The performance of the Low-Profile Visualized Intraluminal Support (LVIS) stent deployed following balloon angioplasty is unknown in treating intracranial atherosclerotic stenosis, and this study was to investigate the safety and efficacy of the LVIS stent in treating intracranial atherosclerotic stenosis in the middle cerebral artery M1 segment.
MethodsThirty-five patients were enrolled with 35 atherosclerotic stenoses at the M1 segment. The stenosis was about 75% in 16 patients, 80% in 15, and 90% in the rest four. The LVIS stent was used to treat these patients.
ResultsThe success rate of stenting was 97.1%. The stenting procedure was failed in one patient because of intraprocedural dissection of the stenotic (75%) segment, resulting in a 30-day periprocedural complication rate of 2.9% (1/35). Before stenting, the stenosis rate ranged 75%–90% (mean 78.9%±4.7%), and after stenting, the diameter of the stented segment was significantly (P<0.0001) increased to 1.5–3.4mm (mean 2.1±0.32mm) ranging 68.2%–100% (mean 94.0%±5.8%) of the normal arterial diameter, with the residual stenosis ranging 0–31.8% (median 4.8%, IQR 2.4%–7.3%). Follow-up was performed at 6–20 months (mean 8.5) after stenting. One patient (2.9%) had occlusion of the stented M1 segment with no symptoms, and two patients (5.7%) had slight asymptomatic instent stenosis (40%) at the M1 segment, with the instent restenosis and occlusion rate of 8.6% (3/35).
ConclusionThe braided LVIS stent can be safely applied for treatment of intracranial atherosclerotic stenosis in the middle cerebral artery with good safety and efficacy immediately after stenting and at follow-up.
Nos propusimos analizar la seguridad y efectividad de la colocación de un stent low-profile visualized intraluminal support (LVIS™) tras angioplastia con balón en pacientes con estenosis ateroscleróticas intracraneales en el segmento M1 de la arteria cerebral media (ACM).
MétodosIncluimos 35 pacientes con estenosis ateroscleróticas en el segmento M1 de la ACM; la estenosis era del 75% en 16 pacientes, del 80% en 15 y del 90% en los 4 restantes. En todos los casos el tratamiento se basó en la colocación de un stent LVIS™.
ResultadosEl stent se implantó con éxito en el 97,1% de los casos; en un paciente, el procedimiento no se pudo llevar a cabo a causa de una disección del segmento estenótico (estenosis del 75%) durante la operación, lo que supone una tasa de complicaciones perioperatorias a los 30 días del 2,9%. Antes de la colocación del stent, el grado de estenosis oscilaba entre el 75 y el 90% (media [DS]: 78,9% [4,7%]). Tras el procedimiento, el diámetro del segmento en el que se había colocado el stent aumentó de forma significativa (P<0,0001) hasta los 1,5-3,4 mm (media: 2,1mm [0,32]), logrando un 68,2-100% (media: 94,0% [5,8%]) del diámetro normal de la arteria, y una estenosis residual del 0 al 31,8% (mediana: 4,8%; p25-p75, 2,4-7,3%). Se realizó un seguimiento de entre 6 y 20 meses (media: 8,5) tras el procedimiento. Un paciente (2,9%) presentó una oclusión asintomática del segmento M1 intervenido, y 2 pacientes (5,7%) presentaron estenosis intrastent asintomáticas leves (40%) en M1, por lo que la tasa de reestenosis intrastent y oclusión fue del 8,6%.
ConclusiónEl stent LVIS™ es un tratamiento seguro y efectivo para las estenosis ateroscleróticas intracraneales en la ACM.
The prevalence of ischemic stroke has elevated steadily in developing countries.1 Among patients with stroke which is the second leading cause of death across the world following ischemic heart diseases, intracranial arterial stenosis caused by atherosclerosis is one of the commonest causes of ischemic stroke, accounting for 30%–50% of ischemic stroke in Asian people.2,3 Currently, in spite of intensive medical treatment, careful control and management of cardiovascular risk factors and lifestyle to prevent repeated ischemic stroke or transient ischemic attack, patients with high-grade (70%–90%) symptomatic intracranial atherosclerotic stenosis are still refractory to aggressive medication, and the rate of one year stroke or death in patients with over 70% symptomatic intracranial stenosis was 12.6% in the medication arm in the SAMMPRIS trial comparing stenting and aggressive management for prevention of recurrent stroke in intracranial stenosis.4 Consequently, endovascular treatment with balloon angioplasty and deployment of stents is considered an alternative choice for treatment of high-grade intracranial atherosclerotic stenosis, with increasing evidence demonstrating encouraging outcomes for endovascular management of this kind of stenosis.5–10 Because arterial dissection and/or elastic recoiling is a frequent sequel after balloon dilatation of high-grade stenoses, a stent is required to stabilize the concerning vessel segment after balloon angioplasty, and deployment of an intracranial stent has been applied widely as a primary therapeutic choice for treating severe intracranial atherosclerotic stenosis which is refractory to medications.11 The Wingspan stent (Stryker Neurovascular, Fremont, CA, USA) has been initially applied for treating intracranial atherosclerotic stenosis, but this stent has some issues related to the excessive radial force and the delivery system.5,7,10 Other stents including the Enterprise stent (Codman Neurovascular, Raynham, MA, USA) have also been used in this aspect,12,13 and the use of the Enterprise stent in treating intracranial atherosclerotic stenosis is an off-labeling application. Drug-eluting stents have also been used for treating patients with symptomatic intracranial atherosclerotic diseases, and current evidence suggests that the deployment of a drug-eluting stent intracranially is a relatively safe and effective method in comparison with non-drug-eluting stents or medical management of intracranial atherosclerotic diseases.14 The functional and physical properties of self-expanding intracranial stents including the Wingspan, Enterprise, Neuroform (Smart Therapeutics, Boston Scientific, Boston, MA, USA), Leo (Balt) and Solitaire (ev3) stents have been compared in vitro and in comparison with the clinical experience of the authors, and it has been found that these stents are fundamentally different and that no stent is superior in all tested aspects including the radial force, wall apposition, bending stiffness, kink resistance, vessel wall coverage, and ease of delivery.15 None of these stents are ideal in the sense of universally meeting all possible requirements, and knowledge of the functional and physical features of these stents are mandatory for safe and successful endovascular treatment. Stent deployment has actually effectively increased the indications of endovascular embolization for cerebral aneurysms, and low-profile devices have enabled endovascular access to distal lesions in small cerebral arteries measuring <2.5mm in diameter.16–18 The Low-Profile Visualized Intraluminal Support (LVIS, MicroVention, Tustin, CA, USA) device is a new neurovascular self-expandable retrievable closed-cell stent system initially designed for stent assistance in coil embolization of intracranial aneurysms with a wide neck. This braided stent provides a choice for aneurysm embolization between conventional stents and flow diverters and has been increasingly used for treating intracranial aneurysms19–22 not only in larger parent arteries but also in smaller arteries with a diameter ranging 1.7–2.4mm.23 We had used the LVIS stent in treating a middle cerebral (MCA) aneurysm concurrent with stenotic parent artery, and after balloon angioplasty of the stenosis, a LVIS stent was deployed for both assistance of coil embolization of the aneurysm and sustaining the lumen of the stenotic parent artery. The LVIS stent displayed good radial support and excellent apposition to the vascular wall at the stenotic segment. This case promoted us to apply more LVIS stents for the treatment of intracranial atherosclerotic stenosis especially at the M1 segment of MCA. We hypothesized that the LVIS stent could be deployed in smaller intracranial arteries with a diameter less than 2.5mm for treating intracranial atherosclerotic stenosis. This study was consequently performed to investigate the safety and effectiveness of the LVIS stent in treating intracranial atherosclerotic stenosis in arteries less than 2.5mm in diameter.
Materials and methodsThis study was approved by the ethics committee of our hospital with all patients having provided the signed informed consent for study participation. In the prospectively maintained database, patients with intracranial atherosclerotic stenoses treated with deployment of the LVIS stent between September 2016 and March 2019 were retrieved and enrolled. The inclusion criteria were symptomatic intracranial arterial stenosis, arterial stenosis>70% confirmed by angiography, recurrent medically refractory transient ischemic attack, recurrent infarction even under medication over one year, and no contraindication for endovascular stent deployment.
In this study, patients treated with deployment of a LVIS stent were administered aspirin (100mg/d) and clopidogrel (75mg/d) three days before the procedure. The procedure was conducted with general anesthesia. Heparinization was maintained with the activated clotting time of 250–300s. After 3-dimensional angiography revealed the anatomy of the stenotic artery and surrounding structures, the distal normal arterial diameter was measured on the 3-dimensional angiography, and a balloon catheter with the diameter 0.1–0.2mm less than the normal arterial diameter was chosen for balloon angioplasty before stent deployment. After dilatation of the stenotic segment using the balloon catheter at 6atm, a microcatheter was navigated to the stenotic location for deployment of the LVIS stent. The Gateway (Stryker Neurovascular, Kalamazoo, MI, USA) or the Maverick (Boston Scientific, Boston, MA, USA) balloon catheter was normally used, and after the microcatheter was in place, the microcatheter was exchanged with the balloon catheter using an exchange microguidewire. The stent was chosen as oversized so as to have enough support. After stenting, clopidogrel (75mg/day) was administered in all patients 3 months before long-term use of aspirin (100mg/day). The thromboelastogram was routinely checked before operation, and if the patient did not respond to the clopidogrel, Ticagrelor was used instead with 90mg twice daily. At follow-up, cerebral angiography was performed for monitoring the status of the stented artery. The follow-up was performed one month following stenting at the outpatient department for clinical symptoms and signs, digital subtraction angiography at 6 months and two years, and computed tomography angiography at 12 months.
Statistical analysisStatistical analysis was performed with the SPSS 18.0 software (IBM, Chicago, IL, USA). Measurement data were presented as mean±standard deviation. A paired t-test was conducted for comparing the diameter of the parent arteries before and after stenting. The Chi-square or Fisher exact test was applied for nominal variables. Univariate analysis was performed to determine the association of the size increments of parent arteries on follow-up angiography with other factors. A P value of <0.05 was set as statistically significant.
ResultsThirty-five patients were enrolled with 35 arterial stenoses at the M1 segment of MCA including 18 males and 17 females with an age range of 27–72 years (mean 58.0±9.4) (Table 1). There were 15 patients with diabetes mellitus, 21 patients with hypertension, 24 with hyperlipidemia, 18 with smoking history, 15 with alcoholic abuse, and 15 with overweight. The stenosis was about 75% in 16 patients, 80% in 15 and 90% in the rest four, with 19 stenoses on the right and 16 on the left side. All patients except one underwent successful deployment of the LVIS stent with the success rate of 97.1% (34/35). Thirty-four LVIS stents (3.5mm×15mm) were deployed in 34 patients (Figs. 1–4). The stenting procedure was failed in one patient because of intraprocedural dissection of the stenotic (75%) segment of artery which was caused by balloon angioplasty, resulting in hemorrhage and a 30-day periprocedural complication rate of 2.9% (1/35). The neurological symptoms were not aggravated after the procedure in this patient.
Patients with intracranial stenosis treated with VLIS stents.
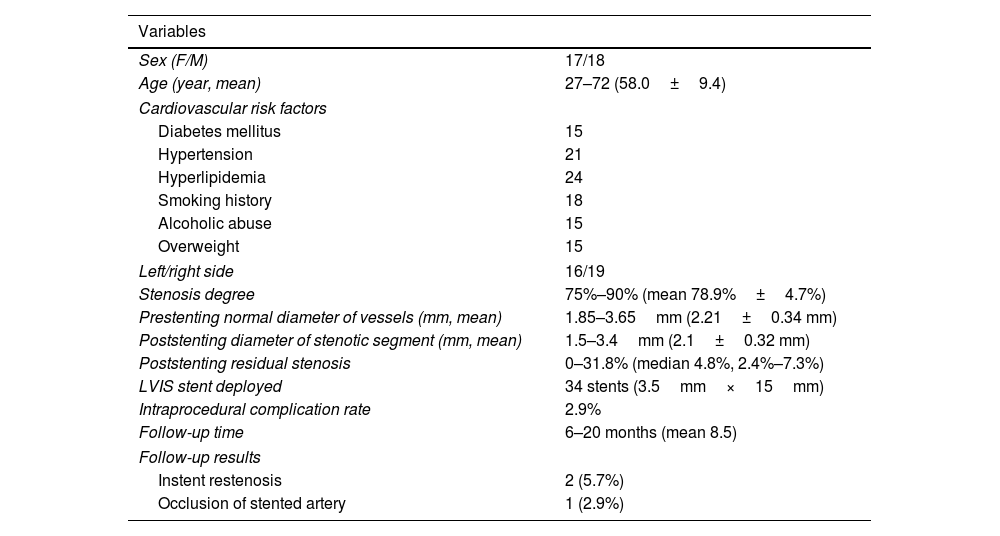
| Variables | |
|---|---|
| Sex (F/M) | 17/18 |
| Age (year, mean) | 27–72 (58.0±9.4) |
| Cardiovascular risk factors | |
| Diabetes mellitus | 15 |
| Hypertension | 21 |
| Hyperlipidemia | 24 |
| Smoking history | 18 |
| Alcoholic abuse | 15 |
| Overweight | 15 |
| Left/right side | 16/19 |
| Stenosis degree | 75%–90% (mean 78.9%±4.7%) |
| Prestenting normal diameter of vessels (mm, mean) | 1.85–3.65mm (2.21±0.34 mm) |
| Poststenting diameter of stenotic segment (mm, mean) | 1.5–3.4mm (2.1±0.32 mm) |
| Poststenting residual stenosis | 0–31.8% (median 4.8%, 2.4%–7.3%) |
| LVIS stent deployed | 34 stents (3.5mm×15mm) |
| Intraprocedural complication rate | 2.9% |
| Follow-up time | 6–20 months (mean 8.5) |
| Follow-up results | |
| Instent restenosis | 2 (5.7%) |
| Occlusion of stented artery | 1 (2.9%) |
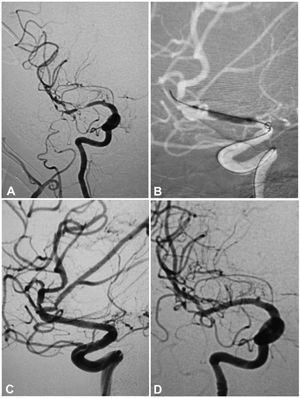
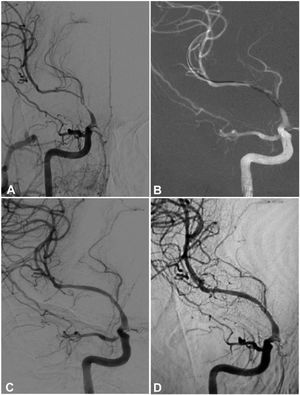
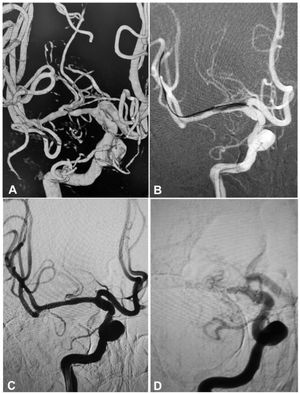
A 53-year-old woman had an atherosclerotic stenosis (78%) at the M1 segment of the right middle cerebral artery (MCA) treated with the angioplasty and stenting of the Low-Profile Visualized Intraluminal Support (LVIS) stent. (A) The stenosis was shown at the M1 segment. (B) A balloon was used to dilate the stenosis before stenting. (C) At the end of the stenting with a LVIS stent (3.5mm×15mm), the stenotic segment was restored to the normal diameter. (D) At 12-month follow-up, the stented segment of artery remained totally open with no instent stenosis.
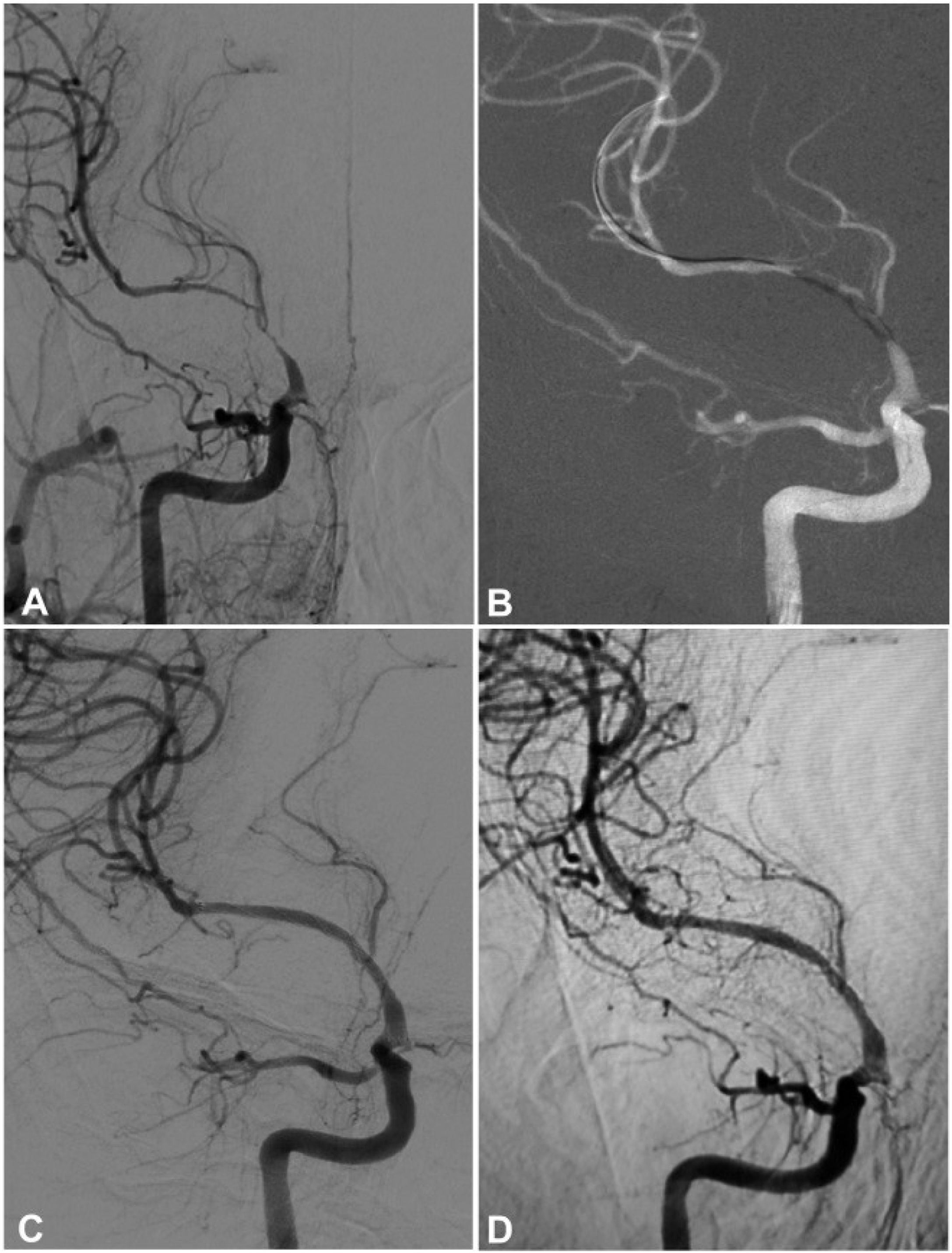
A 64-year-old man had an atherosclerotic stenosis (75%) at the M1 segment of the right middle cerebral artery treated with angioplasty and stenting of the Low-Profile Visualized Intraluminal Support (LVIS) stent. (A) The stenosis was shown at the M1 segment. (B) A balloon was used to dilate the stenosis before stenting. (C) At the end of the stenting with a LVIS stent (3.5mm×15mm), the stenotic segment was almost restored to the normal diameter. (D) At 6-month follow-up, the stented segment of artery remained totally open with slight instent stenosis.
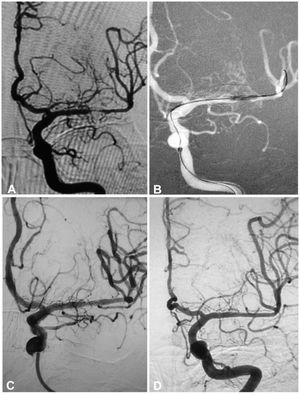
A 55-year-old woman had an atherosclerotic stenosis (83%) at the M1 segment of the left middle cerebral artery treated with angioplasty and stenting of the Low-Profile Visualized Intraluminal Support (LVIS) stent. (A) The stenosis was shown at the M1 segment. (B) A balloon was used to dilate the stenosis before stenting. (C) At the end of the stenting with a LVIS stent (3.5mm×15mm) deployed, the stenotic segment was almost restored to the normal diameter. (D) At 12-month follow-up, the stented segment of artery remained patent.
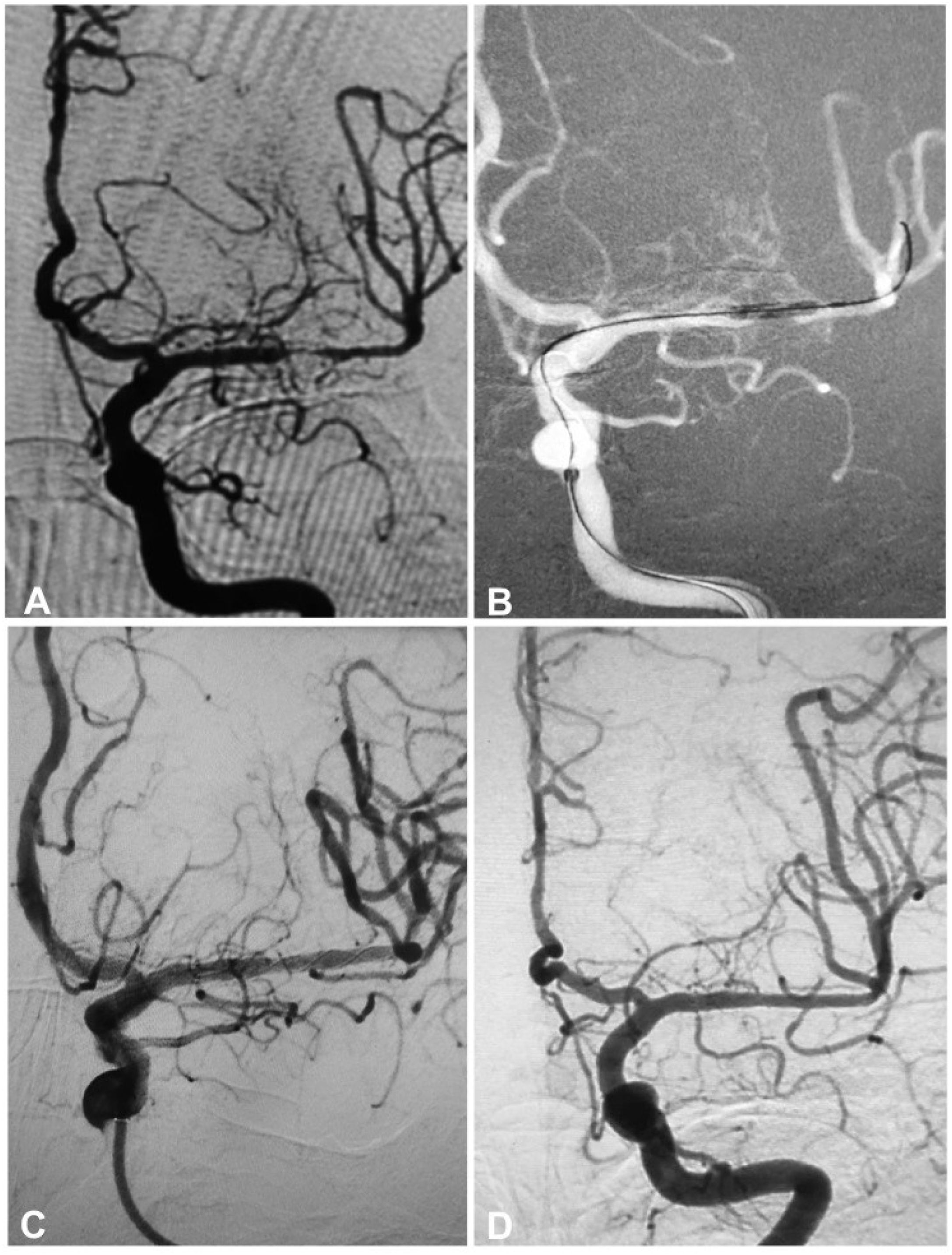
A 54-year-old man had an atherosclerotic stenosis (88%) at the M1 segment of the right middle cerebral artery treated with angioplasty and stenting of the Low-Profile Visualized Intraluminal Support (LVIS) stent. (A) The stenosis was shown at the M1 segment. (B) A balloon was used to dilate the stenosis before stenting. (C) At the end of the stenting with a LVIS stent (3.5mm×15mm), the stenotic segment was almost restored to the normal diameter. (D) At 6-month follow-up, the stented segment of artery was totally occluded with no symptoms.
In one patient, intractable gastrointestinal bleeding took place one month after discharge because of administration of dual antiplatelet medication but without causing severe consequences. No other complications or sequela took place. Balloon angioplasty was performed twice in seven patients, accounting for 20.6% (7/34) of all patients, and once in the other 27 (27/34 or 79.4%) prior to stent deployment. Before stenting, the stenosis rate ranged 75%–90% (mean 78.9%±4.7%), and after stenting, the diameter of the stented segment was significantly (P<0.0001) increased to 1.5–3.4mm (mean 2.1±0.32mm) ranging 68.2%–100% (mean 94.0%±5.8%) of the normal arterial diameter near the original stenosis, with the residual stenosis ranging 0–31.8% (median 4.8%, IQR 2.4%–7.3%). Follow-up was performed at 6–20 months (mean 8.5) after stenting, with angiographic follow-up in all cases. Two patients (5.7%) experienced slight asymptomatic instent stenosis (20%) at the M1 segment (Fig. 2), and one patient (2.9%) had occlusion of the stented M1 segment with no symptoms (Fig. 4). The patient who had intraprocedural hemorrhage caused by balloon angioplasty had the same stenotic degree (75%) at the M1 segment. No further aggravation of stenosis was found in the other patients, and the instent restenosis and occlusion rate was 8.6% (3/35). No dizziness, headache, disturbance of language, transient ischemic attack, ischemic stroke or other neurological symptoms were detected in the patients.
DiscussionBy applying the LVIS stent for the treatment of refractory symptomatic severe intracranial atherosclerotic stenosis, our study demonstrated excellent effects and safety with the success rate of stent deployment of 97.1%, periprocedural complication rate of 2.9%, median residual stenosis of 4.8% following stenting, a non-symptomatic instent restenosis rate of (5.7%), and a stent occlusion rate of 2.9% at follow-up.
The LVIS stent possesses some technical advantages over existing intracranial stents, like the Neuroform (Stryker, Fremont, CA, USA) and Enterprise stents. The LVIS stent has an inner diameter of stent deploying microcatheter measuring 0.017 inches and the smallest available stent delivery system which is also the most commonly used microcatheter for coil delivery. Three radiopaque markers are designed along the whole length of the LVIS stent with three additional radiopaque markers at the proximal and distal ends, allowing operators to visualize its expansion during maneuver. Other stents have markers only at the proximal and distal ends.24 During deployment, the LVIS stent could be visualized in the whole process and thus helpful in the stenting procedure. The LVIS stent has a greater metal coverage but smaller meshes, which, in theory, can better cover and fix smaller fragile plaques and is beneficial for endothelial cells to crawl and cover the foreign body of metal struts. The LVIS stent has an average metal coverage of 11.5% with the pore densities of 0.782pores/mm2 while the average metal coverage is only 5.0% for the Enterprise stent and 10.0% for the Neuroform stent.25 The pore density is 0.276pores/mm2 for both the Neurform and Enterprise stent. A greater pore density is better for fixing small plaques and for endothelial cell covering and growth. The LVIS stent has the highest perpendicular radial force (37.1gf) followed by the LEO stent (34.2gf, Balt, Montmorency, France), the Enterprise stent (15.2gf), and the Neuroform stent (11.4gf).25 The radial force is the force to support the stent expansion against the vessel wall.25 Because the radial force indicates the capacity of the stent to support the expanded artery or coils inside an aneurysm, a high radial force is very important to maintain the arterial lumen after expansion. Moreover, the radial force of the LVIS stent can be adjusted by regulating the size of the pores at maneuver.25 However, too large a radial force may damage the arterial intima and cause in-stent stenoses.
The LVIS stent can be retrieved prior to full deployment, which is an incomparable advantage over the Neuroform and Wingspan stents which are not retrievable because of the open-cell design. However, because of the higher metal coverage of the LVIS stent, the arrangement of the filaments of the braided LVIS stent can be changed to adapt to the altering curvature of the parent vessel and consequently increase the frequency of occlusion of branch vessels originating from the stented segment of parent artery compared with the Neuroform and Enterprise stents which have a smaller metal coverage area.
Li et al.7 had studied Wingspan stenting for severe symptomatic intracranial atherosclerotic stenosis in 433 patients treated at a single medical center with a large volume. In their study, the success rate of intracranial deployment of the Wingspan stent was 99.1% in 429 patients, the mean stenosis rate was relieved from prestenting (82.3±7.6)% to poststenting (16.6±6.6)%, the 30-day periprocedural complication rate was 6.7% (in 29 patients), and poststenting follow-up at 6–69months revealed a ipsilateral stroke rate of 5.5% (20 patients). Xu et al.26 investigated Neuroform stenting for symptomatic intracranial atherosclerotic stenosis in 71 consecutive patients and found that the technical success rate was 100%, the arterial stenosis was improved from prestent 84.2%±9.1% (median 85%, IQR 75%–90%) to poststent 16.9%±10.2% (median 15%, IQR 10%–25%), and the frequency of ipsilateral stroke, intra-cerebral hemorrhage, or death within 30 days was 0%. Miao et al.27 investigate tailored angioplasty and/or stenting for symptomatic intracranial atherosclerotic stenosis and found a technical success rate of 97.5% to 100% for the stenting group and 30-day complication rate of 4.4% with only three patients having stroke in the stenting group. Want et al.28 investigated application of the Enterprise stent in atherosclerotic intracranial arterial stenosis in a series of 60 cases. In their study, the technical success rate of stenting was 100%, the arterial stenosis was improved from prestent 76.3±12.7% to poststent 22.8±4.8%, the perioperative complication rate was 8.3% (in five patients) with no mortality within 30 days, and 13.3% (six) patients had postoperative restenosis at 6.2 months follow-up with five (8.3%) ischemic events. Salik et al.29 studied the medium-term results of undersized angioplasty and stenting for symptomatic high-grade intracranial atherosclerotic stenosis with Enterprise. In their study, the technical success rate was 99%, the degree of pre-procedural stenosis was 92±6% and dropped to 12±10% after stent deployment, one (1.5%) patient had intracranial hemorrhage, and instent restenosis occurred in two patients at 22-month follow-up with no recurrent stroke or ischemic attack. Ma et al.8 studied one-year outcome of stenting in 300 patients for symptomatic intracranial atherosclerotic stenosis and found that the probability of primary outcome (stroke, transient ischemic attack or death) at 1 year was 8.1% (95% CI 5.3%–11.7%). In 76 patients with angiographic follow-up, 27.6% (21/76) had re-stenosis ≥50% and 18.4% (14/76) had re-stenosis ≥70%. No baseline characteristic was associated with the primary outcome.8 Compared with the above studies, our study applying the LVIS stent in the treatment of refractory symptomatic severe intracranial atherosclerotic stenosis had comparable and even better outcomes immediately after the stenting procedure and at follow-up.
Instent restenosis is a critical parameter in evaluating the effect of angioplasty and stenting and recurrent stroke after stenting.9,30 The restenosis was primarily resulted from inflammatory response of the vascular wall to stenting with resultant excessive intimal hyperplasia. Among risk factors for vascular events, lesion location was an increasingly important risk for restenosis, and anterior circulation lesions were more prone to instent restenosis,31 which was confirmed by another study that further revealed that instent restenosis took place more frequently in younger (less than 55 years) patients with the instent restenosis primarily locating in the anterior circulation.32 The authors in this study continued to hypothesize that the symptomatic atherosclerotic stenosis affecting the supraclinoid internal carotid artery segment in younger patients might basically be different from stenoses in other locations and in older patients and might stand for more of an inflammatory arteriopathy of “Moyamoya” type occurring in younger patients than a primarily atherosclerotic process.32 In our study, we investigated only atherosclerotic lesions treated in the M1 segment of the MCA without lesions in the posterior circulation. At follow-up of a mean 8.5 months, one patient (2.9%) had occlusion of the stented M1 segment with no symptoms, and two patients (5.7%) had slight instent stenosis (40%) at the M1 segment with no symptoms, ether.
Because intraprocedural complications most likely occur in the stage of balloon angioplasty before deployment of the LVIS stent, the selection of the expanding balloon is very important, with the diameter of the balloon equal to or slight greater than that of the normal artery distal to the stenosis and the length of the balloon being able to cover the whole stenotic segment. If the balloon is much bigger than the normal diameter of the artery distal to the stenotic segment, dissection of the artery may be resulted from balloon angioplasty leading to intraprocedural hemorrhage. For a long segment of stenosis, the expansion of the stenosis should be performed in different subsections starting from the proximal to the distal section. Eccentric plaques should be expanded only once, and for concentric hard plaques, a bigger balloon should be used for expansion twice before stent deployment.
Some limitations may exist in this study including single center study, retrospective nature, no randomization or control and only Chinese ethnicity. The interpretation of the results of the study should be consequently cautious. Further prospective studies in the future are needed to resolve these issues for better outcomes.
ConclusionThe braided LVIS stent can be safely applied for the treatment of intracranial atherosclerotic stenosis in the M1 segment of the middle cerebral artery with good safety and efficacy immediately after stenting and at follow-up. However, randomized controlled clinical trials with double-blinded design and involvement of multiple medical centers and ethnicities should be performed in the future to confirm the safety and clinical effect of the LVIS stent.
FundingNone declared.
Author contributionData collection: Xin-Yu Li, Ji-Wei Wang, Jian-Feng Liu, Hui Li.
Study design: Cong-Hui Li, Bu-Lang Gao.
Data analysis: Xin-Yu Li, Cong-Hui Li, Bu-Lang Gao.
Supervision: Yang-Yang Tian.
Writing of the article: Bu-Lang Gao.
Conflict of interestWe declare that we have no conflict of interest.
None.