The ‘wine glass’ appearance refers to a characteristic sign wherein the coronal T2-weighted imaging (T2-WI) sequences show hyperintensities of the corticospinal tracts bilaterally.1 This sign has been infrequently observed in motor neuron diseases,1 and rarely in adult-onset leukodystrophies (especially in Krabbe disease),2,3 osmotic demyelination syndrome (ODS),4 celiac disease,5 Lyme neuroborreliosis,6 and human T-cell lymphotropic virus type 1 (HTLV-1) infection,7 among others. The ‘wine glass’ sign has not been described following receipt of any type of vaccine.
We herein report the first case of ‘wine glass’ pattern following COVISHIELD vaccination (ChAdOx1 nCoV-19; recombinant, replication-deficient chimpanzee adenovirus vector vaccine).
Case reportA 47-year-old previously healthy man from rural West Bengal (India) was brought to the emergency department with abrupt onset rapidly progressive stiffness of bilateral lower limbs and clumsiness of both upper limbs for the last five days. It was associated with slurring of speech, gait unsteadiness, and behavioral changes in the form of inappropriate laughter, crying spells and inappropriate intermittent anger outbursts. According to his family members, these symptoms started within five days of the second dose of COVID-19 vaccination (COVISHIELD). There was no cognitive impairment, dysphagia, nasal regurgitation, sensory, visual, or sphincter disturbance. He denied trauma or exposure to neurotoxins, radiation, or drug abuse/addiction. His family history was noncontributory. Neurological examination revealed normal mental status with emotional lability and brisk jaw jerk. There was spastic-ataxic dysarthria with a spastic tongue, without atrophy or fasciculations. Motor system examination showed bilateral symmetrical spasticity involving all four limbs. The muscle power was 4/5 in lower limbs and 5/5 in upper limbs. All deep tendon reflexes were exaggerated. Gait was spastic-ataxic and tests for several cerebellar functions were abnormal. There was no sensory or autonomic dysfunction.
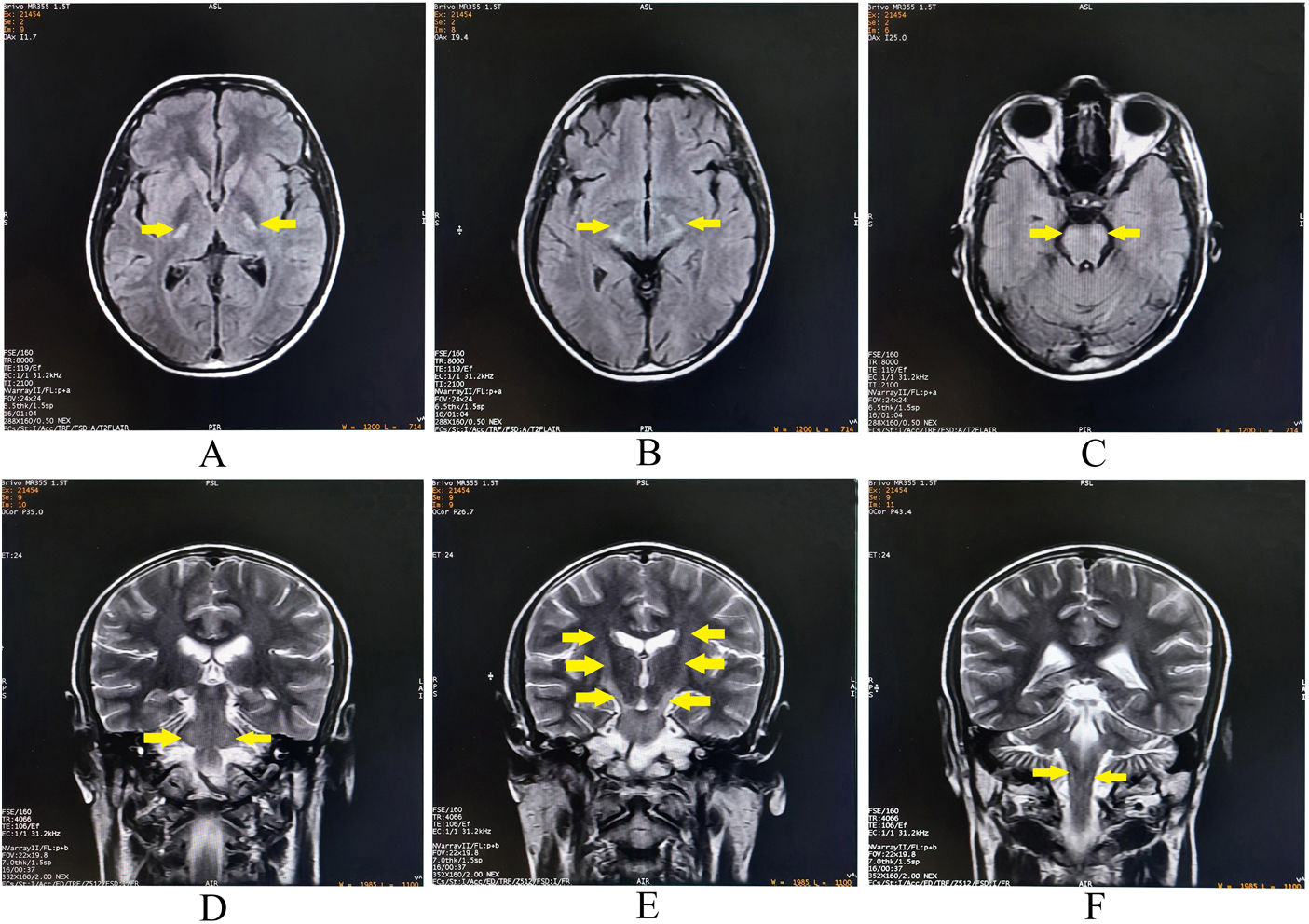
Complete blood cell count, blood glucose, electrolytes, and renal, liver and thyroid function tests, were normal. 1.5T brain magnetic resonance imaging (MRI) (performed on the second day of hospital admission, i.e., seven days after the receipt of the COVISHIELD vaccine) revealed linear, bilateral symmetrical hyperintensities involving the corticospinal tracts on T2-WI, giving a ‘wine glass’ appearance, when seen in the coronal plane (Fig. 1). It also revealed increased T2 signal in the middle cerebellar peduncles, which contain the frontocerebellar tracts (connecting to orbitofrontal and dorsolateral prefrontal cortex) (Fig. 1). No other additional supra-and-infra-tentorial lesions could be identified. MRI of the spinal cord (Fig. 2) and optic nerves were unremarkable. Cerebrospinal fluid study revealed lymphocytic pleocytosis (16cells/μl, all lymphocytes), increased protein (62mg/dL), and mildly increased IgG synthesis without oligoclonal bands. Anti-AQP-4-antibody and anti-myelin oligodendrocyte glycoprotein (MOG)-antibody tests were negative. Tests for syphilis, human immunodeficiency virus (1,2), neurosarcoidosis, neuro-Bechet's, antinuclear antibody profile, and vasculitis and anti-thyroid antibody profile, were negative. The visual evoked potentials were normal. A paraneoplastic work-up including bone survey and contrast enhanced computerized tomographic scan of the thorax and abdomen were also normal. A thorough clinical and electrophysiological examination including nerve conduction study and needle electromyography of all limbs did not reveal any evidence for a motor neuron involvement. He was prescribed bolus intravenous methylprednisolone therapy (1g/day for five days) to which his symptoms responded dramatically in respect to decrease in the pseudobulbar affect, spasticity and ataxia. During discharge, he had no demonstrable neurological deficits except persistent brisk deep tendon reflexes. At three month of follow-up, he had shown no clinical stigmata of either recurrent attack/relapse or worsening of the preexisting neurological deficits; however, mild hyperreflexia persisted.
Brain magnetic resonance imaging revealing bilateral symmetrical hyperintense lesions on axial T2-weighted-fluid-attenuated inversion recovery involving posterior limb of internal capsules (A), crus of midbrain (B), and basis pontis (C). The lesions are hyperintense on coronal T2-weighted images at bilateral middle cerebellar peduncles (D), and along the corticospinal tracts from internal capsules to basis pontis (E, F).
Our patient had bilateral symmetrical hyperintensities involving the corticospinal tracts on coronal T2-WI sequences (‘wine glass’ sign). Wallerian degeneration of the corticospinal tracts in motor neuron diseases (amyotrophic lateral sclerosis and primary lateral sclerosis) remain the most common causes associated with this sign.1,8,9 In a cohort of patients with Krabbe disease, Muthusamy et al.3 observed that this disease may present as isolated involvement of corticospinal tract (resembling the ‘wine glass’ appearance on coronal T2-WI). A case of osmotic demyelination syndrome-associated ‘wine glass’ sign was described by Saroja et al.,4 in a postpartum young female with hypernatremia. The patient presented with fever, altered sensorium and progressive quadriparesis, which recovered completely over a period of seven months.4 Turner et al.5 reported a patient with progressive pure motor hemiparesis, corticospinal tract hyperintensities and denervation pattern on electromyography, mimicking amyotrophic lateral sclerosis, who eventually was diagnosed to be a case of celiac disease.5 The pathogenic mechanism of damage to corticospinal tracts in this disease is unclear. Perivascular inflammation mediated breakdown of the blood–brain barrier, resulting in influx of cross-reacting antibodies (e.g., anti-tissue transglutaminase 2 immunoglobulin A antibodies), could be a possibility.5 Yeung et al.6 reported a 60-year-old man with Lyme neuroborreliosis with progressive gait disturbance, involuntary weight loss, spastic-ataxic paraparesis, sensorimotor axonal polyneuropathy, and ‘wine glass’ sign on MRI. More recently, Gonzalez Trujillo F. et al.7 reported the case of a previously healthy 49-year-old female with progressive quadriparesis and partial bulbar dysfunction with exalted reflexes, positive bilateral Hoffman's sign, and muscular atrophy with spasticity, due to HTLV-1 infection, along with bilaterally symmetrical hyperintensities involving corticospinal tracts.
The analysis of the history, the temporal course and the neurological examination led us to locate the lesion (topographic diagnosis) somewhere in either the bilateral periventricular white matter or brainstem, affecting corticospinal, corticobulbar and fronto-ponto-cerebellar tracts. However, craniospinal junction can also be a rare, but potential site of lesion in some cases. The etiological differential diagnoses in this case were either (1) an acute vascular event, or (2) an acute-onset demyelinating event, both of them having questionable association with the receipt of vaccine.
Adult-onset leukodystrophies, infective encephalomyelitis, and motor neuron diseases tend to have a subacute to chronic course. Osmotic demyelination syndrome was considered as it can present abruptly, and has been documented to cause such pattern of lesions,4 but absence of rapid changes in plasma osmolality, plasma sodium and glucose levels refuted this possibility. Amid the primary demyelinating disorders of the central nervous system, multiple sclerosis, neuromyelitis optica spectrum disorders as well as MOG-associated disorders were ruled out, as the criteria for those ones were not met. Hence, despite extensive clinical, neuroradiological, biochemical and electrophysiological investigations for exclusion of potential differential diagnoses, COVID-19 vaccine-associated long-tract demyelination remained the sole rational diagnosis.
Both COVID-19 infection itself and vaccination against it have been associated with demyelinating disorders of the central nervous system.10–15 However, acute onset long-tract demyelination predominantly affecting the corticospinal tracts resembling a ‘wine glass’ has not been described to date following any vaccination (including COVID-19 vaccines). Whether there is any causal relationship between COVID-19 vaccination and demyelination is unknown at present, but definitely calls for further large-scale, well-designed international studies.
Authors’ contributionsAll authors contributed significantly to the creation of this manuscript; each fulfilled criteria as established by the ICMJE.
Ethics statementInformed written consent was obtained from the patient involved in this study.
DisclosuresR. Ghosh reports no disclosures relevant to the manuscript.
S. Dutta reports no disclosures relevant to the manuscript.
M. Ghosh reports no disclosures relevant to the manuscript.
J. Benito-León reports no disclosures relevant to the manuscript.
Study fundingNone.
Conflict of interestsThe authors declare that they have no conflict of interest.
J. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), European Commission (grant ICT-2011-287739, NeuroTREMOR), the Ministry of Economy and Competitiveness (grant RTC-2015-3967-1, NetMD—platform for the tracking of movement disorder), and the Spanish Health Research Agency (grant FIS PI12/01602 and grant FIS PI16/00451).