Much has been published on syringomyelia related to Chiari malformation. In contrast, little is known about the condition when it is not associated with this malformation, but this presentation of syringomyelia constitutes a different entity and therefore requires specific management. We conducted a literature review to summarise the most accepted and widespread ideas about the pathophysiology, management and other aspects of syringomyelia unrelated to Chiari malformation.
DevelopmentWe reviewed the most relevant literature on this condition, focusing on the pathophysiology, clinical presentation, diagnosis, and treatment.
ConclusionsSyringomyelia unrelated to Chiari malformation is a distinct entity that must be well understood to guarantee correct diagnosis, monitoring, and management. When the disease is suspected, a thorough study should be conducted to identify its aetiology. Treatment must aim to eliminate the cause of the disease; symptomatic treatment should remain a second-line option.
Son muchos los conocimientos y publicaciones existentes sobre la siringomielia relacionada con la malformación de Chiari, pero existe poca difusión de este cuadro cuando no se presenta en relación con dicha malformación. Ello es importante ya que es una entidad propia que precisa de un conocimiento y manejo específico. Presentamos esta revisión con el objetivo de dar a conocer las ideas más aceptadas y difundidas a día de hoy al respecto de la fisiopatología, manejo y otros aspectos de la siringomielia no secundaria a malformación de Chiari.
DesarrolloSe ha realizado una revisión de la literatura más relevante en torno a esta patología, centrándose en su fisiopatología, presentación clínica, estudio diagnóstico y manejo.
ConclusionesLa siringomielia no relacionada con malformación de Chiari es una entidad propia que precisa de un conocimiento adecuado en su profundidad para su sospecha, seguimiento y manejo adecuado. Ante el hallazgo de este cuadro debe realizarse un estudio detallado encaminado a intentar identificar la causa, quedando el tratamiento sintomático como opción de rescate.
Syringomyelia is almost inherently thought to be accompanied by Chiari malformation, due to the large body of literature on the association between these 2 entities. However, syringomyelia is a distinct entity that may be caused by a wide range of conditions. This literature review focuses on the aetiopathogenesis of syringomyelia and the different treatment options for syringomyelia unrelated to Chiari malformation.
Syringomyelia is frequently defined as the development of an expansive, fluid-filled cyst within the spinal cord. Although this is the most widely accepted definition, some authors question its accuracy as it does not consider such conditions as hydromyelia (abnormal widening of the central canal with CSF accumulation, regarded by many authors as a preliminary stage of syringomyelia) or non-pathological widening of the central canal.1 The central canal in these patients is typically linear and fusiform, with a maximum diameter of 2-4mm on the axial plane, usually decreasing with age.
DevelopmentAetiologyBatzdorf1,2 coined the term primary or idiopathic syringomyelia to describe symptoms of syringomyelia in the absence of abnormalities at the level of the foramen magnum. This term has been used more broadly in the literature in reference to symptoms with no specific cause of abnormal CSF circulation.3 One of the most frequent types of secondary syringomyelia is type I Chiari malformation, which accounts for nearly 50% of cases. Spinal cord injury is the cause most frequently addressed in the literature, with syringomyelia developing in 0.5%-4.5% of patients; this rate may be as high as 30%, according to autopsy results from these patients.4 A third group includes infectious syringomyelia, which is most frequent in patients with meningitis secondary to tuberculosis and listeriosis.5 Syringomyelia may also be iatrogenic, in the context of arachnoiditis secondary to surgical or diagnostic procedures (lumbar puncture, myelography). All these scenarios have a common pathogenic mechanism: obstruction of normal CSF circulation.
Risk factorsSome features may be regarded as risk factors for syringomyelia in certain groups. Among iatrogenic risk factors, for example, greater quantities of blood at the surgical site or multiple traumatic punctures are associated with increased risk of fibrosis, which may block CSF circulation. The risk factors for post-traumatic syringomyelia are the most frequently studied. Presence of complete spinal cord injury is probably the most significant risk factor. el Masry and Biyani6 and Curati et al.7 suggest that complete spinal cord lesions (grade A on the American Spinal Injury Association Impairment Scale) double the risk of clinical syringomyelia. Other known risk factors for this type of syringomyelia are spinal canal stenosis >25% and post-traumatic kyphosis >15°. Presence of these risk factors does not imply a need for surgery to prevent the development of syringomyelia.4
PathophysiologyOver the years, numerous theories have attempted to explain the pathogenesis of syringomyelia. We still lack a theory capable of explaining all the possible scenarios that may cause syringomyelia. Although the intramedullary pulse pressure theory proposed by Greitz8 seems to fulfil this goal, it is yet to be universally accepted; some researchers continue to support other theories that have already been rejected by the scientific community.
Traditional and recent theories agree that syringomyelia results from abnormal CSF circulation within the spinal subarachnoid space. Early theories postulated that abnormal CSF circulation was caused by spinal trauma secondary to an ischaemic lesion, leading to spinal cystic degeneration and the formation of a large cavity.9 According to other theories, atrophy of the spinal cord parenchyma causes the central canal to widen and force the remaining parenchyma outward, compressing CSF circulation through the subarachnoid space. The CSF would then be unable to travel down the spinal cord and would therefore enter the parenchyma, accumulating and forming a fluid-filled cavity. These theories prevailed for a long time but are no longer supported as they are physically implausible. If CSF flow stopped, liquid would accumulate above the obstruction, increasing the pressure in that area and leading to CSF accumulation outside the spinal cord, which would compress the parenchyma.10
Other outdated theories have suggested a congenital origin associated with defects in differentiation and neural tube closure11; according to the theories postulated by Gardner12 and Williams,13 syringomyelia is caused by alterations in CSF circulation in the fourth ventricle or the foramen magnum; Heiss and Oldfield propose a similar theory in the context of Chiari malformation, according to which the movement of the cerebellar tonsils has a piston-like effect.
The previously mentioned intramedullary pulse pressure theory, postulated by Greitz in 1995, is probably the most widely accepted and serves as the basis for most current articles. In addition to explaining the symptoms independently of the cause, this theory presents the best performing and currently most widely used treatment option. The intramedullary pulse pressure theory is based on models initially designed to explain the origin and development of chronic hydrocephalus in adults. Before presenting it in detail, we will explain several important yet frequently overlooked concepts. The spinal cord parenchyma shares many physical properties with that of the brain; repeated microdistensions alter its compliance and elasticity (as occurs with brain tissue in chronic hydrocephalus in adults). Contrary to popular belief, CSF is mainly absorbed by capillaries.14
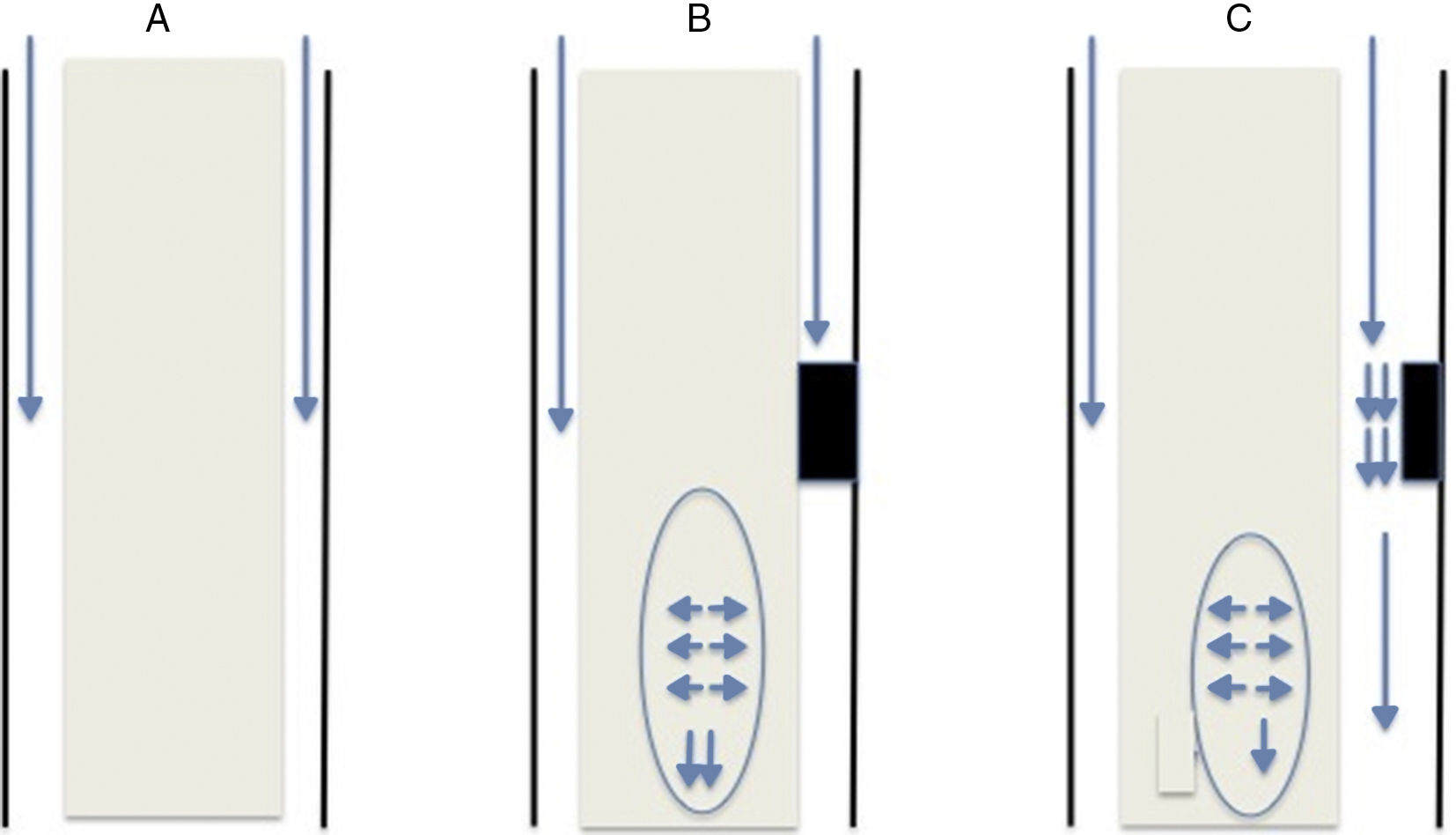
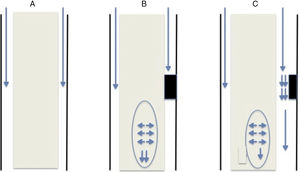
This theory, like previous ones, is based on the obstruction of CSF flow through the spinal subarachnoid space. Obstruction is usually secondary to fibrosis/arachnoiditis and tends to occur in the posterior aspect of the spinal cord: from an anatomical viewpoint, this area contains septa of arachnoid tissue running from the spinal cord to the dura mater,15 which are not as frequent in the anterior aspect. The obstruction may be classified as follows (Fig. 1):
- •
Complete obstruction: when the CSF pressure pulse wave reaches the blocking point, it is transmitted to the spinal cord and continues to descend. Pressure in the subdural space is lower immediately below the obstruction since CSF is not flowing at that point; therefore, the spinal cord parenchyma, which presents increased pressure due to the centrifugal force, widens to fill this space. Not all pressure is transmitted caudally; part of it may be transmitted cranially, which would explain the presence of cysts above the level of the obstruction.
- •
Partial obstruction: when the obstruction is incomplete and allows some CSF flow. This may lead to 2 different physical phenomena. The first is an increase in CSF flow speed due to the narrowness of the channel, resulting in decreased pressure in that region (Bernoulli's principle). The second phenomenon is the Venturi effect, according to which a fluid flowing at a high speed creates a suction effect. Both situations result in dilatation of the parenchyma below the obstruction.
Once the parenchyma is dilated, the extracellular space increases, allowing CSF accumulation. This accumulation compresses the capillary circulation, which has 2 consequences. First, it increases the size of the Virchow–Robin spaces, which forces CSF out of the vessels through which it flows. And second, it decreases the effective surface size of the capillaries that absorb the CSF; CSF therefore remains in the extracellular space, leading to CSF accumulation and a progressive increase in the size of the cavity.9
SymptomsLittle research has been published on the natural history of syringomyelia, especially regarding those cases unrelated to Chiari malformation. Research on post-traumatic syringomyelia is noteworthy. This type of syringomyelia frequently presents with sudden clinical deterioration in patients with previously stable spinal cord lesions; symptoms appear a mean of 9 years after the initial lesion.4 Sensory alterations, pain, and motor weakness constitute the most frequent form of presentation.
Syringomyelia is not associated with specific symptoms. The most common form of presentation is central cord syndrome; this largely depends on the location of the syrinx. Manifestations include ulcers, alterations in temperature sensitivity, and signs of lower motor neuron dysfunction. Bogdanov et al.15 aimed to determine whether syringomyelia secondary to Chiari malformation and that unrelated to Chiari malformation caused different symptoms. In that series, manifestations occurred at similar frequencies regardless of the group. The most frequent symptom was segmental sensory loss (93%), followed by pyramidal signs (82%) and muscle atrophy (60%).
No correlation has been established between syrinx size and location and symptom severity.16 Regarding the clinical manifestations mentioned above, there is only weak evidence in favour of surgical treatment when the patient presents muscle weakness. Paraesthesia or pain do not justify surgery since response to the procedure is not greatly favourable in these patients. Likewise, surgery is not recommended in patients with syringes showing radiological progression but causing no symptoms.4 Follow-up neurophysiological studies may help in treatment decision-making as they may reveal signs of progressive worsening that may not be clinically apparent.
DiagnosisDiagnosis must always be derived from clinical suspicion. Syringomyelia is usually suspected in patients with stable spinal cord lesions or a history of neurological deficits, secondary to spinal cord processes or surgery, who display sudden, progressive clinical deterioration and whose symptoms progress at a slower pace than at onset. There currently is no gold standard for the diagnosis of syringomyelia, although we may extrapolate some general ideas from the literature.
CT-myelography has traditionally been the most frequently used diagnostic test for syringomyelia.17 The technique is no longer in use for several reasons. Firstly, this invasive test may cause iatrogenic syringomyelia as it requires a lumbar puncture, which may cause trauma, and contrast injection, which may induce inflammation and fibrosis. Secondly, most obstructions of CSF circulation in the spinal cord occur in the posterior aspect due to the presence of arachnoid webs, which are not found in the anterior aspect of the spinal cord. This uneven distribution allows the contrast agent to circulate through the anterior aspect, completely filling the subarachnoid space, potentially resulting in false negatives.
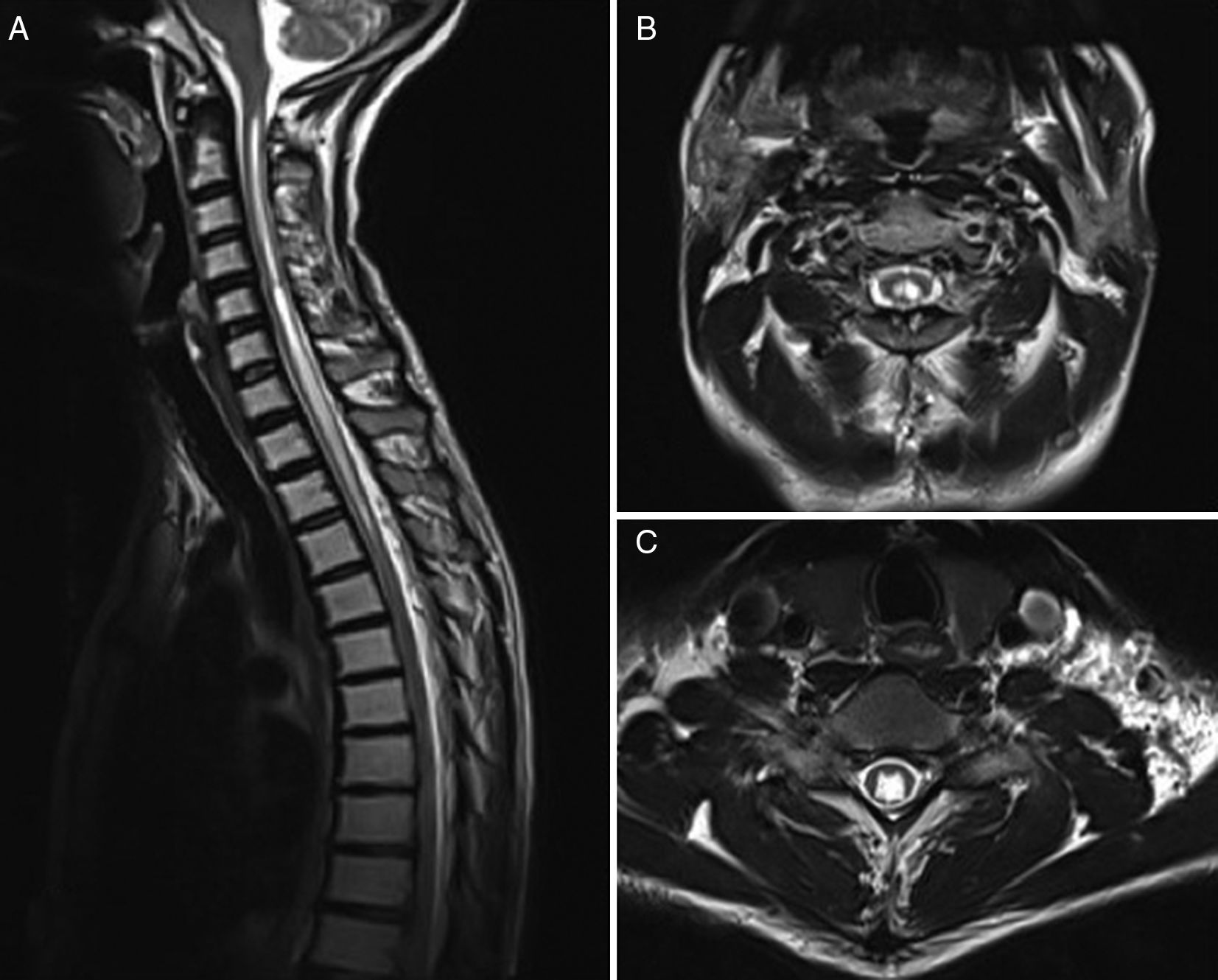
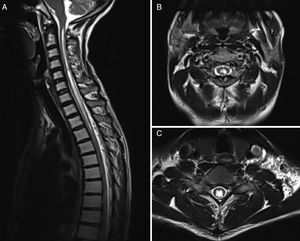
MRI is currently the most widely used imaging technique (Fig. 2), and is complemented by cine MRI. The latter technique detects CSF movement and is extensively used for the study of such other conditions as hydrocephalus, but is rarely used in this field. Mauer et al.18 published a series of over 300 patients with syringomyelia; the cause was unknown in half of the cases. In this subgroup, myelography only identified the point of blockage in 2 patients, whereas cine MRI detected blockage in 33; no clear obstruction was observed in the remaining patients. This study shows the superiority of cine MRI for identifying syringomyelia.
Although some researchers, such as Inoue et al.,19 have suggested that the location of the obstruction may be identified based on syrinx site, shape, and size, it is generally agreed that this is not possible using MRI only.18 No data have been published on such parameters as sensitivity and specificity of the technique. Although cine MRI has been found to be superior to myelography or CT-myelography, it fails to identify the location of the obstruction in a high percentage of cases.
We should note the usefulness of intraoperative echography, mentioned by many authors and used routinely during surgery in many centres. This type of echography helps identify the point of CSF obstruction in many patients in whom the obstruction could not be identified previously, enabling the most appropriate intervention to be designed based on the findings.20 Neurophysiological studies are highly useful for follow-up, enabling the evaluation of any changes that may require a different therapeutic approach.
In summary, when a patient displays radiological signs of a syrinx, physicians should aim to determine the location of CSF flow disruption.
TreatmentMultiple treatment options and strategies have been described; these include conservative treatment, symptomatic treatment, and symptomatic or aetiological surgical treatment. Most studies support aetiological treatment in view of the high rate of poor outcomes and recurrence associated with symptomatic treatment, despite initially positive results.
Spontaneous resolution has been reported in several published cases. Sudo et al.21 were probably the first to describe one of these cases of spontaneous resolution, in 1990. Since then, up to 37 cases have been reported in different series22; although most of these are secondary to Chiari malformation, some are unrelated.23,24 These cases mainly correspond to children, although spontaneous resolution has also been reported in elderly patients. Possible explanations include enlargement of the posterior fossa, age-related atrophy of the cerebellar tonsils, spontaneous destruction of the walls obstructing CSF circulation, and opening of the cyst to the subdural space. Some published cases report improvements with conservative treatment, such as physiotherapy techniques specifically developed for post-traumatic syringomyelia and for cervical curve correction. Patients with isolated symptoms, such as pain or sensory alterations, should receive conservative treatment.
Surgery may be classified into 2 types, depending on the purpose of the intervention: symptomatic surgery, aimed at emptying the syrinx by draining the CSF into other cavities through tubes; and aetiological surgery, which aims to identify the exact location of the obstruction and restore normal CSF circulation. CSF shunting to the pleural cavity or the subdural space is the most widely used symptomatic surgical technique. We should highlight arachnolysis among aetiological surgical techniques; other techniques include spinal cordectomy or bypassing the obstruction.
CSF shunting to the pleural cavity was first performed by Abbe in 1892.25 Although in the field of neurosurgery CSF has traditionally been diverted to the peritoneal cavity, it is usually diverted to the pleural cavity in the context of syringomyelia as this space is closer and the procedure is easier to perform.26 Several studies report favourable short-term outcomes for symptom management and patient function.27,28 CSF may also be diverted to the subdural space. This procedure has been used for many years as it is easier to perform than other techniques; the literature reports good immediate outcomes, with improvements in 72.5% of patients and a low morbidity rate.29,30 It does have limitations, however, as it is not possible to ensure proper CSF flow through the catheter or the absence of flow reversal.
In recent years, these techniques have been criticised for their high recurrence rates (up to 92% at 3 years31) and the need for additional surgical procedures and frequent follow-up consultations. Studies comparing the efficacy of shunting and such other techniques as arachnolysis place much emphasis on this issue. Ghobrial et al.32 reviewed articles published until 2015, finding a total of 410 surgery patients. The authors concluded that older age and the type of intervention (patients undergoing shunting are 7 times more likely to experience recurrences than those treated with arachnolysis) are significantly associated with symptom recurrence.
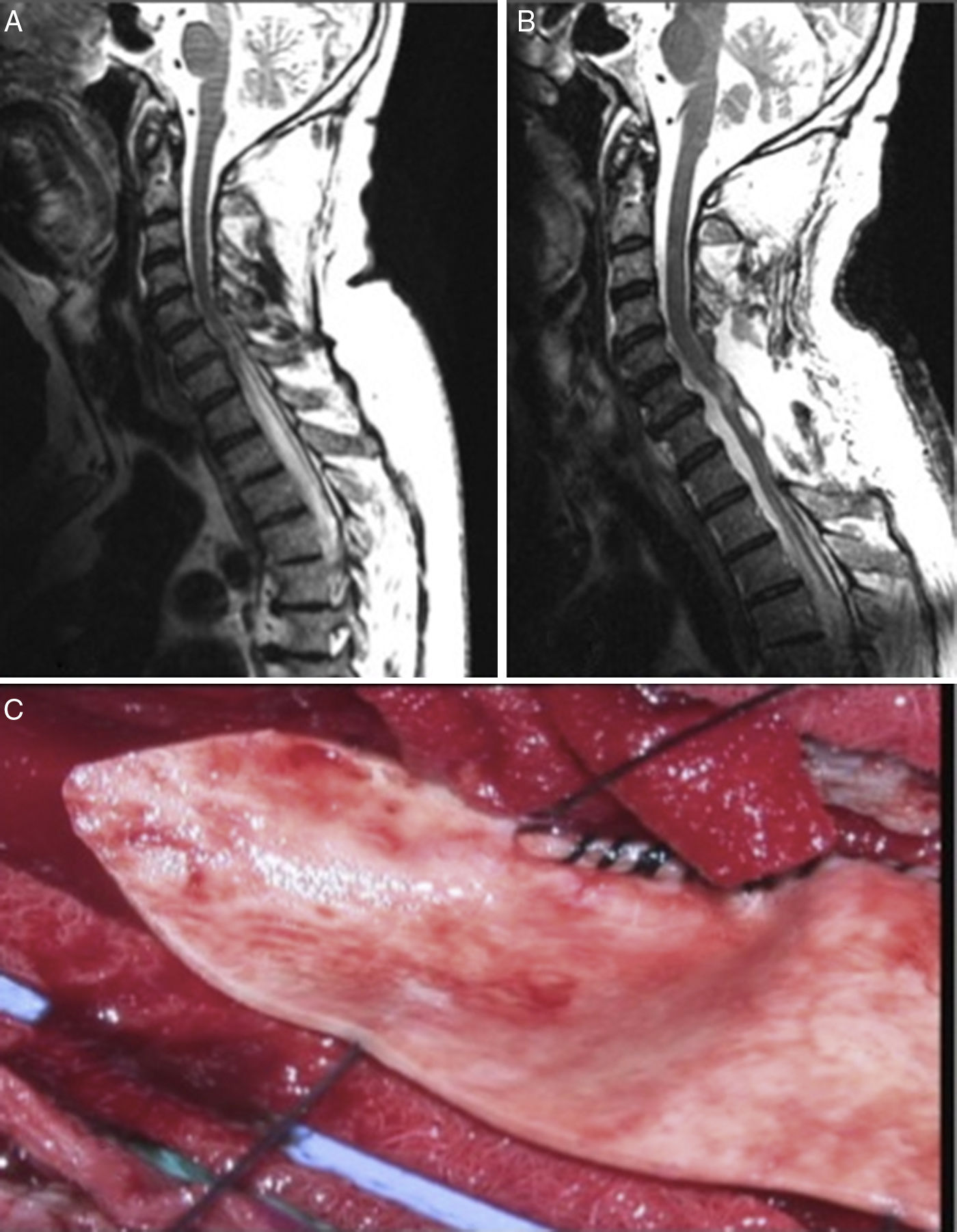
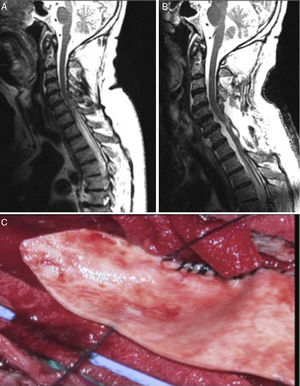
In view of the high rate of poor long-term outcomes associated with CSF shunting, techniques aimed at restoring normal CSF flow (arachnolysis) have become the aetiological treatment of choice. The results of the series published by Klekamp31 are noteworthy. The authors used the following surgical approach: they first performed a laminectomy or laminoplasty at the level affected by the cyst. They then performed an intraoperative echography to identify the location of CSF flow obstruction and opened the dura mater immediately above that point. Subsequently, arachnolysis was performed to destroy adhesions caudally until healthy tissue was found, in order to restore CSF flow. Finally, duraplasty was performed to increase the space available for CSF (Fig. 3).
Klekamp et al.31 classified their patients according to whether arachnoid scarring was focal (<2 levels) or extensive. Patients displayed post-surgical improvements in 95% of cases; 5- and 10-year recurrence rates were 34% and 40%, respectively. Outcomes were much more favourable in the patients with focal arachnoid scarring, with recurrence rates of 17% and 22%, compared to 63% and 69% in the group with extensive arachnoid scarring. Another series published by Klekamp20 focused on post-traumatic syringomyelia; several techniques were used in the same centre, as the surgeon decided to replace shunting with arachnolysis. That series shows that patients undergoing shunting usually need closer follow-up and confirms the clinical effectiveness of both interventions; which show differences in the duration of the effects.
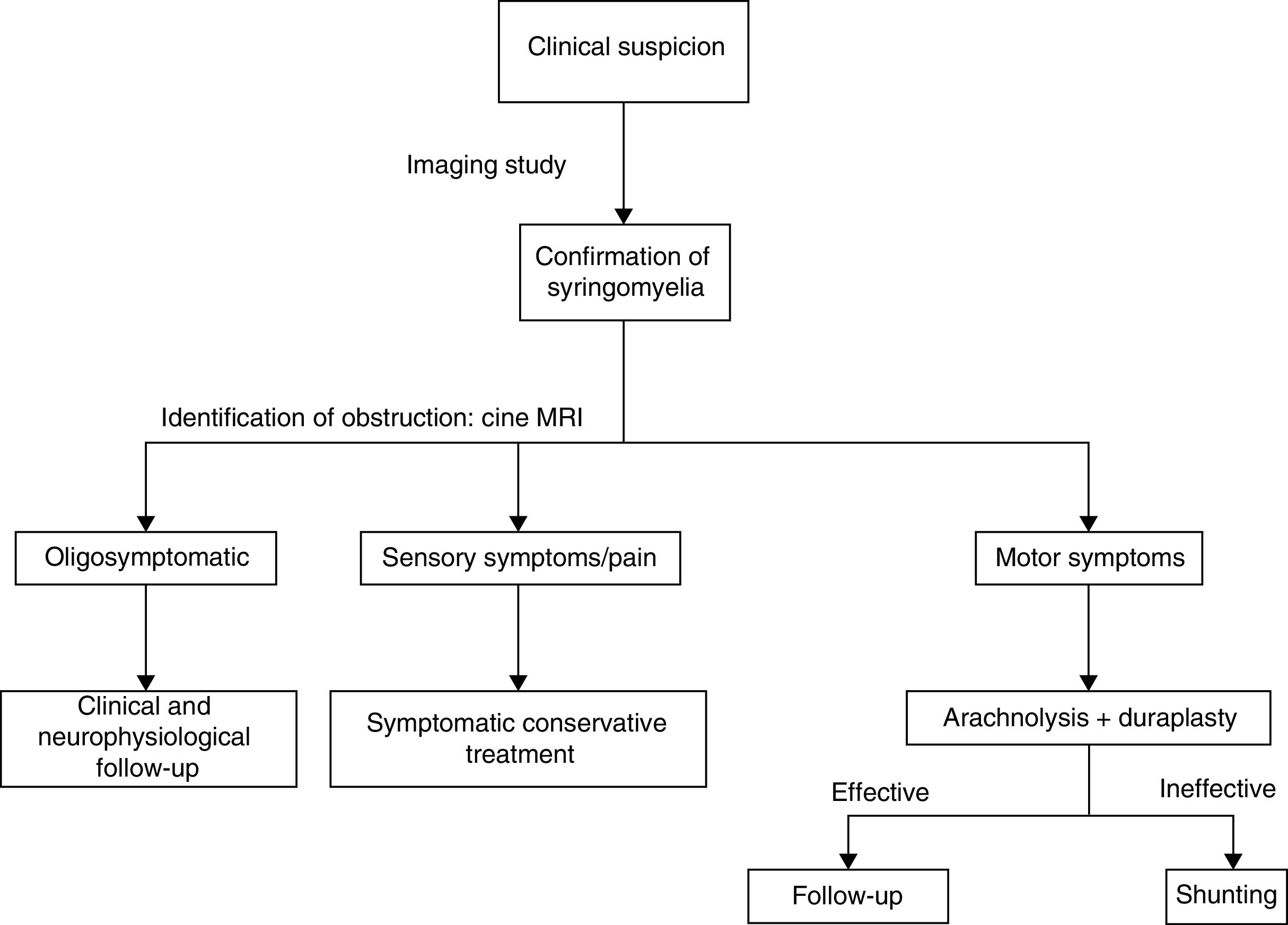
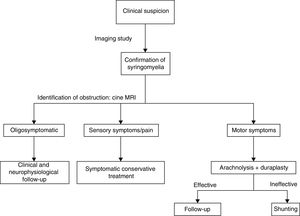
This explains why shunting is now only used as rescue therapy when arachnolysis cannot be performed or has failed. Cordectomy is another rescue technique33 that restores CSF flow and on occasion even enables the communication between the syrinx and the subdural space. This procedure should always be considered the last resort due to its potential psychological impact, and should only be used in patients with complete spinal cord injury. We should also mention other alternative techniques, such as subarachnoid–subarachnoid bypass, in which a tube is inserted into the subdural space to bypass the obstruction cranially and caudally, allowing CSF circulation.34 This technique has been found to have similar results to those of other techniques. The management strategy used in our centre prioritises aetiological treatment, based on the evidence available (Fig. 4).
ConclusionsThough rare, syringomyelia unrelated to Chiari malformation is a distinct entity whose aetiopathogenesis requires further study. A deeper understanding of the condition may help physicians determine which patients are eligible for surgery and choose the most appropriate option for aetiological treatment, as this approach has been found to be more effective than other options in the long term.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Giner J, Pérez López C, Hernández B, Gómez de la Riva Á, Isla A, Roda JM. Siringomielia no secundaria a Chiari. Actualización en fisiopatología y manejo. Neurología. 2019;34:318–325.