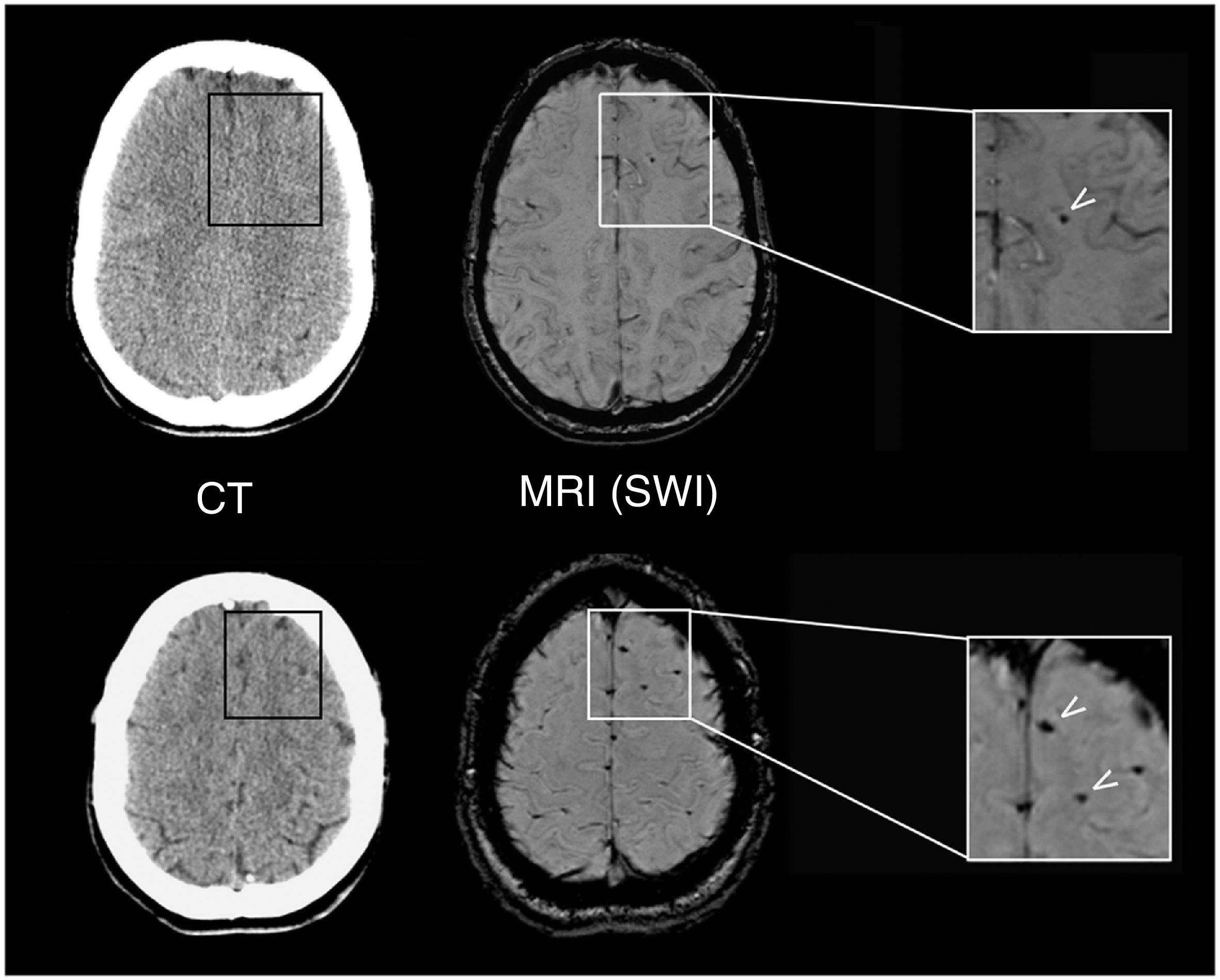
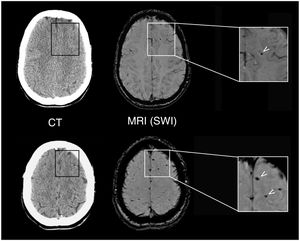
Although all patients showed normal brain CT findings at baseline, MRI revealed lesions suggestive of DAI in 2 patients (10%). Figure 2 shows the lesions observed in a 26-year-old patient with an mTBI with loss of consciousness and PTA following a motorcycle accident. We also observed focal signal alterations of potentially traumatic aetiology in five other patients (25%); aetiology could not be confirmed in all cases, however.
Neuroradiological findings in a 26-year-old patient with mTBI. The results of a brain CT scan (left) performed 2h after the trauma were normal, whereas a brain MRI study at 10 days (right) revealed focal signal alterations (arrows) on SWI sequences, which corresponded to microbleeds indicative of mild DAI.
The results of this pilot study demonstrate that concussive symptoms secondary to mTBI may last days after the trauma occurs potentially interfering with patients’ work or academic performance. Besides the typical symptoms of concussion (headache, vertigo, etc.), these patients may display cognitive alterations, which are observable through the use of specific tools. Finally, MRI revealed structural alterations compatible with DAI or microbleeds in a considerable percentage of patients, despite normal early brain CT results in all patients.
Remarks on the inclusion of patients in the studyOne of the most noteworthy findings was the high number of patients attending the hospital following MHT who were eventually found not to be eligible for inclusion. Age was the most frequent reason for exclusion: 55% of patients treated during the study period were older than 65. This figure is consistent with the recent changes observed in the epidemiology of traumatic brain injury. A significant increase has been observed in the ages of trauma patients worldwide, with falls surpassing traffic accidents as the most frequent cause in this age group.1 The strict screening criteria applied, specifically chosen to eliminate the known confounding factors, reduced the number of potential participants to 6.9% of the patients treated. Only 60% of those meeting the inclusion criteria volunteered to participate in the study. Therefore, the study participants accounted for less than 4% of all patients treated in a 1-year period at this tertiary hospital. In a 2013 study, Luoto et al.25 discuss this issue and note that many hospital studies into mTBI use potentially biased samples, for which reason results cannot be generalised to the entire population of trauma patients.
Diagnostic criteria for mTBIAccording to the classic 2004 World Health Organization criteria,4 diagnosis of mTBI requires a GCS score of 13, 14, or 15. However, more recent diagnostic criteria rule out patients scoring 13 on the GCS. Various authors note that in terms of mortality indicators and complications, the progression of patients scoring 13 is more similar to that of patients with moderate than with mTBI.26 Stein and Ross27 compare initial brain CT findings in a group of 106 patients scoring 13 on the GCS to those of 341 patients diagnosed with moderate traumatic brain injury according to the classic criteria (GCS score 9-12). Both groups showed a similar prevalence of lesions in the brain CT images (44.3 and 40.3%, respectively). These researchers also found that 20% of patients with pathological brain CT findings required surgical treatment; for this reason, they propose that trauma patients scoring 13 on the GCS should be reclassified as having moderate rather than mTBI.27
The present study also required normal brain CT findings among the inclusion criteria applied. The exclusion of patients with pathological brain CT findings ensures a more homogeneous sample, as patients displaying brain injury on CT scans are followed up according to different protocols, which are more similar to those used for cases of moderate traumatic brain injury. The absence of brain injury in conventional emergency department examination tests enabled us to study patients with more benign symptoms (GCS scores of 14 or 15 and normal CT results).
Clinical assessment of mTBI: current toolsIn order to address the issue of why some patients present post-concussive syndrome, it is necessary to better understand the clinical presentation of acute-phase mTBI. The prognostic value of such traditional descriptors as presence and duration of loss of consciousness and PTA are not well established; neither phenomenon is well understood from a pathophysiological perspective in the context of mTBI. The validity and predictive power of PTA is very uncertain in these patients, despite being a defining characteristic of concussion and a critical element of routine hospital assessment.16
We used the SCAT2, originally designed to assess trauma in sport, to obtain a complete register of participants’ clinical information. Patients continued to present a high number of post-concussive symptoms at 1-2 weeks after trauma, although severity tended to decrease. The SCAT2 results at the time of hospitalisation and at 1-2 weeks support the use of the tool in the standardised monitoring of post-concussive symptoms, as it also provides a general overview of these patients’ cognitive status. However, evaluation of the clinical symptoms assessed in this study (loss of consciousness and balance) is less reliable in the hospital context civilian mTBI than in sport, as it is often based on information provided by patients themselves or on witness accounts. Furthermore, balance cannot be assessed in patients with injuries to the lower limbs which prevent them from standing. Results of balance assessments are more variable in the general adult population than among young athletes: 10% of a sample from a healthy Canadian population and approximately 65% of a Finnish control group had low scores for balance tests.16 These results suggest that we should reconsider the value of the SCAT2 balance subtest in future studies aiming to validate the scale's use outside the context of sport.
Neuropsychological evaluation resultsWhile traumatic brain injury can affect almost any aspect of brain function, the most significant consequence is probably dysfunction of frontal systems, which are of particular importance for executive function (planning and organisation, working memory, flexibility, and behavioural inhibition, among others). For this reason, researchers such as Chen and d’Esposito28 and Stuss29 define traumatic brain injury as a disorder of cognitive control.
The results of our detailed neuropsychological evaluation of patients with mTBI and controls show that learning SIN capacity and memory are mildly impaired in the first 2 weeks after an mTBI. Other indicators of attention (which also have an executive component, due to the involvement of working memory and inhibition) add to the results that show the presence of subtle (...) subtle cognitive deficits in this group of patients.
These are the results of an exploratory analysis, and statistical significance for differences between the patient and control groups was set at P<.05, with no strict post hoc correction. However, they are consistent with the results of a recent systematic review, which concluded that cognitive deficits are consistently associated with mTBI in the first 2 weeks after trauma occurs.11 The study also found a limited association between loss of consciousness and reduced information processing speed. However, while nearly 70% of the patients in our study had experienced loss of consciousness, the results do not clearly confirm impairment of information processing speed.
Normal brain computed tomography findings and lesions on the magnetic resonance imagesPatients with mTBI, and particularly those with persistent symptoms, may present brain injuries that can go unnoticed on brain CT scans. SWI sequences identified traumatic structural lesions in 10% of the patients who underwent MRI studies. In another five patients (25%), MRI displayed lesions which were not observable on conventional brain CT scans, although aetiology was unclear in some cases.
Although other studies have found a correlation between the total volume of lesions detected on SWI sequences and clinical indicators of severity,30 no clear association has been established between the presence of these lesions and cognitive recovery following trauma. Several studies report that the presence of lesions in neuroimaging studies of patients with mTBI is associated with poorer results for such cognitive functions as memory. However, as the great majority of the neuropsychological tests applied yield similar results for groups of patients with and without lesions observable through neuroradiology, some authors assert that these patients do not require different treatment.31
These brain lesions occur during the acute phase of mTBI and persist indefinitely. Identification of these lesions provides information not only about severity, but also about which neurobehavioural systems may be affected. Advanced MRI studies provide information on the distribution of a brain injury and enable the creation of more effective assessment and treatment strategies, similar to the role these studies play in the rehabilitation of patients with ischaemic stroke.
In conclusion, despite our study's limitations, and contrary to the received wisdom in the clinical setting, our results confirm that some cases of mTBI should not be considered banal injuries. Despite normal CT findings, advanced MRI studies showed that 10%-35% of patients had lesions that were potentially indicative of DAI. Both during the acute phase and at 1-2 weeks, our patients displayed alterations in their overall neurocognitive status, compared to the control group. The results of the neuropsychological evaluation show that these patients’ cognitive status continues to be affected in the medium term, with symptoms including memory and executive attention issues. One of the study's main limitations is its relatively short follow-up period. As symptoms can persist for months following mTBI, potentially even becoming permanent, longer follow-up periods are necessary. Future studies should address this matter.
Our findings show that the typical management of patients attending hospital with mTBI (usually an assessment and discharge without follow-up) may not be appropriate in all cases. Structured recording of post-concussive symptoms and neuropsychological assessment provide very important information on the alterations these patients may display for at least the first 2 weeks after trauma. Despite the need for larger samples, our results support the use of the SCAT2 questionnaire as part of routine clinical care.
FundingThis study was partially funded by the following organisations: MAPFRE foundation (project 2012-04, awarded to M.A.P.), the Fondo de Investigación Sanitaria (project PI13/02397, lead researcher: M.A.P.), and Institut de Recerca Vall d’Hebron (grant no. PRED-VHIR-2012-26, awarded to A.R.). This study is part of A.R.’s doctoral thesis.
Conflicts of interestThe authors have no conflicts of interest to declare.
We would like to thank Dr Núria Bargalló for her invaluable assistance with the MRI studies. We are also grateful to the nursing team at the emergency department's neurotraumatology unit for their assistance in selecting patients and to Noelia Montoya, Lourdes Expósito, and Mercedes Arribas for their constant support.
Please cite this article as: Rădoi A, Poca MA, Cañas V, Cevallos JM, Membrado L, Saavedra MC, et al. Alteraciones neuropsicológicas y hallazgos neurorradiológicos en pacientes con conmoción cerebral postraumática. Resultados de un estudio piloto. Neurología. 2018;33:427–437.