Timing is one of the most important modifiable prognostic factors in the management of status epilepticus. Epilepsia partialis continua (EPC) is a status epilepticus subtype of highly variable, occasionally prolonged, duration. The aim of this study was to analyse the relationship between EPC duration and outcomes.
MethodsWe performed an observational prospective study of all patients with EPC admitted to our tertiary hospital between 1 September 2017 and 1 September 2018.
ResultsThe sample included 10 patients, of whom 9 were women; median age was 74 years. The most frequent aetiology was cerebrovascular disease (n = 6). EPC onset occurred outside the hospital in 5 patients, with a median time to hospital admission of 4 hours. The median time to treatment onset (TT) for all patients was 12.3 hours. The median time from treatment onset to EPC control (TC) was 30 hours; TC showed a strong positive correlation with TT (Spearman’s rho = 0.88). Six patients presented hyperglycaemia at onset; this was positively correlated with TC (rho = 0.71). All 6 patients with hyperglycaemia presented a brain injury explaining the EPC episode.
ConclusionsDelays were observed in different phases of EPC management, which was related to longer duration of the episode. Glycaemia was also related to episode duration, probably acting as a triggering factor rather than as the aetiology.
El tiempo de actuación médica es uno de los factores pronósticos modificables más importantes en el estatus epiléptico (EE). La epilepsia parcial continua (EPC) es un tipo de EE con duración muy variable, en ocasiones prolongada. Nuestro objetivo fue analizar la relación entre el tiempo de evolución de la EPC y su pronóstico.
MétodosEstudio observacional prospectivo de todos los pacientes con EPC que ingresaron en un hospital terciario en el periodo comprendido del 01/09/2017 al 01/09/2018.
ResultadosSe incluyeron 10 pacientes con EPC, nueve mujeres, con una mediana de edad de 74 años. La etiología más frecuente fue la patología cerebrovascular (n = 6). El inicio de la EPC fue extrahospitalario en cinco pacientes, siendo la mediana del tiempo hasta el ingreso hospitalario (TH) 4 horas. En la serie global, la mediana del tiempo hasta el inicio del tratamiento (TT) fue 12,3 horas. La mediana del tiempo hasta el control desde el inicio del tratamiento (TC) fue 30 horas, y presentó una correlación positiva alta con el TT (rho Spearman 0,88). Seis pacientes presentaban hiperglucemia al inicio del episodio, y ésta asoció una correlación positiva con el TC (rho Spearman: 0,71). Los seis pacientes con hiperglucemia tenían una lesión cerebral que justificaba el origen de la EPC.
ConclusionesIdentificamos un retraso en diferentes fases del manejo de la EPC, lo que se relacionó con una mayor duración del episodio. La glucemia también se relacionó con la duración del episodio, con un papel probablemente más desencadenante que etiológico.
Epilepsia partialis continua (EPC) is a type of focal status epilepticus first described in 1894 by Russian neuropsychiatrist Aleksei Yakovlevich Kozhevnikov, who reported on a “special form of cortical epilepsy.”1 EPC was initially defined as continuous, rhythmic jerks or movements affecting a single part of the body. Immediately after it was first described, and subsequently during the 20th century, attempts were made to apply this definition to other forms of focal status epilepticus not featuring impaired awareness and presenting with continuous symptoms (aura continua), whether somatosensory, visual, olfactory, auditory, or cognitive.2–4 At present, we lack a universally accepted definition.
After the First London Colloquium on Status Epilepticus was held in 2007,5 a European multicentre EPC study group was formed, which defined EPC as “a condition of continuously repeated fragments of epileptic seizures (motor or sensory), with preserved consciousness, lasting ≥ 1 hour, and representing locally restricted epileptic activity.”6 The study group also proposed a classification of EPC according to its clinical course (Table 1). In 2015, the International League Against Epilepsy published the latest definition and classification of status epilepticus,7 according to which EPC is classified as a type of focal motor status epilepticus. The document calls attention to the possibility of irreversible brain damage resulting from prolonged epileptic seizures. However, no study has analysed the association between progression time and prognosis of EPC.
Classification of EPC proposed by the European multicentre EPC study group.
| Type 1: solitary event |
| a. In patient with history of epilepsy |
| b. Acute symptomatic EPC in patient with no history of epilepsy |
| Type 2: chronic repetitive nonprogressive |
| a. With frequent brief episodes (> 1 episode per month, lasting < 24 h) |
| b. With rare long episodes (1 episode or less per month, lasting > 24 h) |
| Type 3: chronic persistent nonprogressive |
| a. Starting as type 2 |
| b. Immediately persistent |
| Type 4: chronic progressive |
EPC: epilepsia partialis continua.
Source: Mameniškienè et al.6.
Although no population-based epidemiological data are available, the prevalence of EPC is estimated to be lower than 1 case per 1 000 000 population in the United Kingdom, according to a clinical series published in the literature8; no data are available on the incidence of the condition.
The causes of EPC include stroke, central nervous system tumours, malformations of cortical development, autoimmune encephalitis, and central nervous system infection.9,10 Uncomplicated hyperosmolar hyperglycaemia is a possible cause of EPC, although the precise association with the disease is unknown, and some authors regard it rather as a trigger factor.6,11 In any case, correction of hyperglycaemia is the treatment of choice, especially considering that EPC is usually refractory to antiepileptic drugs (AED).12
The purpose of this study was to describe the clinical characteristics of a series of patients with EPC, focusing on the relationship between progression time and response to treatment. We also analysed the presence of hyperglycaemia and its relationship with EPC aetiology and outcome.
MethodsWe conducted a prospective observational study of a cohort of patients with EPC admitted to Hospital Clínico San Carlos (Madrid, Spain) between 1 September 2017 and 1 September 2018. Our centre, a tertiary-level university hospital located in the city of Madrid and serving a population of 370 501 in 2017, keeps a prospective registry of all patients admitted to hospital with status epilepticus. Our study was approved by our hospital’s clinical research ethics committee (project no. 18-407E); all patients or their relatives signed informed consent forms prior to inclusion.
We recruited all patients aged ≥ 18 years and diagnosed with EPC who were admitted to any department of our hospital during the study period. Diagnosis was based on the latest definition of EPC proposed by the European multicentre EPC study group, mentioned previously: continuously repeated fragments of epileptic seizures (motor or sensory), with preserved consciousness, lasting ≥ 1 hour.6
We gathered the following data on each episode: patient demographic data (age, sex); history of epilepsy; dependence at baseline according to the modified Rankin Scale (mRS)13; history of diabetes mellitus and blood glucose level at onset of the episode; aetiology, type of EPC according to the classification proposed by the European study group, and severity of the episode according to the Status Epilepticus Severity Score (STESS)14; and setting at EPC onset (in-hospital vs pre-hospital). Time was recorded in hours and regarded as a continuous quantitative variable, similarly to practices followed in the code stroke protocol. Symptom onset (or, if this was not witnessed, the last known time without symptoms) was regarded as the reference time point. We calculated time to hospital admission (time to admission), time to assessment by the neurology department (time to neurological examination), time to treatment onset (time to treatment), and time from treatment onset to resolution of the episode (time to resolution), with resolution defined as symptom resolution persisting for at least 24 hours.
Data were also gathered on the sequence of pharmacological treatments administered and the doses. All patients underwent at least one electroencephalography (EEG) study (either routine EEG or prolonged video EEG). All EEG recordings were analysed by at least 2 neurologists specialising in epilepsy and certified in EEG interpretation by the Spanish Society of Neurology.
The outcome variables were time to resolution and morbidity and mortality during hospitalisation and at 3 months after discharge. The evaluation of morbidity was based on mRS scores and the appearance of new neurological symptoms (cognitive or motor sequelae).
Statistical analysisStatistical analysis was performed using the IBM SPSS software (version 23). Statistical significance was set at P < .05.
We initially performed a descriptive analysis of the sample. Given the small size of our sample, we assumed that data were not normally distributed. Qualitative variables are presented as frequencies and quantitative variables as medians with quartiles 1 and 3 (Q1-Q3).
To compare qualitative variables, we used the chi-square test or the Fisher exact test, when more than 25% of the expected values were below 5. Quantitative variables were compared with the Mann–Whitney U test for comparisons between 2 groups or the Kruskal–Wallis test for comparisons between more than 2 groups. We used the non-parametric Spearman rho test to evaluate the correlation between 2 quantitative variables. Multivariate analysis could not be performed due to the small size of the sample.
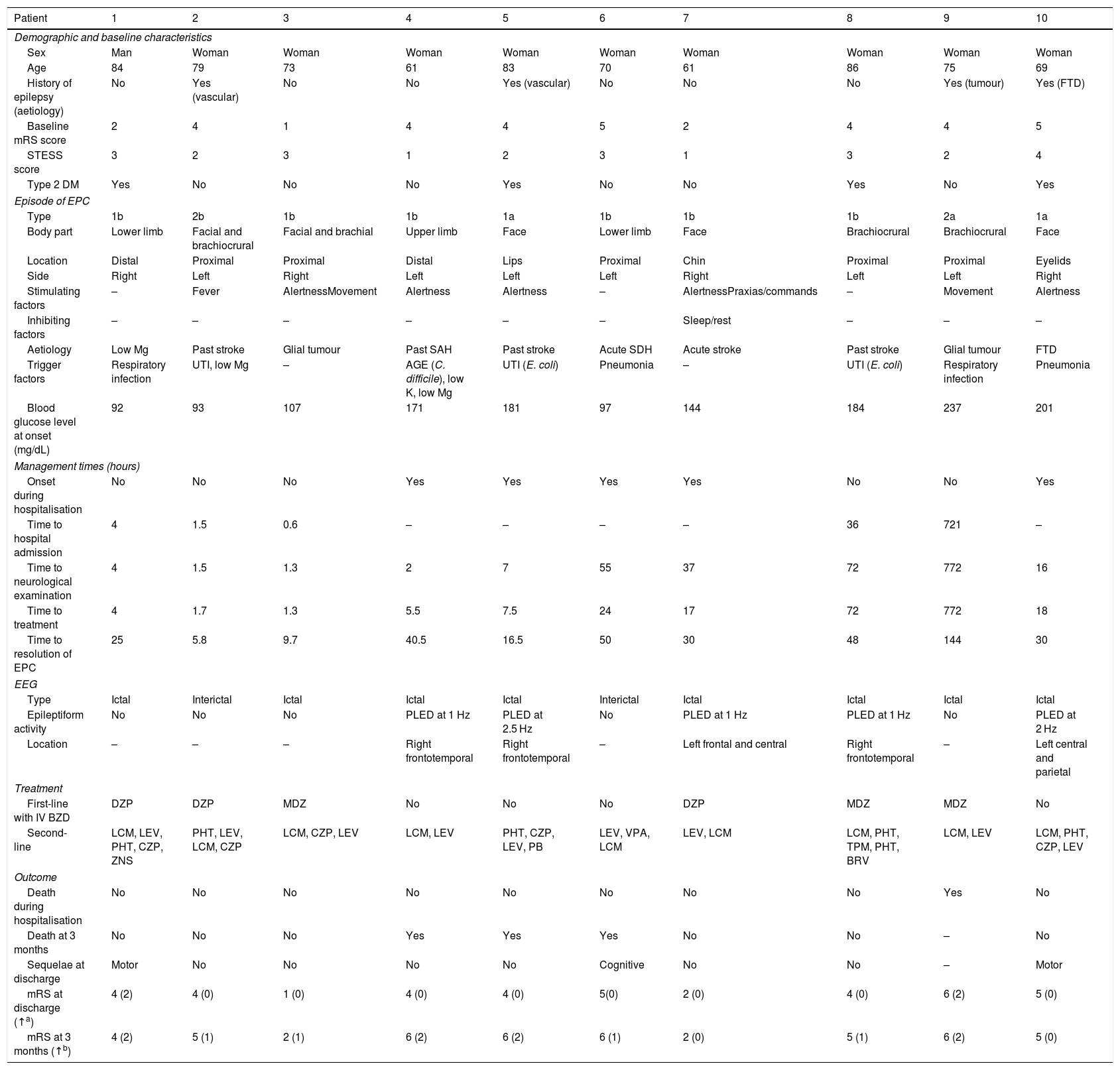
ResultsDuring the study period, a total of 51 patients were admitted to our hospital with a diagnosis of status epilepticus, 10 of whom presented EPC. Table 2 provides descriptive data for our series.
Descriptive results from our series.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Demographic and baseline characteristics | ||||||||||
| Sex | Man | Woman | Woman | Woman | Woman | Woman | Woman | Woman | Woman | Woman |
| Age | 84 | 79 | 73 | 61 | 83 | 70 | 61 | 86 | 75 | 69 |
| History of epilepsy (aetiology) | No | Yes (vascular) | No | No | Yes (vascular) | No | No | No | Yes (tumour) | Yes (FTD) |
| Baseline mRS score | 2 | 4 | 1 | 4 | 4 | 5 | 2 | 4 | 4 | 5 |
| STESS score | 3 | 2 | 3 | 1 | 2 | 3 | 1 | 3 | 2 | 4 |
| Type 2 DM | Yes | No | No | No | Yes | No | No | Yes | No | Yes |
| Episode of EPC | ||||||||||
| Type | 1b | 2b | 1b | 1b | 1a | 1b | 1b | 1b | 2a | 1a |
| Body part | Lower limb | Facial and brachiocrural | Facial and brachial | Upper limb | Face | Lower limb | Face | Brachiocrural | Brachiocrural | Face |
| Location | Distal | Proximal | Proximal | Distal | Lips | Proximal | Chin | Proximal | Proximal | Eyelids |
| Side | Right | Left | Right | Left | Left | Left | Right | Left | Left | Right |
| Stimulating factors | – | Fever | AlertnessMovement | Alertness | Alertness | – | AlertnessPraxias/commands | – | Movement | Alertness |
| Inhibiting factors | – | – | – | – | – | – | Sleep/rest | – | – | – |
| Aetiology | Low Mg | Past stroke | Glial tumour | Past SAH | Past stroke | Acute SDH | Acute stroke | Past stroke | Glial tumour | FTD |
| Trigger factors | Respiratory infection | UTI, low Mg | – | AGE (C. difficile), low K, low Mg | UTI (E. coli) | Pneumonia | – | UTI (E. coli) | Respiratory infection | Pneumonia |
| Blood glucose level at onset (mg/dL) | 92 | 93 | 107 | 171 | 181 | 97 | 144 | 184 | 237 | 201 |
| Management times (hours) | ||||||||||
| Onset during hospitalisation | No | No | No | Yes | Yes | Yes | Yes | No | No | Yes |
| Time to hospital admission | 4 | 1.5 | 0.6 | – | – | – | – | 36 | 721 | – |
| Time to neurological examination | 4 | 1.5 | 1.3 | 2 | 7 | 55 | 37 | 72 | 772 | 16 |
| Time to treatment | 4 | 1.7 | 1.3 | 5.5 | 7.5 | 24 | 17 | 72 | 772 | 18 |
| Time to resolution of EPC | 25 | 5.8 | 9.7 | 40.5 | 16.5 | 50 | 30 | 48 | 144 | 30 |
| EEG | ||||||||||
| Type | Ictal | Interictal | Ictal | Ictal | Ictal | Interictal | Ictal | Ictal | Ictal | Ictal |
| Epileptiform activity | No | No | No | PLED at 1 Hz | PLED at 2.5 Hz | No | PLED at 1 Hz | PLED at 1 Hz | No | PLED at 2 Hz |
| Location | – | – | – | Right frontotemporal | Right frontotemporal | – | Left frontal and central | Right frontotemporal | – | Left central and parietal |
| Treatment | ||||||||||
| First-line with IV BZD | DZP | DZP | MDZ | No | No | No | DZP | MDZ | MDZ | No |
| Second-line | LCM, LEV, PHT, CZP, ZNS | PHT, LEV, LCM, CZP | LCM, CZP, LEV | LCM, LEV | PHT, CZP, LEV, PB | LEV, VPA, LCM | LEV, LCM | LCM, PHT, TPM, PHT, BRV | LCM, LEV | LCM, PHT, CZP, LEV |
| Outcome | ||||||||||
| Death during hospitalisation | No | No | No | No | No | No | No | No | Yes | No |
| Death at 3 months | No | No | No | Yes | Yes | Yes | No | No | – | No |
| Sequelae at discharge | Motor | No | No | No | No | Cognitive | No | No | – | Motor |
| mRS at discharge (↑a) | 4 (2) | 4 (0) | 1 (0) | 4 (0) | 4 (0) | 5(0) | 2 (0) | 4 (0) | 6 (2) | 5 (0) |
| mRS at 3 months (↑b) | 4 (2) | 5 (1) | 2 (1) | 6 (2) | 6 (2) | 6 (1) | 2 (0) | 5 (1) | 6 (2) | 5 (0) |
AED: antiepileptic drug; AGE: acute gastroenteritis; BRV: brivaracetam; BZD: benzodiazepines; CZP: clonazepam; DZP: diazepam; EPC: epilepsia partialis continua; FTD: frontotemporal dementia; low K: hypokalaemia; low Mg: hypomagnesaemia; LCM: lacosamide; LEV: levetiracetam; MDZ: midazolam; mRS: modified Rankin Scale; PB: phenobarbital; PHT: phenytoin; PLED: periodic lateralised epileptiform discharges; SAH: subarachnoid haemorrhage; SDH: subdural haematoma; STESS: Status Epilepticus Severity Score; TPM: topiramate; UTI: urinary tract infection; VPA: valproic acid; ZNS: zonisamide.
Median age (Q1-Q3) in our sample was 74 years (69-82); 9 patients were women. At baseline, the median mRS and STESS scores were 4 (2.5-4) and 2.5 (2-3), respectively. Four patients had history of epilepsy, which was not refractory in any case; these patients were using a median of one AED (1-2).
All patients presented motor symptoms, and the most frequent type of EPC was 1b (acute symptomatic EPC in patients with no history of epilepsy; n = 6). The body part most frequently involved was the face (n = 3). The most frequent aetiology of EPC was cerebrovascular disease (n = 6); these lesions were old in 4 patients and recent in 2. EPC was secondary to glioblastoma multiforme in 2 cases and presented in the context of frontotemporal dementia in one case; in the latter case, no structural cause was found and the episode was attributed to severe hypomagnesaemia (0.7 mg/dL).
Half of the episodes of EPC presented while the patients were hospitalised for other reasons. Median time to admission for patients with pre-hospital EPC onset was 4hours (1.5-36). In the whole sample, median time to neurological examination was 11.5hours (2.5-50.5) and median time to treatment was 12.3hours (4.4-22.5).
All patients underwent at least one EEG study, and 8 underwent prolonged video EEG monitoring. Median duration of video EEG monitoring was 4hours (1-6.5). In 8 patients, EEG studies were performed during the episode of EPC: 5 patients presented periodic lateralised epileptiform discharges at a median of 1.5Hz (1-2.3), whereas the remaining 3 did not present EEG patterns compatible with status epilepticus (2 presented focal slow activity, which was initially generalised, and another patient showed a normal EEG trace). In the remaining 2 patients, the EEG study was performed after the episode had resolved, and no epileptiform activity was detected. Diagnosis of EPC was certain in the 5 patients showing EEG patterns not compatible with status epilepticus, since they presented unequivocal symptoms of EPC.
In all cases, determination of blood glucose level at onset of EPC was considered to have been performed under fasting conditions, since more than 4 hours had passed since the last meal. Six patients presented hyperosmolar nonketotic hyperglycaemia (blood glucose level ≥ 126 mg/dL) at the onset of the episode, with a median blood glucose level of 182.5mg/dL (164.3-210). Of these, 3 patients had history of type 2 diabetes mellitus, 2 presented acute hyperglycaemia secondary to pneumonia, and one was diagnosed with type 2 diabetes mellitus during hospitalisation.
Six patients received intravenous benzodiazepines as the first-line treatment: 3 patients received diazepam and 3 were treated with midazolam. The median number of AEDs used for second-line treatment was 3.5, and the drug most frequently used as the first second-line treatment was lacosamide (n = 6), at a median dose of 450mg/day (300-600). The 2 most frequently used AEDs were lacosamide and levetiracetam; both were administered to 9 of the 10 patients. None of the patients received third-line treatment with anaesthetics or required admission to the intensive care unit.
Regarding progression, EPC was controlled in all cases, with a median time to resolution of 30hours (14.8-48.5). Seven patients presented complications (median of 3.5 per patient), with the most frequent being pneumonia (n = 5), followed by metabolic alterations (n = 4) and encephalopathy (n = 3, due to phenytoin in 2 patients and to combination therapy with clonazepam and brivaracetam in another).
Only one patient died during hospitalisation, after the episode was controlled, due to complications (aspiration pneumonia affecting the base of the right lung, and severe acute and chronic malnutrition). Of the 9 surviving patients, 6 recovered completely and 3 presented sequelae (motor sequelae attributable to the aetiology of EPC in 2 cases and cognitive sequelae in one). In one patient, sequelae resulted in a 2-point decrease in the mRS score at 3 months after discharge. Median mRS score at discharge was 4 (3.5–5).
At 3 months after discharge, 4 patients had died. Of the remaining 6 patients, 4 scored a median of one point lower on the mRS (1-1.8) than at baseline, and 2 showed no change from baseline scores. Median mRS score at 3 months after discharge was 5 (3.5-6).
Correlation between time to resolution and other variablesTime to resolution showed a strong positive correlation with time to admission in patients with pre-hospital onset of EPC (Spearman rho = 0.90; P = .037). In the whole series, we observed a strong positive correlation between time to resolution and time to neurological examination (Spearman rho = 0.84; P = .002) and time to treatment (Spearman rho = 0.88; P < .001). Time to resolution was also found to be moderately correlated with blood glucose level at onset of EPC (Spearman rho = 0.71; P = .11).
Likewise, linear regression revealed a significant association between time to resolution and the remaining time variables (time to admission, time to neurological examination, and time to treatment): time to resolution increased by 0.2 hours for each one-hour increment in time to admission (P = .007), time to neurological examination (P < .001), or time to treatment (P < .001).
Time to resolution was not correlated with any other quantitative (age, baseline mRS score, number of AEDs, STESS score) or qualitative variable (age, history of diabetes mellitus, history of epilepsy, type of EPC, setting at EPC onset, delay in identifying EPC, use of benzodiazepines as first-line treatment, mortality, or sequelae).
Correlation between mortality and other variablesOnly one patient died during hospitalisation. This patient presented the longest times of the series, with a time to admission of 721 hours (vs median of 2.8 hours in survivors [0.8-28]), a time to treatment of 772hours (vs 7.5hours [2.9-21], and a time to resolution of 144hours (vs 30hours [13.1-44.3]). However, the correlation could not be analysed statistically since only this one patient died during hospitalisation.
Mortality at 3 months was significantly higher among patients not receiving benzodiazepines as the first-line treatment (P = .018), and was also higher among patients with in-hospital onset of EPC, although this difference was not statistically significant (P = .071).
Neither mortality during hospitalisation nor mortality at 3 months was correlated with other variables.
Correlation between morbidity and other variablesPresence of sequelae at discharge seems to be associated with higher STESS scores (P = .057). Female sex was associated with increased risk of higher mRS scores at discharge (P = .046).
No correlation was observed between morbidity at discharge or at 3 months and other quantitative or qualitative variables.
DiscussionOur results reveal delays at different stages of EPC management with respect to the times recommended by international guidelines on the management of status epilepticus (no specific guidelines for EPC have been issued due to a lack of published data).7,15–17 Furthermore, delays in management times (time to admission, time to neurological examination, and time to treatment) have been associated with longer EPC duration.
Previous studies have analysed the correlation between progression time and prognosis of status epilepticus. In 1994, Towne et al.18 reported higher mortality rates among patients with status epilepticus lasting over an hour. This threshold was decreased to 30 minutes in 2005 by Eriksson et al.19 in a study including children with status epilepticus, and replicated in 2016 by Cheng20 in adult patients. In 2018, our research group published the first prospective study including time as a continuous quantitative variable.21 We observed considerable diagnostic and treatment delays in the management of adults with non-convulsive status epilepticus, with admission and treatment delays being strongly correlated with duration of status epilepticus. The present study provides further evidence of the correlation between treatment delay and duration of status epilepticus, in this case EPC.
In the light of the above, we would have expected to find an association between delayed resolution of the episode and higher mortality. However, we were unable to test this hypothesis due to the small size of our sample and the fact that only one patient died. Interestingly, that patient presented the longest management times, although this may simply be coincidence. Mortality increased considerably at 3 months of follow-up. This fact, together with the long management times observed in our series, suggests that EPC is comparable to other types of status epilepticus in terms of severity, underscoring the need for early treatment.
In our series, the most frequent aetiology of EPC was cerebrovascular disease, both ischaemic and haemorrhagic, with EPC occurring either immediately after the cerebrovascular event or long afterwards. Other articles report different results, with cerebrovascular aetiology being rare9 or at least considerably less frequent than in our series.10,22 Furthermore, we found a high percentage of patients with uncomplicated hyperosmolar hyperglycaemia at onset of EPC, and a positive correlation between blood glucose level and time to resolution. However, the median blood glucose level in our sample was lower than those reported in other studies, with the highest value being below 250 mg/dL. All patients presenting hyperglycaemia at the onset of EPC showed structural brain lesions on neuroimaging studies that explained EPC. Therefore, hyperglycaemia may have acted as a trigger rather than as an aetiological factor in our series, unlike in other studies that found hyperglycaemia to be the most or one of the most frequent aetiologies.9,10
Although our study was not designed to analyse epidemiological aspects of EPC, we were able to estimate the relative prevalence of the condition among all cases of status epilepticus, using data from the prospective registry of status epilepticus of our centre. During the study period, a total of 51 patients with status epilepticus were admitted to our hospital, showing a relative prevalence of EPC of 19.6%. Although this is an indirect estimate, it represents a high proportion of the total number of cases of status epilepticus, which stands in contrast with the low prevalence estimates reported in other studies.8 This may be explained by the fact that EPC is underdiagnosed, as reported previously,12,23 or because ours is a reference hospital with a stroke unit, which increases the prevalence of cerebrovascular disease in our centre.
The small size of the sample and our study’s single-centre design prevent us from extrapolating our results. These factors, together with the small number of deaths during follow-up, constitute limitations for our statistical analysis. Furthermore, gathering outcome data at discharge increases the likelihood of information bias, since the moment of discharge may be heterogeneous and be influenced by factors linked to hospitalisation and other personal circumstances. The prospective design of our study was intended to compensate for the imprecision inherent to time variables.
ConclusionsEPC is a type of status epilepticus with high relative prevalence; in our series, the most frequent aetiology was cerebrovascular disease. We found a high percentage of patients with hyperglycaemia at onset of EPC; blood glucose level was positively correlated with time to resolution of the episode. However, all patients with hyperglycaemia at onset of EPC showed structural brain lesions explaining the episode, which suggests that high blood glucose levels act as a trigger rather than as an aetiological factor. Lastly, management times in our series were long, which resulted in longer duration of status epilepticus.
FundingNone.
Conflicts of interestThe authors have no conflicts of interest to declare.
Dr Álvaro Gutiérrez Viedma was awarded the Fernando de Castro grant for young post-residency researchers by Universidad Complutense de Madrid.
Please cite this article as: Gutiérrez-Viedma Á, Romeral-Jiménez M, Serrano-García I, Parejo-Carbonell B, Cuadrado-Pérez ML, Sanz-Graciani I, et al. La relevancia del tiempo en la epilepsia parcial continua. Neurología. 2022;37:263–270.