The analysis of the core biomarkers of Alzheimer’s Disease (AD) in the cerebrospinal fluid (CSF) is recommended in the clinical units where it is available. Because of the absence of universal validated values, the determination of specific cut-off points for each center and its population is recommended. The main objective of the CORCOBIA study was to determine the cut-off points of core AD CSF biomarkers for several centers (Parc de Salut Mar, Barcelona and Hospital General de Granollers), which work with the same reference laboratory (Laboratori de Referència de Catalunya).
MethodsProspective study including cognitively unimpaired individuals (CU, n = 42), subjects with amnestic mild cognitive impairment (aMCI, n = 35) and patients with dementia due to Alzheimer’s Disease (AD, n = 48), in whom clinical and neuropsychological assessment, neuroimaging, APOE genotyping and lumbar puncture to analyse amyloid beta peptides (Aβ42, Aβ40), total tau (tTau) and phosphorylated Tau (pTau181) using the Lumipulse G600II (Fujirebio) was performed. The values of sensitivity (SE), specificity (SP), predictive values and area under the curve (AUC) were calculated, determining the cut-off point according to the Youden index by comparing the CU and AD groups.
ResultsThe resulting cut-offs and their AUC were the following: Aβ42 750 pg/mL (AUC 0.809); Aβ42/Aβ40 0.062 (AUC 0.78); pTau181 69.85 pg/mL (AUC 0.81); tTau 522.0 pg/mL (AUC 0.79); Aβ42/tTau 1.76 (AUC 0.86); Aβ42/pTau181 10.25 (AUC 0.86).
ConclusionsThe determination of cut-off points of core AD CSF biomarkers for the participating centers allows a better diagnostic accuracy. The ratio CSF Aβ42/pTau181 shows the highest AUC and better balance between sensitivity and specificity.
El análisis de biomarcadores bioquímicos de Enfermedad de Alzheimer (EA) en líquido cefalorraquídeo (LCR) se recomienda como parte del diagnóstico en los centros en los que está disponible. Ante la ausencia de valores universales validados, se recomienda la determinación de puntos de corte (PdC) específicos para cada centro y su población. El objetivo principal del proyecto CORCOBIA fue determinar los PdC de biomarcadores de EA en LCR para varios centros (Parc de Salut Mar de Barcelona y Hospital General de Granollers), que trabajan con el mismo laboratorio de referencia (Laboratori de Referència de Catalunya).
MétodosEstudio prospectivo incluyendo sujetos cognitivamente sanos (CS, n = 42), con deterioro cognitivo ligero amnésico (DCLa, n = 35) y con demencia tipo Alzheimer (DTA, n = 48), realizando valoración clínica y neuropsicológica, neuroimagen, genotipado APOE y punción lumbar para analizar los péptidos beta amiloides (Aβ42, Aβ40) y las proteínas tau total (tTau) y tau fosforilada (pTau181) mediante el autoanalizador Lumipulse G600II (Fujirebio). Se calcularon los valores de Sensibilidad (S), Especificidad (E), valores predictivos y área bajo la curva (ABC), determinando el punto de corte según índice de Youden comparando los grupos de CS y DTA.
ResultadosLos PdC y ABC fueron los siguientes: Aβ42 750 pg/mL (ABC 0.809); Aβ42/Aβ40 0.062 (ABC 0.78); pTau181 69.85 pg/mL (ABC 0.81); tTau 522.0 pg/mL (ABC 0.79); Aβ42/tTau 1.76 (ABC 0.86); Aβ42/pTau181 10.25 (ABC 0.86).
ConclusionesLa determinación de PdC para biomarcadores de EA en LCR para los centros participantes permite una mejor precisión diagnóstica, siendo la ratio Aβ42/pTau181 en LCR el parámetro con mayor ABC y mejor balance entre sensibilidad y especificidad.
Determining biochemical core biomarkers of Alzheimer’s disease (AD) in cerebrospinal fluid (CSF) leads to more accurate diagnosis of the disease.1 It is recommended in the current diagnostic guidelines for centres with the necessary resources.2,3 The determination of CSF levels of Aβ42, Aβ42/Aβ40, total Tau protein (tTau), and Tau phosphorylated at threonine 181- (pTau181) was included in the revised National Institute on Aging and Alzheimer’s Association (NIA-AA) diagnostic criteria for AD in 2011 and subsequently in the clinical practice guidelines in our healthcare system.2–5 In the research setting, a system has been proposed for the classification of the results of these biomarkers to facilitate their interpretation (A/T/N system, NIA-AA 2018).6,7 Over the past decade, the determination of these biomarkers has progressively been incorporated into the diagnosis of cognitive impairment, especially in specialissed units. The commercialisation of standardised autoanalysers, methods, and reactants in recent years has favoured their implementation in clinical laboratories. However, given the lack of universal values due to different population-related and methodological factors (preanalytical and analytical factors), determination of specific cut-off points (COP) for each centre and population is still recommended.2
In this context, the main aim of the CORCOBIA study (correlation of cognitive tools with biomarkers of Alzheimer’s disease) was to determine COPs for the main CSF biomarkers of AD for the population from the participating centres (Hospital del Mar and Centre Emili Mira at Parc de Salut Mar, and Hospital General de Granollers), which work with the same reference laboratory (Laboratori de Referència de Catalunya). We conducted a systematisation of the preanalytical and analytical phases and analysed the correlation between standardised cognitive tools (Neuronorma battery)8 and CSF biomarkers. This study describes the results from the determination of COPs for the biomarkers studied.
MethodsThe CORCOBIA study was approved by the ethics committee of the Institut Hospital del Mar d’Investigacions Mèdiques (project code 2014/5638) and complies with the principles of the Declaration of Helsinki on medical research (2013). The study was conducted jointly between Hospital del Mar (Parc de Salut Mar, Barcelona) and the Laboratori de Referència de Catalunya, together with 2 collaborating healthcare centres: Centre Emili Mira (Parc de Salut Mar, Santa Coloma de Gramenet) and Hospital General de Granollers.
ParticipantsWe included patients assessed at the different participating centres with the following diagnoses (established according to the 2011 NIA-AA criteria): cognitively unimpaired (CU), amnestic mild cognitive impairment (aMCI),3 and mild AD dementia.4,9 All participants signed an informed consent form that had previously been approved by the ethics committee; in patients with mild AD dementia, the informed consent was signed both by the participant and the family member responsible for them.
The selection criteria for all participants were as follows: Inclusion criteria: a) age between 60 and 85 years; b) sufficient vision, hearing, and physical conditions to perform the evaluations; c) minimum level of schooling to undergo neuropsychological evaluation; d) stable medical condition and pharmacological treatment in the 3 months before the start of the study. Exclusion criteria: a) lack of consent or inability of the patient to adequately collaborate in the study; b) any central nervous system disease other than that under study that may affect cognition; c) psychiatric condition that, in the opinion of the researcher, affects the cognitive capacities of the participant (for example, major depressive episode, bipolar disorder, decompensated dysthymia, schizophrenia, psychotic episode (according to the DSM-V criteria); d) hypothyroidism and/or vitamin deficits (B12, folic acid) or any other medical condition that, in the opinion of the researcher, may interfere with cognitive function; e) any medical condition that constitutes a contraindication for lumbar puncture (eg, active treatment with anticoagulants; haematological disorder affecting coagulation, such as significant thrombocytopaenia or coagulation disorders; acute liver failure; lumbar spine disorders for which lumbar puncture entails high risk); f) alcohol abuse or consumption of substances that, in the opinion of the researcher, may interfere in the cognitive assessment; g) presence of severe hypoacusis, significant amblyopia, or blindness; and h) presence of any situation that may, in the opinion of the researcher, result in unsuitability for the study.
Participants were assigned to different groups according to the following criteria: I) AD dementia: patients with moderate cognitive impairment according to the Global Deterioration Scale (GDS),10 who also met diagnostic criteria for probable AD dementia according to the 2011 NIA-AA criteria4; II) aMCI: patients with mild cognitive impairment according to the GDS, with a primary amnestic profile3;III)CU: subjects with normal results in a neuropsychological study after adjusting for age and level of schooling.
ProceduresPatients with clinical diagnosis of aMCI and mild AD dementia were assessed at the neurology consultations of the 3 collaborating centres. The sample of CU participants included patients’ companions (42.9%) and subjects with subjective cognitive complaints with normal cognitive performance for their age and level of schooling in the neuropsychological assessment (57.1%).
Participants were asked to attend an information session, sign an informed consent form, and undergo an extensive standardised neuropsychological assessment (Neuronorma battery),8 a magnetic resonance imaging (MRI) study (in individuals with evidence of cognitive impairment), APOE genotyping, and a lumbar puncture (LP).
Lumbar puncture and CSF analysisLP (L3-L4 or L4-L5 intervertebral spaces) was performed between 08:00 and 10:00, with patients in fasting conditions, using a 22 G needle (diameter of 0.7 mm). The preanalytical procedure was performed according to the recommendations published by Vanderstichele et al.,11 as follows:a) CSF was stored in a 10 mL inert polypropylene tube (Sarstedt Ref. 62610201) for delivery to the laboratory; b) CSF was centrifuged at room temperature (2000 g for 10 min); c)supernatant was aliquoted in 500 µL inert polypropylene microtubes (Starstedt Ref. 72.730.006); d)aliquots were immediately transferred with dry ice to the reference laboratory; and e) aliquots were frozen at –80 °C. The maximum time permitted between the LP and freezing, first with dry ice and later in freezers at –80 °C, was 4 and 12 hours, respectively.
Participants were recruited between November 2014 and November 2019. Biomarker determination was conducted between May 2018 and December 2019, according to the following procedure: a) thawing of CSF aliquots for 30 min at room temperature; b) homogenisation of each aliquot in a shaker for 10 s; c) quantification in a Lumipulse G600II analyser (Fujirebio, Belgium) to measure the biomarkers Aβ42, Aβ40, tTau, and pTau181 (pg/mL) using a chemiluminescence enzyme immunoassay (CLEIA) with in vitro diagnostic reagents (Fujirebio).
APOE genotypingThe molecular study of the APOE genotype was conducted by analysing samples of genomic DNA by real-time PCR allelic discrimination assay with a RealType kit of the APOE gene (Progenie Molecular), identifying the presence of the rs7412 (g8041C > T) or rs429358 (g7903 T > C) polymorphisms, which define the APOE diplotype for the ɛ2, ɛ3, and ɛ4 alleles, which in turn code for the APOE-ɛ2, APOE-ɛ3, and APOE-ɛ4 isoforms, respectively.
Statistical analysisCut-off points for AD CSF biomarkersWe obtained COPs by using ROC curves to analyse the ability to classify participants as CU or patients with AD dementia; we chose the COP with the highest Youden index. We performed a descriptive analysis of the demographic characteristics of these 2 groups. The Shapiro-Wilk test was used to test for normality and means were compared using the t test (for parametric data) or the Mann-Whitney U test (for non-parametric data), as appropriate. We used the chi-square test to compare the frequencies of categorical variables. P-values < .05 were considered statistically significant. To determine COPs for each of the main AD CSF biomarkers (Aβ42, tTau, and pTau181) and biomarker ratios (Aβ42/Aβ40, Aβ42/tTau, and Aβ42/pTau181), we obtained the ROC curves, the areas under the ROC curves (AUC), confidence intervals (95% CI), and the sensitivity (SE), specificity (SP), positive predictive value (PPV), and negative predictive value (NPV) associated with each COP. We selected the COP with the highest Youden index (Youden index: SE + SP – 1).
Classification of patients with aMCI according to the A/T/N systemThe COPs calculated were applied to the patients with a clinical diagnosis of aMCI, using the NIA-AA’s amyloid/Tau/neurodegeneration (A/T/N) classification (2018) to classify them into the following categories: A–T–N– (normal AD biomarkers); A+T–N– (AD continuum: Alzheimer-type pathological change); A+T+N± (AD continuum: AD); A+T–N+ (AD continuum: concomitant suspected non-Alzheimer’s pathologic change); A–T+N+, A–T+N–, A–T–N+ (non-AD pathologic change).7 These variants were determined according to the COP of the biomarkers included in this study. For instance, patients were classified as A+ when the Aβ42 and/or Aβ42/Aβ40 value was below the COP, and as T+ and N+ when pTau181 and tTau were above the COP, respectively. We performed a descriptive analysis of the sociodemographic data for each group of subjects included in each A/T/N variant, with numerical variables (age and years of schooling) expressed as means and standard deviations (SD) of the numerical variables, and the dichotomous categorical variable (sex) expressed as a percentage.
ResultsWe included a total of 125 participants assessed at the different sites (105 at Hospital del Mar, 15 at Hospital General de Granollers, and 5 at Centre Emili Mira), with the following diagnoses: 42 CU, 35 aMCI, and 48 mild AD dementia.
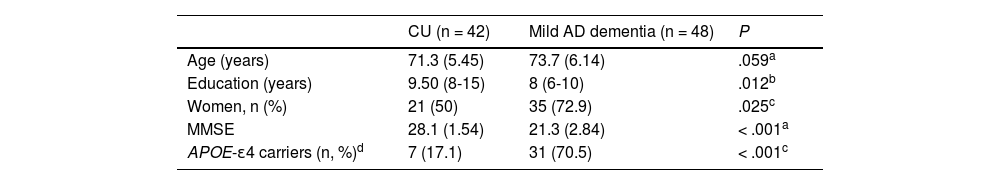
Cut-off points for AD CSF biomarkersThe sample used to calculate COPs included 90 participants (42 CU and 48 with mild AD dementia). Mean age (SD) was 72.5 (5.9) years, and 62.2% were women. As shown in Table 1, patients with AD dementia were slightly older than CU participants, although this difference was not statistically significant (71.3 vs 73.7; P = .059). We did observe statistically significant differences in the level of schooling and sex, with fewer years of schooling and a higher percentage of women in the group of patients with mild AD dementia than in the CU group (8 vs 9.5, and 72.9% vs 50%, respectively; P < .05). Furthermore, we observed significantly better performance in the Mini–Mental State Examination (MMSE)12 (28.1 vs 21.3; P < .001) and a lower percentage of APOE-ɛ4 carriers in the CU group than in the AD dementia group (17.1% vs 70.5%; P < .001).
Characteristics of the sample for the calculation of the cut-off points (n = 90).
| CU (n = 42) | Mild AD dementia (n = 48) | P | |
|---|---|---|---|
| Age (years) | 71.3 (5.45) | 73.7 (6.14) | .059a |
| Education (years) | 9.50 (8-15) | 8 (6-10) | .012b |
| Women, n (%) | 21 (50) | 35 (72.9) | .025c |
| MMSE | 28.1 (1.54) | 21.3 (2.84) | < .001a |
| APOE-ɛ4 carriers (n, %)d | 7 (17.1) | 31 (70.5) | < .001c |
Age and MMSE score are expressed as means (standard deviation) and the level of schooling as the median (p25-p75).
APOE-ɛ4: APOE-ɛ4 carriers (in homo- or heterozygosis); CU: cognitively unimpaired; MMSE: Mini–Mental State Examination.
The median time between sample collection and analysis was 5.5 months (range: 0-17.3 months). Of all samples, 61.1% were jointly analysed in May 2018, and monthly series were subsequently performed until the remaining 38.9% were completed.
Table 2 includes the descriptive data from the CSF measurements of biomarkers (Aβ42, Aβ42/Aβ40, tTau, pTau181, Aβ42/tTau, and Aβ42/pTau181) and the results of the comparison between the CU group and the AD dementia group; all biomarkers showed statistically significant differences (P < .001).
CSF measurement (n = 90).
| CU (n = 42) | Mild AD dementia (n = 48) | Pa | |
|---|---|---|---|
| Aβ42 (pg/mL) | 1061 (679.0-1526.7) | 611.5 (511.5-716.5) | < .001 |
| Aβ42/Aβ40 | 0.083 (0.051-0.109) | 0.044 (0.038-0.055) | < .001 |
| tTau (pg/mL) | 391.0 (283.2-516.0) | 715.5 (468.7-979.0) | < .001 |
| pTau181 (pg/mL) | 52.7 (41.1-71.8) | 120.0 (79.0-170.9) | < .001 |
| Aβ42/tTau | 2.86 (1.55-5.09) | 0.81 (0.54-1.37) | < .001 |
| Aβ42/pTau181 | 24.7 (10.9-38.0) | 4.76 (3.12-9.04) | < .001 |
Variables are expressed as medians (p25-p75).
Aβ: beta-amyloid protein; CSF: cerebrospinal fluid; CU: cognitively unimpaired; pTau181: Tau phosphorylated at threonine (position 181); tTau: total Tau protein.
Fig. 1 shows the ROC curves of the biomarkers analysed, with AUC values ranging from 0.78 to 0.86. The values selected to determine the optimal COP for the main AD CSF biomarkers were the following: Aβ42 = 750 pg/mL, tTau = 522 pg/mL, pTau181 = 69.9 pg/mL; and the ratios Aβ42/Aβ40 = 0.062, Aβ42/tTau = 1.755, and Aβ42/pTau181 = 10.3. Table 3 includes the SE, SP, predictive values, and Youden indices. SE was above 80% for all biomarkers and ratios, with the exception of tTau (70.8%). SP was above 70% in all cases except for the Aβ42/Aβ40 ratio (64.3%). The Aβ42/pTau181 ratio obtained the highest Youden index (0.62), with an AUC of 0.86.
Cut-off points for the set of CSF biomarkers.
| Cut-off point | Sensitivity | Specificity | PPV | NPV | Youden | AUC (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|
| Aβ42 (pg/mL) | 750.0 | 83.3% | 71.4% | 76.9% | 78.9% | 0.548 | 0.81 (0.72-0.90) | < .001 |
| Aβ42/Aβ40 | 0.062 | 89.6% | 64.3% | 74.1% | 84.4% | 0.539 | 0.78 (0.68-0.88) | < .001 |
| tTau (pg/mL) | 522.0 | 70.8% | 78.6% | 79.1% | 70.2% | 0.494 | 0.79 (0.70-0.89) | < .001 |
| pTau181 (pg/mL) | 69.9 | 81.3% | 76.2% | 79.6% | 78.0% | 0.574 | 0.81 (0.72-0.90) | < .001 |
| Aβ42/tTau | 1.75 | 87.5% | 73.8% | 79.2% | 83.8% | 0.613 | 0.86 (0.78-0.94) | < .001 |
| Aβ42/pTau181 | 10.25 | 83.3% | 78.6% | 81.6% | 80.5% | 0.619 | 0.86 (0.78-0.94) | < .001 |
Aβ: beta-amyloid protein; AUC: area under the ROC curve; CI: confidence interval; CSF: cerebrospinal fluid; NPV: negative predictive value; PPV: positive predictive value; pTau181: Tau phosphorylated at threonine (position 181); tTau: total Tau protein.
Twenty-five (71.4%) of the individuals in the aMCI group (n=35) were included in the AD continuum category: 5 (14.3%) individuals in the Alzheimer-type pathological change group (A+T–N–), and 20 (57.1%) in the AD group (A+T+N±). Furthermore, 9 participants (25.7%) presented normal results for AD biomarkers (A–T–N–), and one (2.86%) subject was included in the non-AD pathologic change group (A–T+N+). Table 4 shows the frequency and percentage of subjects in each A/T/N profile and their sociodemographic characteristics.
Sociodemographic characteristics and A/T/N profiles in patients with aMCI (n = 35).
| A/T/N profile | n (%) | Age | Education level | Sex (% of women) |
|---|---|---|---|---|
| AD continuum | ||||
| A+T–N– | 5 (14.9) | 71.6 (4.93) | 10.6 (4.56) | 40 |
| A+T+N– | 2 (5.7) | 78.5 (3.54) | 7.00 (1.41) | 0 |
| A+T+N+ | 18 (51.4%) | 74.1 (4.42) | 9.33 (4.10) | 77.8 |
| Non-AD pathologic change | ||||
| A–T+N+ | 1 (2.9) | 75.0 | 8 | 100 |
| Normal results in AD biomarkers | ||||
| A–T–N– | 9 (25.7) | 76.2 (3.15) | 7.78 (2.59) | 22.2 |
Numerical variables are expressed as means (standard deviation) and the dichotomous variable as a percentage.
AD: Alzheimer disease; aMCI: amnestic mild cognitive impairment.
Six (4.8%) participants in the study experienced some adverse event related to the LP (5 of them presented headache after puncture, and one presented dizziness); all of them fully recovered. No severe adverse events were reported. No adverse events associated with the remaining procedures of the study were observed.
DiscussionDespite the development of semi-automatic analysis systems to determine AD CSF biomarkers, no universal reference values have been established for their interpretation. This study describes the COPs obtained after a prospective multi-centre study with the aim of reliably applying them in our population in clinical practice.
Cut-off points for AD CSF biomarkersWe obtained COPs by using ROC curves to analyse the ability to classify participants as CU or patients with AD dementia; we chose the COP with the highest Youden index. The COP of a specific biomarker may be selected using different statistical approaches, including normal values in a healthy population, controlling the desired SE and SP, maximising accuracy, or using ROC curves (graphical representation of SE vs 1 – SP of the possible COPs), with the latter approach frequently being used in clinical research. The selection of a COP after a capacity analysis using ROC curves according to the highest Youden index leads to an optimal balance between SE and SP,13,14 which we consider appropriate for clinical practice for the diagnosis of AD as a part of a complex diagnostic process.
The calculation of COPs using a case-control approach has been used in several previous studies,15–21some of which use the same analysis platform.18–21 With the aim of optimising the reliability of clinical diagnosis, our study uses inclusion and exclusion criteria that meet the clinical practice recommendations for the diagnosis of AD,3,4,8 as well as an extensive neuropsychological battery for the correct characterisation of subjects. Likewise, we used restrictive criteria regarding the degree of cerebrovascular disease (brain MRI with leukoaraiosis with a score equal to or lower than 1 on the Fazekas scale, beyond other more frequent criteria such as the absence of strategic or large vessel infarcts). Among the case-control studies proposing COPs for the Lumipulse platform, we may highlight those published by Leitão et al.18 (80 patients with AD dementia vs 40 controls), Bayart et al.20 (44 patients with AD dementia vs 42 healthy controls, determining Aβ42 and tTau levels only), and the important multi-centre study recently published by Goborn et al.21 (321 patients with AD dementia vs 342 controls). The COPs referred in the technical specifications of the Lumipulse G system (manufactured by Fujirebio) at the time of this study were obtained by comparing 60 patients with AD dementia against 40 controls. These COPs were slightly lower than those obtained in this study, with the exception of the Aβ42/Aβ40 ratio, which yielded a similar result (Aβ42 < 526 pg/mL, tTau > 409 pg/mL, pTau > 50.2 pg/mL in the study by Goborn et al.21; Aβ42 < 599 pg/dL, tTau > 404 pg/dL, pTau > 56.5 pg/dL, Aβ42/tTau < 1.275, Aβ42/pTau < 8.10, Aβ42/Aβ40 < 0.069 in the technical specifications). These differences may be due to a certain variability in the demographic characteristics of the sample, including a younger age than in our cohort (for example, mean age was 62.2 and 66.8 years in the control group and the AD dementia group, respectively, in the study by Goborn et al.21) as well as differences regarding the selection criteria for the control group. Our selection criteria for the control group included an extensive psychometric assessment but, unlike other studies,18,20,21 did not include such other neurological diseases as chronic headache or polyneuropathies, nor such disorders as epilepsy, multiple sclerosis, or major psychiatric disorders, as was the case for the control group mentioned in the technical specifications. Furthermore, we applied the same inclusion criteria for all participants, minimising variability in the control group, unlike in the multi-centre study mentioned above.21
Other studies have opted to define the COPs of AD CSF biochemical biomarkers according to concordance with the findings of a brain positron emission tomography (PET) study with amyloid radiotracers or fluorodeoxyglucose (FDG) showing a pattern of hypometabolism suggestive of AD22–29; some of these studies were performed at centres in our healthcare system.23,24 Of these, we considered specially relevant the study published by Alcolea et al.,23 including 94 participants (35 with MCI, 12 with AD dementia, 41 with other dementias or neurodegenerative diseases, and 6 healthy controls), which obtained COPs for AD CSF biomarkers using the same analysis platform as in the present study, according to the results of an amyloid PET study (18F-Florbetapir).23 The COPs obtained (Aβ42 > 916 pg/dL, tTau > 456 pg/dL, pTau > 63 pg/dL, Aβ42/Aβ40 < 0.062) are slightly different from our own for Aβ42 and tTau, and very similar for pTau. Interestingly, the Aβ42/Aβ40 ratio is the same in both studies. In this case, the differences observed, though minor, may be due to the use of a different approach (a clinically heterogeneous sample was included).
Regarding the time between sample collection and analysis, although sample collection began in 2014 and the cited recommendations mentioned a sample stability of 2 years.11 a subsequent study by Willemse et al.30 revealed that levels of Aβ42, tTau, and pTau181 would remain stable for 12 years at a temperature of –80º.
Although the study also included a group of patients with aMCI, their data were not used to calculate the COP, as diagnosis of MCI is relatively unstable, and some patients classified as such do not progress to dementia or even obtain normal results in subsequent psychometric tests.31 However, including this group with MCI has enabled us to apply the results obtained and study their classification capacity in accordance with the A/T/N system.
Differences in demographic characteristics between the groups used to obtain the COPs (CU and AD dementia) are consistent with the known risk factors for the disease. The group with dementia was slightly older than the reference group, although this difference in mean ages was less than 3 years and was not statistically significant. We did observe statistically significant differences in sex and level of schooling. These differences are consistent with the increased risk of AD dementia observed in women and subjects with a lower level of schooling, as reported in the literature.32–34 Regarding APOE genotyping, we observed differences regarding the percentage of carriers of the APOE-ɛ4 variant, which was higher in the AD dementia group; this is to be expected due to the increase in AD risk in carriers of this allelic variant.35,36
In terms of the values obtained for the different biomarkers and ratios combining them, we observed statistically significant differences between the 2 groups for all biomarkers, which is in line with the clinical diagnosis. All biomarkers and ratios present AUCs greater than 0.78, with the AUC obtained by combining biomarkers of amyloidosis and Tau protein deposition being particularly noteworthy (Aβ42/pTau181 and Aβ42/tTau, AUC 0.86); this is in line with previous studies.37,38Overall, SE and SP levels were above 70%, with most COPs presenting values higher than 75% for both statistical measurements. The biomarkers with the highest SE were those indicating amyloidosis (Aβ42 and Aβ42/Aβ40), especially the Aβ42/Aβ40 ratio (SE: 89%), although their SP values were more modest (71% and 64%, respectively). This may be due to the different time pattern of both biomarkers, with the decrease in markers of amyloidosis occurring earlier than the increase in tTau and pTau181 levels39 in preclinical stages of the disease. Another potential explanation is the inclusion of participants with low CSF amyloid peptide levels due to cerebral amyloidosis not directly associated with AD. We observed a better SP for the 2 biomarkers of Tau protein deposition, tTau and pTau81 (78.6% and 76.26%, respectively). The combination of biomarkers of amyloidosis and tauopathy using the Aβ42/pTau181 ratio obtained the highest AUC values and the best balance between SE and SP, which is advisable for the diagnosis of an irreversible disease, without overlooking the need to detect patients in early stages. As shown by previous studies, the Aβ42/pTau181 ratio is considered a very accurate CSF biomarker.40–42 In general, our findings support the use of COPs obtained in clinical practice, preferentially those obtained by combining different biomarkers.
A/T/N variants in patients with aMCIMost of the patients in the aMCI group presented abnormal AD biomarker profiles, meeting diagnostic criteria for aMCI due to AD.3 This high percentage may be associated with the extensive formal neuropsychological assessment performed in this study, which leads to an optimal classification of participants; this in turn supports the role of neuropsychological assessment in everyday clinical practice. Our results have enabled more accurate aetiological diagnosis in patients with a clinical diagnosis of aMCI, and are similar to those reported in different studies on the applicability of the A/T/N classification system in other cohorts.43–48
LimitationsRegarding the limitations of our study, we consider that including a larger number of subjects in each group would have increased the study’s statistical power. Furthermore, although we assumed that the biomarker levels were within normal ranges in the control group, we may not rule out the inclusion of some individuals in preclinical stages of AD. This possible selection bias is inherent to the strictly clinical and psychometric classification used, although the use of an extensive neuropsychological battery may minimise this. Another limitation of our study is the lack of longitudinal data, especially in the control group. Likewise, despite the optimisation of the diagnostic study with an appropriate protocol and the experience of the participating centres, an anatomopathological study of patients with AD dementia that may confirm this aetiology is not available. Furthermore, including some subjects with subjective cognitive complaints results in a higher risk of including subjects in preclinical stages of AD,49 although the available evidence on this subject has expanded in the years since the study design and was not considered at that time. Despite the risk of including subjects in the preclinical stage of AD in the control group, the comparison between groups with more distant psychometric results may have reduced its effect, considering the presence of biomarker levels presumably farther from normality in subjects with AD dementia. Lastly, recruitment difficulties, especially regarding the control group, and certain logistical problems associated with the creation of a systematic, reliable system for sending samples, led to a considerable delay in the initially planned execution schedule.
On the other hand, the design of the present study includes a detailed protocol for clinical, neuropsychological, and neuroimaging assessment that helps to correctly characterise patients. Our study was conducted in the context of everyday clinical practice, where greater heterogeneity is observed in demographic and clinical characteristics, comorbidities, and pharmacological treatments than in research cohort studies. This approach provides a greater external validity for the application of the COPs described in other specialised units.
ConclusionsIn this study, we determined COPs for AD CSF biomarkers through a clinical approach, obtaining statistical values that support their reliable application at the participating centres with a low risk of adverse events. The combination of biomarkers of amyloidosis and tauopathy using the Aβ42/pTau181 ratio provides the best balance between sensitivity and specificity.
Conflicts of interestThe authors have no conflicts of interest to declare.
MSC has consulted and sat on advisory boards for Roche Diagnostics International Ltd. and has presented sessions in symposia sponsored by Roche Diagnostics, S.L.U and Roche Farma, S.A.
FundingThis study has received no specific funding from any public, commercial, or non-profit organisation.
MSC has received funds from the European Research Council in the framework of the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 948677) and a Marie Skłodowska-Curie grant (No. 847648 [LCF/BQ/PR21/11840004]), as well as from the Instituto de Salud Carlos III (PI19/00155) and Fundació La Caixa (ID 100010434).
ING and JR have received funds from the Instituto de Salud Carlos III (ISCIII-FEDER, PI21/00194).
The authors would like to thank the participants and their families for their collaboration in the study, as well as all those professionals from different centres who have participated in the study (nurses and nursing assistants at day hospitals and laboratory technicians).