Some studies have shown that CSF amyloid-beta 1-42 (Aβ1-42), total tau (T-tau) and tau phosphorylated at threonine 181 (P-tau181p) proteins are useful diagnostic markers for distinguishing between clinically stable mild cognitive impairment (MCI) patients and those who will develop Alzheimer's disease (AD).
Our objective was to test the ability of this technique to discriminate in our cohort of MCI patients, according to the clinical outcome, one year after the lumbar puncture.
Material and methodsA total of 36 MCI patients were included from the local hospital memory clinic. Using INNO-BIA Alzbio-3 reagents from Innogenetics, we measured CSF Aβ1-42, T-tau and P-tau181p proteins, and calculated the T-tau/Aβ1-42 and P-tau181p/Aβ1-42 ratios. This project was approved by the local ethics committee.
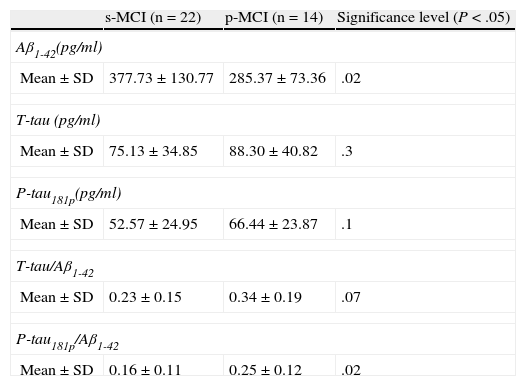
ResultsOne year after the lumbar puncture, 14 MCI patients (38%) developed AD. These patients had lower Aβ1-42 protein levels (285.3ng/ml vs 377ng/ml, P<.02) and higher P-tau181p/Aβ1-42 ratio (0.25 vs 0.16, P<.02) than the clinically stable patients.
ConclusionsOur MCI patients with lower Aβ1-42 protein levels and an increased P-tau181p/Aβ1-42 ratio progressed quickly to AD. These results may help to identify those MCI patients with a poorer prognosis.
En muchos artículos recientes, el análisis de las proteínas Aβ1-42, tau total (T-tau) y tau fostorilada (P-tau) en LCR puede discriminar entre los pacientes con deterioro cognitivo leve (DCL) estables y aquellos otros que van a progresar a enfermedad de Alzheimer (EA). Nuestro objetivo fue comprobar la capacidad de estas proteínas del LCR para discriminar, entre nuestros pacientes DCL, según la evolución clínica en el año siguiente a la punción lumbar.
Material y métodosSe incluyó a 36 pacientes DCL amnésico (criterios de Petersen 2006) procedentes de la consulta de deterioro cognitivo del Hospital General de Alicante. Usando los reactivos INNO-BIA Alzbio-3 (Innogenetics), cuantificamos las proteínas Aβ1-42, T-tau, P-tau181p en LCR, y calculamos los cocientes T-tau/Aβ1-42 y P-tau181p/Aβ1-42. El estudio fue aprobado por el comité ético de investigación del Hospital General de Alicante.
ResultadosEn los 12 meses posteriores a la punción lumbar, 14 pacientes DCL (38%) evolucionaron a EA. Estos pacientes, presentaron menores niveles de Aβ1-42 (285,3 vs. 377,7ng/ml, p<0,02), y un aumento en el valor del cociente P-tau181p/Aβ1-42 (0,25 vs. 0,16, p<0,02) que los pacientes que se mantuvieron estables. No hubo diferencias significativas en el resto de variables estudiadas.
ConclusionesNuestros pacientes DCL que presentaron niveles reducidos de la proteína Aβ1-42 y elevación del cociente P-tau181p/Aβ1-42 en LCR, evolucionaron rápidamente a EA. Estos resultados pueden ayudar a conseguir el objetivo de identificar de forma precoz a los pacientes DCL con peor pronóstico.
Many authors consider mild cognitive impairment (MCI) as the most important risk factor for the development of Alzheimer's disease (AD).1 Thus, 40–60% of patients with MCI develop AD within the next 5 years,2 while another significant percentage maintain a stable form of cognitive impairment.3
Different complementary techniques have been used for several years in an attempt to identify MCI patients who will develop dementia, in order to treat them early or at least to delay and mitigate the devastating effects of the disease. Among the aforementioned complementary techniques the main technique is the analysis of Aβ1-42, T-tau and P-tau proteins in cerebrospinal fluid (CSF), as well as the ratios of Aβ1-42 and both tau proteins, as they reflect the relationship between both AD pathogenic pathways. Their study has led to numerous works being published4–7 and also to different meta-analysis of their predictive ability for the diagnosis of AD.8–10
Moreover, recent publications have used these biomarkers to recognise the MCI patients who will progress rapidly to dementia,11,12 and even to identify the patients already suffering dementia who will evolve more rapidly.13
The aim of this work is to verify whether this technique is capable of differentiating our stable MCI patients from those who progressed to AD in the months following the lumbar puncture (LP).
Materials and methodsStudy designThis was a cohort study.
Study subjectsThe study included 36 patients with amnestic MCI diagnosed according to 2006 Petersen criteria,3 all from the cognitive impairment consultation at Hospital General Universitario in Alicante, some of whom had been monitored for several years. Their study included physical and neurological examinations, a neuropsychological study, Yesavage's geriatric depression scale (15 items), blood tests, brain structural imaging and LP. The patients were monitored in the outpatient clinic with periodic reviews at least every 6 months. These reviews evaluated the development of AD according to the NINCDS-ADRDA criteria14 and GDS scale. According to the progression at 12 months after LP, we differentiated patients into those who were stable (s-MCI) and those who progressed to AD (p-MCI).
Inclusion criteriaAmnestic MCI patients over 55 years. They signed informed consent prior to inclusion in the study and performance of LP.
Exclusion criteriaThe presence of dementia or any other neurological, psychiatric or systemic disease which could cause cognitive impairment, anticoagulant therapy and lack of informed consent were grounds for exclusion. An evaluation with Yesavage's geriatric depression scale over 5 points was also considered as reason for exclusion.
ProceduresThe neurologist in charge emitted a diagnosis of pure or multi-domain amnestic MCI, according to the Petersen criteria.3 Subsequently, a neuropsychological study was conducted that included assessment of memory, language, executive functions, attention and visuo-constructive abilities through the Mini Mental State Examination (MMSE), Rey auditory–verbal learning test, trail making test (TMT) and informer test (INT). Alterations of functions were defined as a result Z of −1.5 or less, which represented at least 1.5 standard deviations below the mean of the control subjects, in at least 1 of the tests used to study each function. The criteria for considering the change from s-MCI to p-MCI was a decline in MMSE over 2 points and/or an in increase in INT over 7 points.
Neuroimaging was performed in all patients with MCI (cerebral MRI in most cases or only cerebral CT scan in 5 of them), in order to rule out other brain lesions which could be responsible for the clinical symptoms.
Obtaining and analysing cerebrospinal fluidThis was conducted between February 2008 and February 2009. The samples were obtained between 10:00 and 14:00h. The LP was conducted by the neurologist responsible using a 20×3(1/2) needle. The CSF was collected in standard tubes and was frozen at −80°C in less than 1h. When the CSF contained blood, it was centrifuged prior to freezing. One tube was collected to perform biochemical analysis and cell count prior to obtaining the freezing tubes. In no case did this cell count show over 100 red blood cells.
After the LP, patients were advised to avoid Valsalva manoeuvres for at least 3 days.
Quantifying Aβ1-42, T-tau and P-tau181p cerebrospinal fluid protein levelsThis was performed using Luminex xMAP technology with INNO-BIA AlzBio3 reagents from Innogenetics (Ghent, Belgium). The details of this combination of immunoassay reagents and analytical platform have been published previously.15
All samples were analysed simultaneously at the end of recruitment and in a blind manner for clinical data. The samples from 19 patients were analysed in duplicate.
Study variablesWe studied the levels of CSF proteins Aβ1-42, T-tau and P-tau181p, as well as the ratios T-tau/Aβ1-42 and P-tau181p/Aβ1-42. These ratios are being used by various authors widely, as they seem to reflect the relationship between the amyloid and tau pathways of the disease.16
Statistical analysisThe reliability of the technique was calculated using the intraclass correlation coefficient.
We used the Kolmogorov–Smirnov test to analyse the distribution type of each variable.
For comparison between 2 groups, we used the Student's t-test for parametric variables and the Mann–Whitney U-test for nonparametric variables.
In addition, we performed ROC curve analysis to determine the best cut-off values for the measurement of variables. The best cut-off value was defined in response to higher sensitivity above all. We then obtained the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for each cut-off point and each variable.
We used a P<.05 level of statistical significance for all hypothesis contrasts. The statistical analysis was conducted using the SPSS v.10.0 software.
Ethical criteriaThe pharmaceutical companies that funded this project had no role in its design, data collection or interpretation or in the writing of this work.
This project was approved by the clinical research ethics committee of Hospital General Universitario in Alicante, after obtaining a civil liability insurance policy.
ResultsReliability of the techniqueIn the 19 cases analysed in duplicate, the intraclass correlation coefficient was 0.94 for protein Aβ1-42, 0.96 for protein T-tau and 0.95 for protein P-tau.
Comparison of s-MCI group vs p-MCI groupBy studying the evolution towards dementia of our patients in the first 12 months after the completion of a LP, we observed that 14 (38%) of them had progressed to dementia. When comparing these groups, we observed:
- –
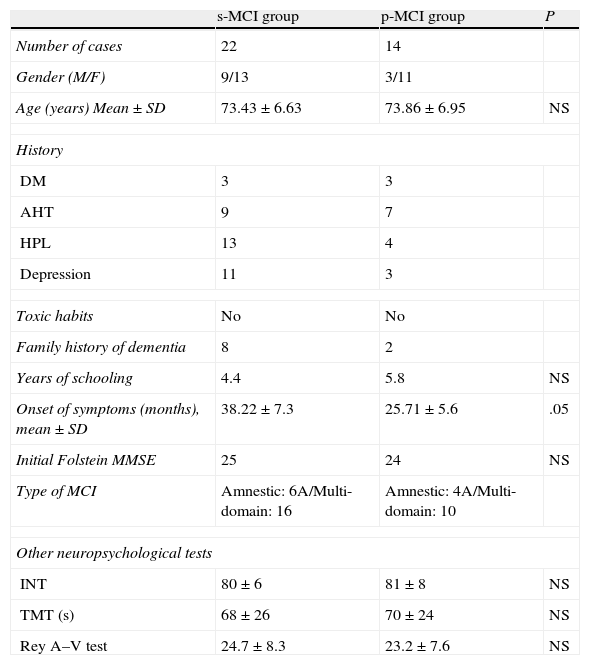
Clinical-demographic characteristics (Table 1): there were no significant differences among the ages of both groups or among most of the variables studied. However, s-MCI patients had a higher percentage of history of depression (46% vs 18%) and a longer evolution time from the onset of symptoms until the completion of a LP.
Table 1.Clinical and demographic characteristics of the s-MCI and p-MCI groups.
s-MCI group p-MCI group P Number of cases 22 14 Gender (M/F) 9/13 3/11 Age (years) Mean±SD 73.43±6.63 73.86±6.95 NS History DM 3 3 AHT 9 7 HPL 13 4 Depression 11 3 Toxic habits No No Family history of dementia 8 2 Years of schooling 4.4 5.8 NS Onset of symptoms (months), mean±SD 38.22±7.3 25.71±5.6 .05 Initial Folstein MMSE 25 24 NS Type of MCI Amnestic: 6A/Multi-domain: 16 Amnestic: 4A/Multi-domain: 10 Other neuropsychological tests INT 80±6 81±8 NS TMT (s) 68±26 70±24 NS Rey A–V test 24.7±8.3 23.2±7.6 NS AHT: arterial hypertension; DM: diabetes mellitus; HPL: hyperlipidemia; INT: informer test; NS: not significant; Rey A–V test: Rey auditory–verbal learning test; TMT: trail making test.
- –
Comparison of protein levels and ratios (Table 2): distinctions between the 2 groups were notable for the protein Aβ1-42 (P<.02) and the ratio P-tau/Aβ1-42 (P<.02). The remaining variables studied did not reach statistical significance.
Table 2.Comparison of the concentrations of the 3 biomarkers and quotients between biomarkers for s-MCI and p-MCI patients.
s-MCI (n=22) p-MCI (n=14) Significance level (P<.05) Aβ1-42(pg/ml) Mean±SD 377.73±130.77 285.37±73.36 .02 T-tau (pg/ml) Mean±SD 75.13±34.85 88.30±40.82 .3 P-tau181p(pg/ml) Mean±SD 52.57±24.95 66.44±23.87 .1 T-tau/Aβ1-42 Mean±SD 0.23±0.15 0.34±0.19 .07 P-tau181p/Aβ1-42 Mean±SD 0.16±0.11 0.25±0.12 .02 - –
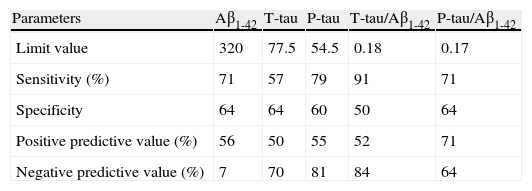
Table 3 shows the results of the ROC curves for each of the variables studied. The sensitivity and NPV values for the cut-off points selected were above 70% in 4 and 3 variables, respectively.
Table 3.Results of the ROC curves for the different parameters used.
Parameters Aβ1-42 T-tau P-tau T-tau/Aβ1-42 P-tau/Aβ1-42 Limit value 320 77.5 54.5 0.18 0.17 Sensitivity (%) 71 57 79 91 71 Specificity 64 64 60 50 64 Positive predictive value (%) 56 50 55 52 71 Negative predictive value (%) 7 70 81 84 64
Our MCI patients who progressed to AD in the first year after LP presented an average Aβ1-42 protein level lower than that of the group of patients who were clinically stable. These results seem useful to identify the MCI patients with a worse prognosis and are in line with some studies published recently.11,12 Landau et al.11 described an increased P-tau181p/Aβ1-42 ratio as a predictor of rapid cognitive decline and, in our experience, we observed this fact in MCI patients with poor prognosis. This ratio has been used by different authors as a reflection of the relationship between the amyloid and tau pathways,11,16 both altered in AD.
Furthermore, we found a decrease in protein Aβ1-42 in this same group of patients. This finding is now considered as the first to appear in AD patients, even before they develop MCI, thus giving greater consistency to our results with respect to those obtained by Blom et al.12 These authors reported an increase in the levels of T-tau and P-tau in patients who progressed to AD rapidly.
In our experience, although there are no statistically significant differences in P-tau181p protein levels between both groups, we observed that they were higher in the p-MCI group. The elevation of this biomarker in CSF is currently considered the most specific finding of AD, both in clinical studies7,9 and in those confirmed by autopsy.17
Analysing the results of the ROC curves, we found high sensitivity and NPV for P-tau and the ratio T-tau/Aβ1-42. The remaining results were more discreet, but we must not forget that the only difference between these patients may be their rate of progression to AD. In any case, these findings emphasise the importance of quantifying all 3 biomarkers, as well as the ratios used.
The new criteria for research in AD suggest that both MRI, PET and biomarkers in CSF may support the diagnosis of AD in amnestic MCI patients.18 However, CSF analysis has some advantages over the other 2 techniques. On the one hand, it is cheaper and easier to obtain than PET. Furthermore, the latter currently studies only the amyloid pathway and its use is still very restricted. On the other hand, changes in CSF reflect AD pathology before volumetry.19 Therefore, this technique seems especially preferable for a very early diagnosis of AD,19 although both methods are complementary and most publications that combine the predictive ability of CSF analysis with other techniques such as MRI agree on this point.20
From a methodological point of view, the use of the Luminex xMAP technique seems to offer some advantages over CSF analysis by ELISA. The simultaneous quantification of the 3 biomarkers improves the technical management and quality control of tests, according to some authors.21 In our case, conducting all quantifications simultaneously, obtaining CSF in a reduced time period and the small age range between the groups significantly decreased confusion factors. The results obtained in the intraclass correlation coefficients for the 3 biomarkers reflect the high reliability of the technique used.
Among the limitations of this study, we note:
- –
Short monitoring time. However, the differences obtained between patients who progressed in the first 12 months after the LP speak to a likely pattern of rapid evolution to AD, as described in recent literature.11,12 This could support the performance of this test not only for diagnosis, but also for prognostic purposes.
- –
Not all patients included in the study had been diagnosed recently. However, we know that these biomarkers, especially protein Aβ1-42, become altered very early in the evolution of AD and do not present significant changes when a control test is performed after a few years.22
- –
This is an invasive test. However, there have been no adverse effects on the population studied. This is consistent with the data published by other authors23 when such tests are performed on patients under good conditions and specific efforts are subsequently avoided for a limited time.
In summary, patients with MCI who have reduced levels of protein Aβ1-42 progress rapidly to dementia, while those with higher levels remain stable for a longer time.
Conflict of interestsThis work was funded by Novartis Laboratories Spain and Grunenthal Spain.
The authors wish to thank the Anaesthesia, Urology and Traumatology Services at Hospital General Universitario in Alicante for their help; as well as María de los Ángeles Miguelsanz, from the Immunology Laboratory, for the excellent management of the samples; and Margarita Ruiz Vegara for her generous collaboration (in memoriam).