Patients with stroke associated with non-valvular atrial fibrillation (NVAF) are a specific group, and their disease has a considerable social and economic impact. The primary objective of the CONOCES study, the protocol of which is presented here, is to compare the costs of stroke in NVAF patients to those of patients without NVAF in Spanish stroke units from a societal perspective.
Materials and methodsCONOCES is an epidemiological, observational, naturalistic, prospective, multicentre study of the cost of the illness in a sample of patients who have suffered a stroke and were admitted to a Spanish stroke unit. During a 12-month follow-up period, we record sociodemographic and clinical variables, score on the NIH stroke scale, level of disability, degree of functional dependency according to the modified Rankin scale, and use of healthcare resources (hospitalisation at the time of the first episode, readmissions, outpatient rehabilitation, orthotic and/or prosthetic material, medication for secondary prevention, medical check-ups, nursing care and formal social care services). Estimated monthly income, lost work productivity and health-related quality of life measured with the generic EQ-5D questionnaire are also recorded. We also administer a direct interview to the caregiver to determine loss of productivity, informal care, and caregiver burden.
Results and conclusionsThe CONOCES study will provide more in-depth information about the economic and clinical impact of stroke according to whether or not it is associated with NVAF.
Los pacientes con ictus asociado a fibrilación auricular no valvular (FANV) constituyen un grupo específico con gran repercusión social y económica. El objetivo principal del estudio CONOCES, cuyo protocolo se presenta en ese trabajo, es comparar los costes del infarto cerebral en los pacientes con FANV frente a los pacientes sin FANV en el ámbito sanitario español ingresados en unidades de ictus, utilizando la perspectiva de la sociedad.
Materiales y métodosCONOCES es un estudio epidemiológico, observacional, naturalístico, prospectivo y multicéntrico de los costes de la enfermedad, en una muestra de pacientes que ha sufrido un ictus establecido e ingresado en una unidad de ictus, en el ámbito sanitario español. El periodo de seguimiento será de 12 meses. Se recogerán variables sociodemográficas, clínicas, la escala de ictus del NIH, el nivel de discapacidad, el grado de dependencia funcional mediante la escala de Rankin modificada y el consumo de recursos sanitarios (hospitalización en el primer episodio, reingresos, rehabilitación ambulatoria, material ortoprotésico, medicación para la prevención secundaria, consultas médicas, atención de enfermería, servicios sociales de atención formal). También se registrará la renta mensual estimada, la pérdida de productividad laboral y la calidad de vida relacionada con la salud con el cuestionario genérico EQ-5D. Por último se entrevistará directamente al cuidador para conocer la pérdida de productividad, los cuidados informales prestados y la sobrecarga del cuidador.
Resultados y conclusionesLa aportación del estudio CONOCES permitirá profundizar en las diferencias del impacto tanto económico como clínico del ictus en función de su asociación con la FANV.
Strokes are events that result in severe disability among survivors. Stroke is the leading cause of dependency in adult patients and the world's second most common cause of dementia,1 making it one of the diseases with the highest social and economic costs.2,3 In 2009, Spain's National Statistics Institute (INE in Spanish) ranked stroke as the second most frequent cause of death in the Spanish population as a whole, and the leading cause in women.4 Its prevalence in Spain is estimated at 7% of the urban population older than 65, and its incidence is 128 people per 100000 in the general population.5,6
Non-valvular atrial fibrillation (NVAF) is a common heart arrhythmia in elderly patients, as well as being the most frequent cardioembolic cause of ischaemic stroke.7 The prevalence of NVAF changes with age, varying from 1% to 9%, and it causes 15% of all strokes.8 The risk of stroke in patients with atrial fibrillation is 5 times higher than that in subjects without NVAF.9 Furthermore, strokes in patients with NVAF have a poorer prognosis, with a risk of death double that of other patients.10,11 The condition is also associated with greater degrees of dependency and disability among survivors.12 All this evidence indicates that subjects with NVAF constitute a specific subset of stroke patients affected by greater economic and social setbacks.
Hospital costs related to cerebrovascular disease in Spain were estimated at 1.526 billion euros in 2004.13 Adding the indirect costs due to loss of productivity and direct non-healthcare related costs gives us estimates approaching 6 billion euros per year.14 Different studies carried out in Sweden and Germany have indicated that stroke associated with NVAF generates higher costs.15,16 Despite there being literature on the costs of stroke in Spain,7 we do not have specific information about the impact of NVAF on stroke costs in our country. On the other hand, the improvements in hospital care brought about by the efforts of stroke units have both decreased mortality and improved patient prognosis. These changes have not yet been analysed sufficiently.17,18 The CONOCES study was created to respond to a need for information about the overall costs of stroke, broken down by aetiology and adapted to the current model of stroke care.4,5
The main purpose of the CONOCES study is to compare stroke costs in patients with NVAF to those of patients without NVAF in hospital stroke units in Spain, from a societal perspective. The secondary objectives of the study are to compare characteristics of ischaemic stroke in patients with and without NVAF in terms of mortality, health-related quality of life (HRQoL), neurological sequelae and recurrence of stroke or other vascular episodes, and estimated burden for the patient's primary carer in the first year following the stroke. An additional objective is using simulation to project patient follow-up data from the first year after stroke to the patient's death in order to calculate the lifetime cost of a stroke.
Methods/designDesign and type of studyCONOCES is an observational, naturalistic, prospective, and multi-centre epidemiological study investigating the costs of disease in a group of patients who have experienced established stroke and who have been admitted to a stroke unit within the Spanish health care system.
Selection criteriaPatient selection criteria are as follows: aged over 18, a clinical diagnosis of a first established ischaemic or haemorrhagic stroke, time from stroke onset less than 24hours, admission to a stroke unit, consent to participate in the study, and signed informed consent sheets from both the patient and the primary carer. The primary carer is defined as the person who was not a professional care provider but had assumed responsibility for the patient's care from the time of the stroke. Exclusion criteria are the patient's or the carer's refusal to participate in the study; diagnosis of transitory ischaemic attack; and prior history of stroke.
Sample sizeIn calculating sample size, the main study variable was the mean healthcare cost incurred by patients with stroke and NVAF 12 months after the episode. Considering the €2982 difference in mean healthcare costs reported by the Brüggenjürgen et al. study,16 which found a mean cost of €11799 (SD 8292) per patient with NVAF and €8817 (SD 7251) per patient without NVAF, with an alpha error of 5%, a statistical power of 85%, and a percentage of losses to follow-up of 28%, the total number of patients to be included was calculated at 320, or 160 with NVAF and 160 without. Sample size calculations were performed using GRANMO® software version 7.04, and GPower® 3.0.10.
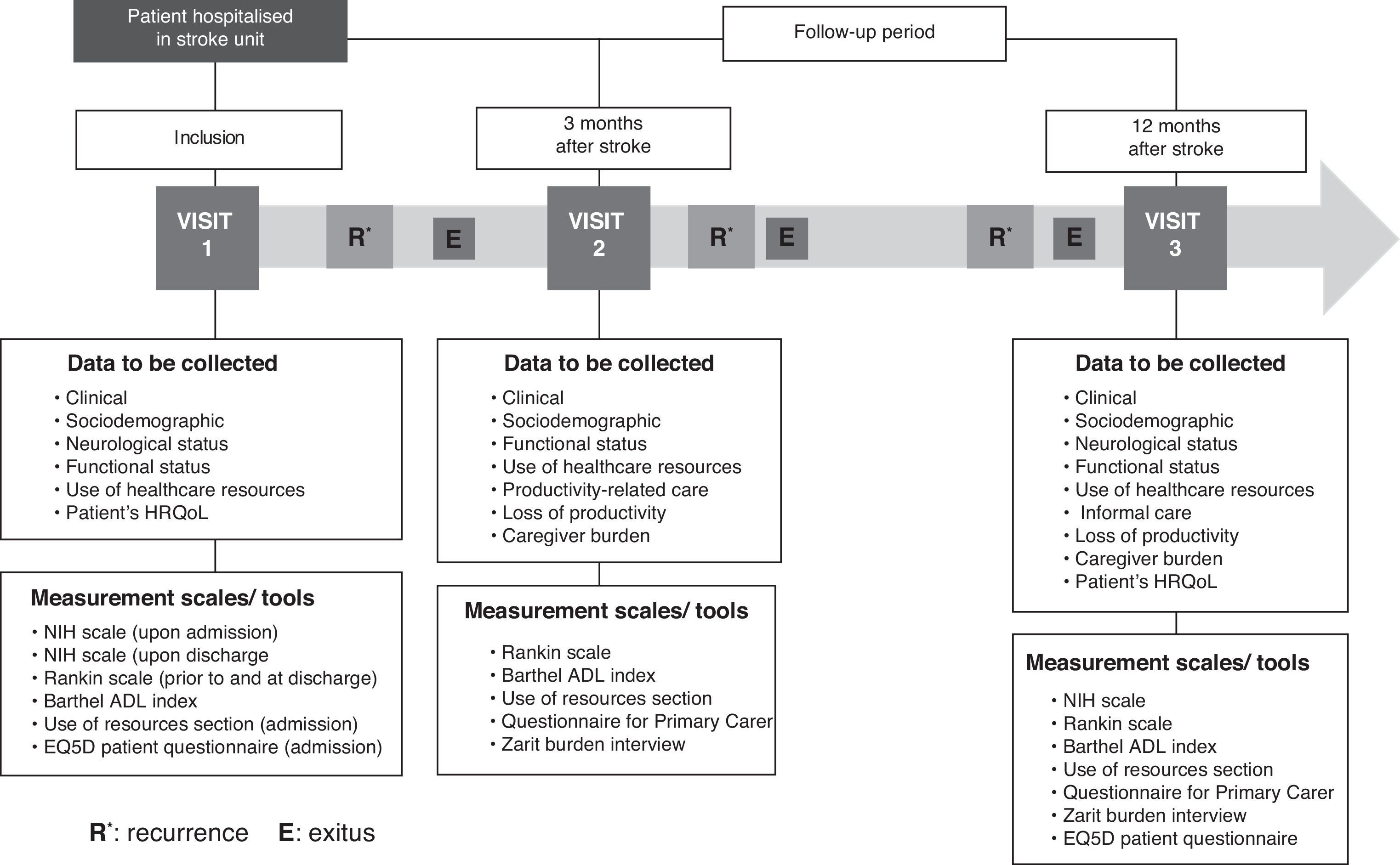
Sample selection and patient follow-upBetween 1 November 2010 and 3 May 2011, researchers at each of 16 participating centres recruited patients in the stroke units, using prospective, consecutive sampling. Recruitment proceeded independently of whether or not patients had NVAF provided that they met inclusion criteria. When one of the patient groups was full, doctors continued recruiting patients from the other group until the sample size was met. The initial visit or inclusion in the study took place while the patient was in hospital immediately following the stroke. The follow-up period after inclusion lasted 12 months. During that time, the patient had 2 follow-up appointments (at 3 and 12 months), coinciding with the check-ups typically scheduled for these patients. At 6 months after the stroke, patients received a telephone call to arrange the third and last appointment and minimise losses to follow-up. Prior to starting the study, we ran a pilot test of the electronic data capture device using data from the initial visit.
Study variablesFig. 1 shows the patient follow-up process along with field work. Patients’ information was gathered through 3 different channels and recorded in an electronic data capture device (eDCD). Firstly, doctors gathered the following variables from medical histories and interviews with patients and/or primary carers: sociodemographic data (age, sex, level of studies, employment status, marital status, lifestyle habits such as tobacco and alcohol use and engaging in regular physical exercise); clinical data (comorbidities, medications, stroke date, stroke classification, complementary tests); neurological state upon admission to the stoke unit and at discharge using the NIH stroke scale,19 also used for patients who had to be readmitted due to recurrence; functional status according to disability measured by the Barthel index20 and degree of functional dependency according to the modified Rankin scale21; and use of healthcare resources (hospital stay at time of first episode, readmission, outpatient rehabilitation, orthotic/prosthetic material, medications for secondary prevention, medical transport, doctors’ appointments, nursing care, and formal social services).
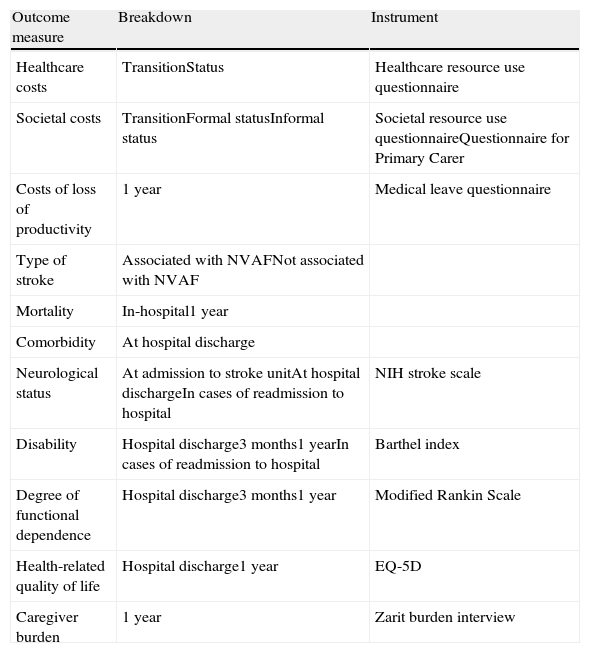
Secondly, doctors held interviews with the patient, or with the carer in the case of patients lacking the cognitive ability to answer questions, to record the following data: estimated monthly income, loss of productivity at work, and HRQoL, measured using the generic questionnaire EQ-5D.22 Thirdly, the carer was interviewed to register loss of productivity, informal care provided (Questionnaire for Primary Carer),23 any assistance by a secondary carer, and carer burden using the Zarit burden interview.24Table 1 provides a summary of measurable results and the instruments used to measure them.
Outcome measures and instruments used in the study.
| Outcome measure | Breakdown | Instrument |
| Healthcare costs | TransitionStatus | Healthcare resource use questionnaire |
| Societal costs | TransitionFormal statusInformal status | Societal resource use questionnaireQuestionnaire for Primary Carer |
| Costs of loss of productivity | 1 year | Medical leave questionnaire |
| Type of stroke | Associated with NVAFNot associated with NVAF | |
| Mortality | In-hospital1 year | |
| Comorbidity | At hospital discharge | |
| Neurological status | At admission to stroke unitAt hospital dischargeIn cases of readmission to hospital | NIH stroke scale |
| Disability | Hospital discharge3 months1 yearIn cases of readmission to hospital | Barthel index |
| Degree of functional dependence | Hospital discharge3 months1 year | Modified Rankin Scale |
| Health-related quality of life | Hospital discharge1 year | EQ-5D |
| Caregiver burden | 1 year | Zarit burden interview |
For patients who experienced stroke recurrence, the eDCD was also used to record data about that patient's clinical and functional situation and the healthcare resources that were used as a result of readmission to the stroke unit and the hospital. Furthermore, the NIH scale was repeated upon readmission and discharge, and the modified Rankin scale and the Barthel index upon discharge from the neurology department.
Estimating and calculating costs of strokeThe societal perspective adopted in this study implies including both the healthcare and social costs of the disease. The latter include costs derived from formal and informal care alike. The cost components have been categorised as either transition or incident costs, and as prevalence or health status costs.25 Transition costs include those that are associated with an acute event, whether at the time of hospitalisation or those incurred in the following months. By health status costs, we refer to costs associated with a characteristic that persists throughout the patient's life time and which entails consumption of resources.
Health costs will be measured by multiplying unit costs of healthcare resources by the number of natural units used. Unit costs will be obtained from a number of Spanish sources, including the database for Spanish healthcare costs (eSalud),26 official fee schedules from Spain's autonomous communities (especially those in which participating stroke units are active), and published preliminary studies. Drug costs will be obtained from the latest schedules updated by the Official College of Pharmacists of Madrid.27,28 Costs will be updated according to the year in which the analysis was performed and the corresponding result summaries were generated.
Transitional costs include those of hospitalisation at the time of the initial episode and readmission to hospital during follow-up. Hospitalisation costs were obtained using a microcost method that involved identifying cost components (hospital stay in days, specific treatments, diagnostic tests) and calculating their sum. Health status costs were defined as any cost derived from outpatient follow-up, such as consultations having to do with monitoring progress after the stroke, visits to the general practitioner and the neurologist, visits to the emergency department, care provided by nursing staff, homecare provided by the Hospital at Home unit (UHD in Spanish), home visits by the primary care team, rehabilitation sessions, speech therapy sessions, complementary tests, patients’ long-term medications, and orthotic/prosthetic material.
Transitional social costs were calculated based on information provided by the patient and/or the primary carer with regard to costs of any modifications made to the patient's residence, workplace, and vehicles as a result of functional limitations caused by stroke. Formal social costs were estimated based on the register of social services provided to the patient, such as attending a day care centre; stays in a public, subsidised, or private residence; stays in social care centres; adapted medical transport; and homecare. Unit costs were then multiplied by the number of times or natural days that the patient had used formal resources.
In assigning monetary value to time spent providing informal care, we used the substitution/replacement technique to evaluate cost of time spent by carers.23,29 The variable in use is the mean salary of the person who would have to substitute the informal carer in all daily activities. We will refer to data presented in the Quarterly Survey of Labour Costs (ETCL in Spanish) and the Salary Structure Survey (EES in Spanish), both prepared by the National Statistics Institute. Salaries for primary carers will be adjusted to the list of activities defined by the Questionnaire for the Primary Carer. Community-based activities and household tasks will be calculated using the hourly salary given in section O (Other social activities and community services; personal services). For personal care activities, which require increased dedication and are more labour-intensive, we will draw on Section N (Healthcare activities and social services). The sensitivity analysis will employ different unit cost scenarios, including hourly costs of homecare services in the form of official data provided by IMSERSO (Spanish institute for social services and the elderly).30
We will also estimate loss of productivity associated with temporary or permanent disability caused by stroke, and loss of labour associated with premature death. To that end, we shall use the EES survey and information gathered using questionnaires.
Applicable legal frameworkAll researchers are required to abide by the Declaration of Helsinki and act in accordance with international laws regulating epidemiological studies and compiled in the International Guidelines for Ethical Review of Epidemiological Studies.31,32 They will also follow the Spanish Ministry of Health and Social Policy directives for post-approval observational studies of drugs for human use.33 The study protocol and all other necessary documents were delivered to the Clinical Research Ethics Committee at Hospital Clínic, Barcelona, which granted its approval.
Data protectionInformation relating to patient identity is kept strictly confidential. Under no circumstances may the identity of the patients be revealed or published. Patient data recorded in the eDCD during the study were made anonymous and dissociated from personal data by means of a code (patient number). Only the researcher is able to associate data with an identified or identifiable individual. Data remain protected under the terms of Spanish Organic Law 15/1999, ratified on 13 December, regarding personal data protection.34
Statistical considerationsSPSS software version 11.0 for Windows will be used for data analysis. For all statistical tests performed with outcome variables, the significance level is established at P=.05. The types of lost data will be described in the final report of results from the study.
Statistical analysis will be completed in several stages. The descriptive analysis for all study variables will distinguish between patients with and without NVAF. Intra-group differences are detected using the Mann–Whitney test, t-test, or ANOVA (or Kruskal–Wallis) for continuous variables. Categorical variables are analysed with a proportion comparison test. Significant differences between patients with and without NVAF are estimated using contrast tests such as the t-test for comparing means, the Mann–Whitney test for comparing medians, and the chi-square or Fisher exact tests for comparing homogeneity of patient distributions for levels of the variable between the 2 study populations (NVAF and non-NVAF), and to compare proportions of independent populations. Associations between variables will be calculated using the Spearman rank correlation for quantitative variables transformed into ranks or ordinals and the Pearson product moment coefficient for continuous variables. The association between cost and the different clinical variables will be analysed using multiple linear regression models in which the dependent variable was cost per patient and independent variables were those found to be significant by the bivariate descriptive analysis, evaluating any potential interactions between covariates. Tests of significance and residuals are applied in accordance with the selected model.
Modelling with discrete-event simulationCosts incurred by the disease will be modelled at the 3- and 5-year marks, and throughout the patient's lifetime. A probabilistic model for discrete events simulation (DES) will be used, and data emerging from the study will be fed into the model. These models let us describe the natural history of a stroke and monitor a sample of patients. DES is a mathematical model for representing the natural history of a disease. It is characterised by its flexibility, which permits the incorporation of complex events in patients and patient progress, and its ability to deliver pertinent results.35 The model that will be used to represent stroke has already been validated,36 and it enables us to calculate care costs for a cohort with the characteristics listed for the CONOCES sample from the date of the event to the patient's death with and without a yearly discount rate (discount rate applied to base case: 3%). Association with NVAF will serve as the descriptive variable for estimating long-term differences.
Anticipated resultsThe CONOCES study will provide, first of all, information about the societal costs of stroke in Spain; second, health outcomes for these patients; third, the association of health costs and outcomes with NVAF, and fourth, projected costs of stroke in these patients’ lifetimes. Table 1 displays outcome measures and the instruments that will be used to determine them. Table 2 shows the different types of results we hope to obtain. The scientific data we hope to obtain include stroke-related hospital costs based on use of resources; estimated healthcare costs incurred over the 12 months after the event; and formal and informal societal costs arising in the year following the event. We will also determine stroke-related losses of labour in Spain among patients in their first year following the stroke.
Final results of CONOCES study.
| Costs |
| Hospital costs of stroke based on resource consumption |
| Healthcare costs up to 12 months after event |
| Formal and informal social costs in the first year after stroke |
| Loss of productivity in Spain among patients who had experienced a stroke in the previous year. |
| Health outcomes in patients with and without NVAF at 3 months and at 1 year after the event |
| Degree of disability |
| Degree of functional dependence |
| Neurological status |
| Health-related quality of life |
| Burden on the caregiver |
| Results from the simulation model |
| Healthcare and social costs generated in the patient's lifetime by ischaemic and haemorrhagic stroke in Spain, in patients with and without NVAF. |
Regarding health outcomes, we will estimate degree of disability, degree of functional dependence, neurological status, HRQoL, and the burden assumed by the caregiver upon a stroke patient's hospital discharge in patients with and without NVAF and at the 3-month and 1-year marks after the event.
Although follow-up time will be 1 year, we plan to use simulation techniques to compare the healthcare and societal costs of ischaemic and haemorrhagic stroke in Spain in patients with and without NVAF over a patient's lifetime.
DiscussionExperts indicate that society underestimates the magnitude of health problems when they are not quantified.37 Measuring the economic and health impact of neurological diseases involves specific difficulties because these diseases are closely linked to disability.38 Unlike measuring outcomes in cancer patients, which is essentially based on changes in survival, a large part of the overall cost of stroke is due to its consequences in terms of the number of years lived with disability (YLD). The Global Burden of Disease Study, published by the WHO in 1996, included mortality and disability together in its calculations of disability-adjusted life years.39 Despite these efforts, the burden of stroke-related disability is still not given sufficient consideration by entities assigning funds for the purpose of research.38 Similarly, the economic analyses that have been carried out in Spain do not examine the diseases generating the highest costs. It has been shown that studies published in Spain under-represent neurological entities such as stroke or Alzheimer disease, despite the fact that these diseases incur some of the highest costs.40 Our study is directed at responding to the lack of economic studies focusing on stroke patients.
Hospital care for patients with stroke has changed in the last 10 years with the implementation of Spain's stroke care plans.7,41 The main pillar of this approach is the care provided by stroke units.17,18 However, studies examining in-hospital mortality tendencies only study those patients who received care as much as 8 or even 10 years ago.42 In order to have an updated view of outcomes and costs, we must examine a sample of patients who have experienced current care conditions. Understanding the new realities in patient care is a necessity, and since the CONOCES study focuses on a sample of patients in stroke units, the care these patients receive is quite homogeneous. The study's limitations are its inclusion of only patients in stroke units and other limitations inherent to its naturalistic design. These features provide considerable external validity, but less internal validity than would be the case with experimental designs.
In addition to providing specific treatment and controlling potential complications, hospital treatment for stroke patients aims at identifying aetiological factors that may point to the most effective means of secondary prevention. One such aetiological factor is NVAF, since this diagnosis permits use of anticoagulant treatment. This being the case, doctors require studies providing more in-depth analysis of the clinical and economic effects of stroke broken down by aetiology. Our study is designed to examine the association of stroke and NVAF, which gives rise to poorer clinical outcomes and is associated with higher costs.
Conflicts of interestThis study is financed with an unconditional grant provided by Boehringer Ingelheim España.
The research consultants (José Álvarez-Sabín, Jaime Masjuan Vallejo, Javier Mar, and Juan Oliva) and stroke unit coordinators participating in this study (Juan Francisco Arenillas, María Teresa Martínez-Zabaleta, Mariano Rebollo, Aida Lago, Tomás Segura, José Castillo, Jaime Gállego, Carmen Jiménez-Martínez, Juan Ignacio López-Gastón, Francisco Moniche, Ignacio Casado-Naranjo, Juan Carlos López-Fernández, Carmen González-Rodríguez y Bernardo Escribano) have received fees from Boehringer Ingelheim España for their participation in this study. Miguel Ángel Casado and María Yébenes are employed by PORIB, the company entrusted with managing the study. Virginia Becerra and Nuria González-Rojas are employed by Boehringer Ingelheim España.