The aim of this research is to present the clinical characteristics of phantom limb pain (PLP) in patients with amputation.
MethodsA retrospective cross-sectional observational study of patients with lower limb amputation is presented. Patients between 18 and 80 years of age with unilateral or bilateral amputation between the years 2015 and 2019 were included. Demographic data, medical history, data related to the amputation, and related abnormal sensations were collected.
Results43 patients (34 men) and 53 amputees were studied, with a mean age of 62 years, with a time elapsed since amputation of 28 months. The most frequent cause of amputation was ischemic (70%). Twenty-three (60%) patients had PLP that began 1 month after amputation with a mean intensity of 3.9 on the VAS scale, in 15 patients the PLP was daily, three patients recognised the disappearance of PLP. 91% of the patients presented non-painful sensations in relation to the phantom limb. No differences were found in the development of the PLP between the 1st and 2nd amputation. A significant association was found between the development of PLP and residual limb pain.
ConclusionsPLP is a prevalent pathology among amputee patients, therefore multidisciplinary care with an active neurologic participation is essential. Studies are needed to deepen the knowledge of the factors that favour the development of PLP in order to focus early and targeted therapies to prevent the appearance of PLP.
El objetivo de este trabajo es presentar las características clínicas del dolor de miembro fantasma (DMF) de pacientes amputados.
MaterialSe presenta un estudio observacional transversal retrospectivo de pacientes con amputación de miembro inferior. Se incluyeron pacientes entre 18 y 80 años con amputación uni o bilateral entre los años 2015 y 2019. Se recogieron datos demográficos, antecedentes médicos, datos relacionados con la amputación y sensaciones anormales relacionados.
ResultadosSe estudiaron 43 pacientes (34 varones) y 53 amputaciones, con una edad media de 6,2 años con un tiempo trascurrido desde la amputación de 28 meses. La causa más frecuente de amputación fue la isquémica (70%). Veintitrés (60%) pacientes presentaban DMF que se inició 1 mes tras la amputación. En 3 pacientes se encontró una resolución completa del DMF. El DMF presentaba una intensidad media de 3,9 en la escala EVA con una frecuencia diaria en 15 pacientes. El 91% de los pacientes presentaban sensaciones no dolorosas en relación con el miembro fantasma. No se encontraron diferencias en el desarrollo del DMF entre la 1ª y 2ª amputación. Se encontró una asociación significativa entre el desarrollo de DMF y el dolor de miembro residual.
ConclusionesEl DMF es una patología prevalente entre pacientes amputados con tendencia a cronificarse por lo que es imprescindible su atención multidisciplinar con la participación neurológica activa. Se precisan estudios que profundicen en el conocimiento de los factores favorecedores del desarrollo del DMF para focalizar terapias precoces y dirigidas para prevenir la aparición de DMF.
The concept of the phantom limb was first proposed in the 16th century by the French surgeon Ambroise Paré. However, it was Weir Mitchell who, in 1872, provided the first detailed description of the phenomenon, using the term “sensory ghost” to refer to these abnormal sensations.1 The term “phantom limb” was later coined by Bailey and Moersch2 in 1941 to describe the development of abnormal sensations in a limb following amputation, the disorder known today as phantom limb syndrome (PLS). Among the abnormal sensations described in the amputated limb, phantom limb pain (PLP) has the greatest impact on the physical, psychological, and economic spheres, as well as on patient quality of life.3,4
Although many years have passed since the first descriptions of this phenomenon, there is still no consensus on the underlying mechanisms of PLP or the most appropriate treatment approach for these painful sensations.5 Schone et al.6 have highlighted the challenges of assessing pain in patients with PLP,6 as these studies have to evaluate the main dimensions of pain, including the sensory, emotional, and cognitive domains. Furthermore, given the unique characteristics of this type of pain, a wide range of factors are involved in the modulation of PLP perception, including genetic, psychological, and sociocultural variables.7,8 Pain assessment is particularly complex in patients with PLP since this pain originates in the amputated limb, and therefore the sensorineural circuits of pain change drastically after amputation. Another challenge in the assessment of PLS is the presence of other abnormal sensations following amputation, such as residual limb pain (RLP) or non-painful phantom sensations (NPPS), which hinder clinical diagnosis of PLP.6
Several studies have investigated the frequency and risk factors for PLP after amputation, with highly variable results. In a recent meta-analysis, Limakatso et al.9 estimated the prevalence of PLP at 64%, with lower rates in developing countries. The authors also identified several risk factors for PLP, including presence of pain before surgery, proximal amputation, lower limb amputation, and presence of RLP or NPPS.9
Therefore, research into the frequency and clinical characteristics of PLP in different sociocultural and economic settings is needed to increase our understanding of this phenomenon. The purpose of this study is to present the frequency, risk factors, and clinical characteristics of PLP in a Spanish population with lower limb amputations.
Material and methodsWe conducted a retrospective, observational, descriptive, cross-sectional study of patients who underwent lower limb amputation at Hospital Universitario Central de Asturias, a tertiary hospital in Spain. We included patients aged 18–80 years who underwent unilateral or bilateral lower limb amputation between 1 January 2015 and 31 December 2019. For patients with bilateral amputation, we also gathered data on the first amputation, regardless of whether it was performed before 2015. We excluded patients with interphalangeal amputations and those with cognitive impairment preventing them from completing the questionnaire. Patients with amputations were identified through the clinical records department, and subsequently contacted by telephone and invited to participate in the study. Interviews took place between November 2019 and February 2020. The questionnaire was completed in person at the hospital, with the exception of 5 patients who were unable to travel and were interviewed at their homes.
The study was approved by the research ethics committee of the region of Asturias (code 86/19). All participants provided written informed consent. Data confidentiality was ensured at all times in accordance with the Declaration of Helsinki and Spanish data protection legislation (Organic Law 3/2018 of 5 December, on the protection of personal data and guarantee of digital rights).
An ad hoc questionnaire was used to gather the following data:
- 1
Demographic data and medical history related to the amputation3,10
- 2
Data related to the amputation (eg, age at the time of amputation, time since the procedure). Level of amputation (hip disarticulation, transfemoral amputation, knee disarticulation, transtibial amputation, ankle disarticulation, or partial foot amputation).11 Cause of amputation (ischaemic, traumatic, infectious, or tumoural).10,12 Use of prosthetic devices.12
- 3
Clinical data on painful or non-painful sensations related to the amputation. Presurgical pain was defined as presence of pain in the limb to be amputated prior to surgery.3,6,9 Postsurgical pain was defined as pain experienced within 7 days of surgery.13 PLS was defined as the set of sensations perceived in the amputated limb.2,12,14 PLP was defined as the set of painful sensations perceived in the amputated limb.14 RLP was defined as the set of chronic painful sensations reported by the patient in the part of the limb that remains after amputation.3,6,9 NPPS was defined as the set of non-painful sensations perceived in the amputated limb.12,14 We analysed the presence, duration, timing of onset, and frequency of each abnormal sensation. Pain intensity was assessed with the visual analogue scale (VAS), which scores pain intensity from 0 (no pain) to 10 (worst imaginable pain).15 PLP was characterised with the Spanish-language Pain Questionnaire,16 a tool based on the validated Spanish-language version of the McGill Pain Questionnaire,17 using pain descriptors grouped into 3 categories: sensory, evaluative, and affective. This scale helps patients to identify and describe their painful sensations using a common language. Based on their experiences, patients were asked about external factors that triggered pain.12,18
- 4
Data related to psychiatric disorders. Presence of depression was assessed using the Hamilton Depression Rating Scale.19 This scale includes 22 items, each scored on a scale from 0 to 4. Total scores are interpreted as follows: 0–7, no depression; 8–13, mild depression; 14–18, moderate depression; 19–22, severe depression; 23–52, very severe depression with suicide risk. Anxiety was evaluated with the Zung Self-Rating Anxiety Scale.20 It consists of 20 items, each scored on a scale from 1 to 4. Total scores are interpreted as follows: 20–28, no anxiety; 29–41, mild anxiety; 42–53, moderate anxiety; 53–80, severe anxiety.
- 5
Treatment-related data. Data were also gathered on pharmacological treatments for the symptomatic management of PLP.5,9,21
We performed a descriptive analysis of the sample. Continuous variables are expressed as mean and standard deviation, and categorical variables as absolute frequencies and percentages. In patients with bilateral amputation, we performed a descriptive analysis of the characteristics of both amputations.
Univariate binary logistic regression models were applied to analyse the association between PLP and other amputation-related factors (eg, presurgical and postsurgical pain, RLP, use of prosthetic devices, depression). Results are presented as odds ratios, with 95% confidence intervals and P values. Statistical significance was set at P < .05.
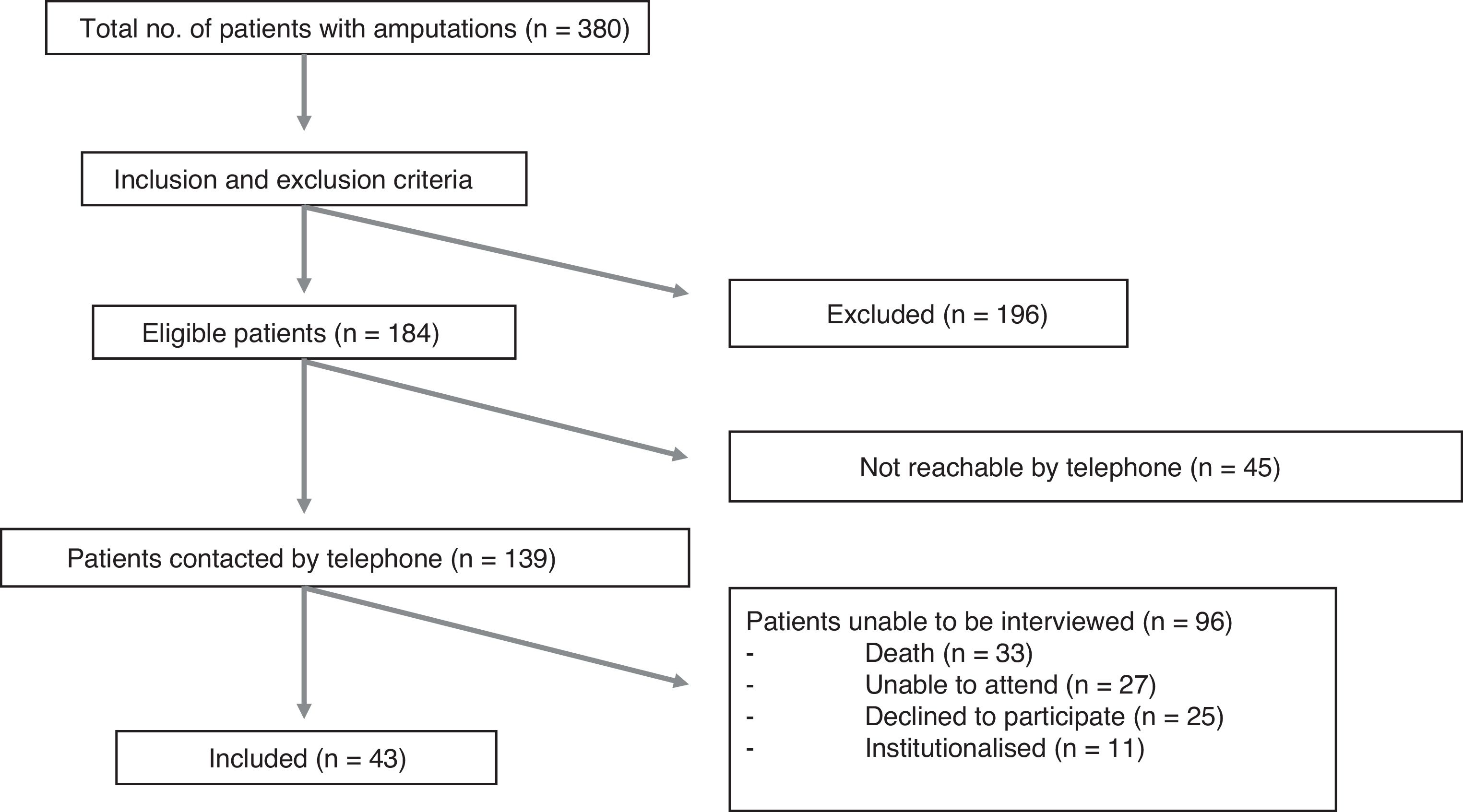
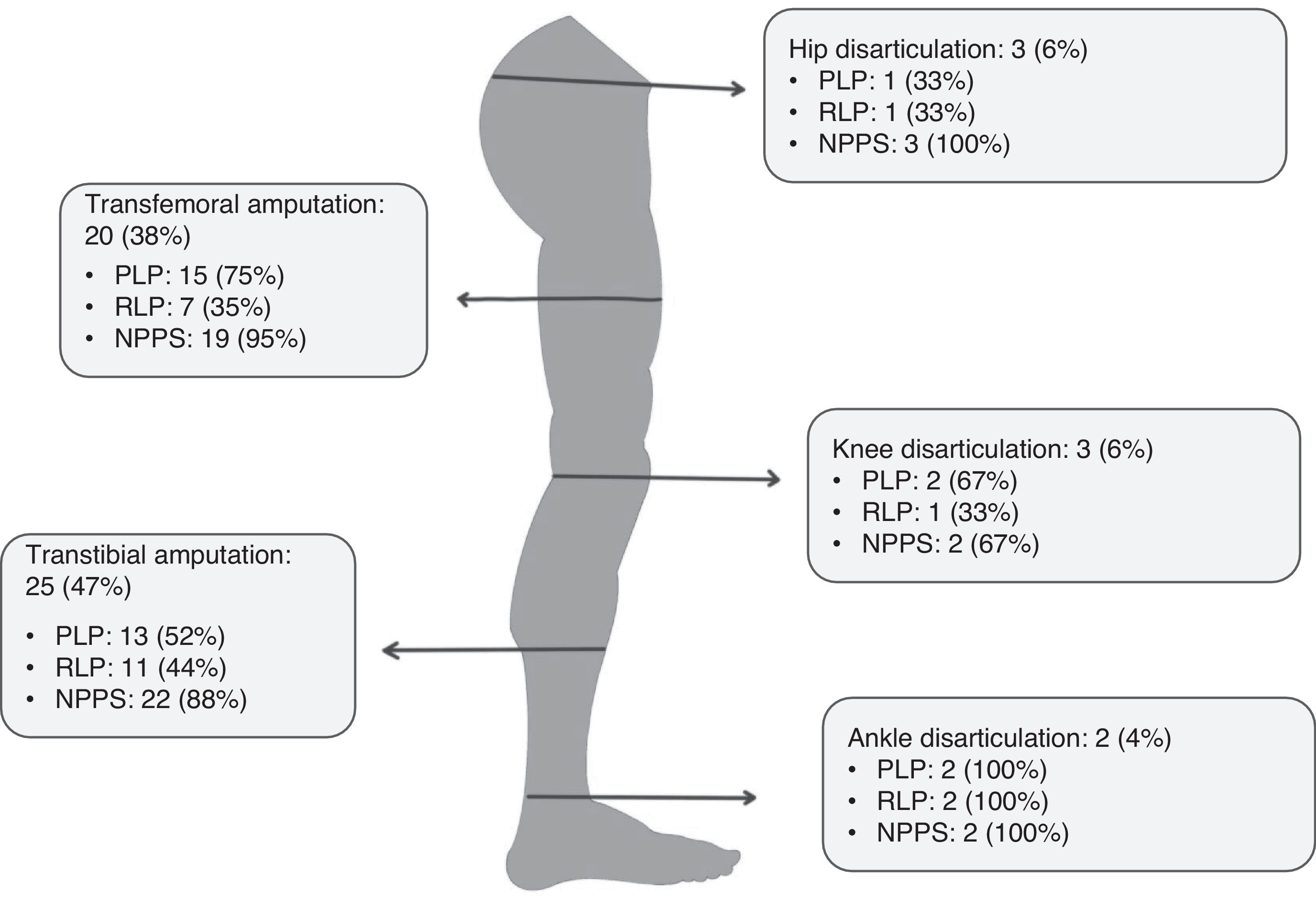
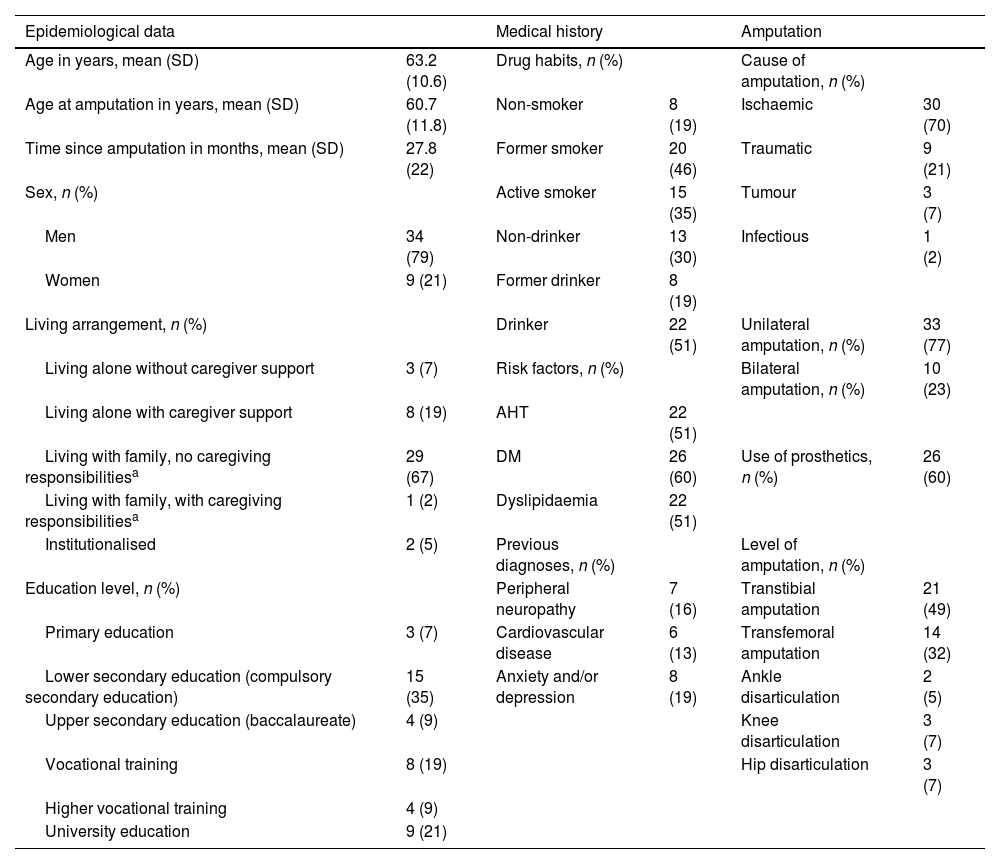
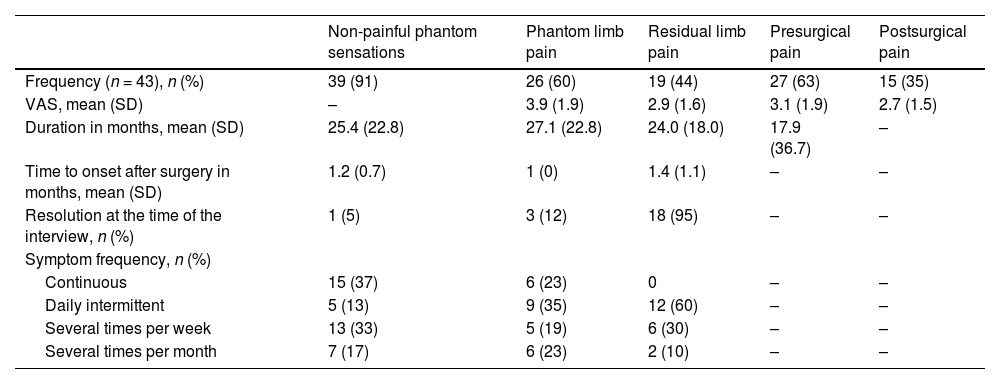
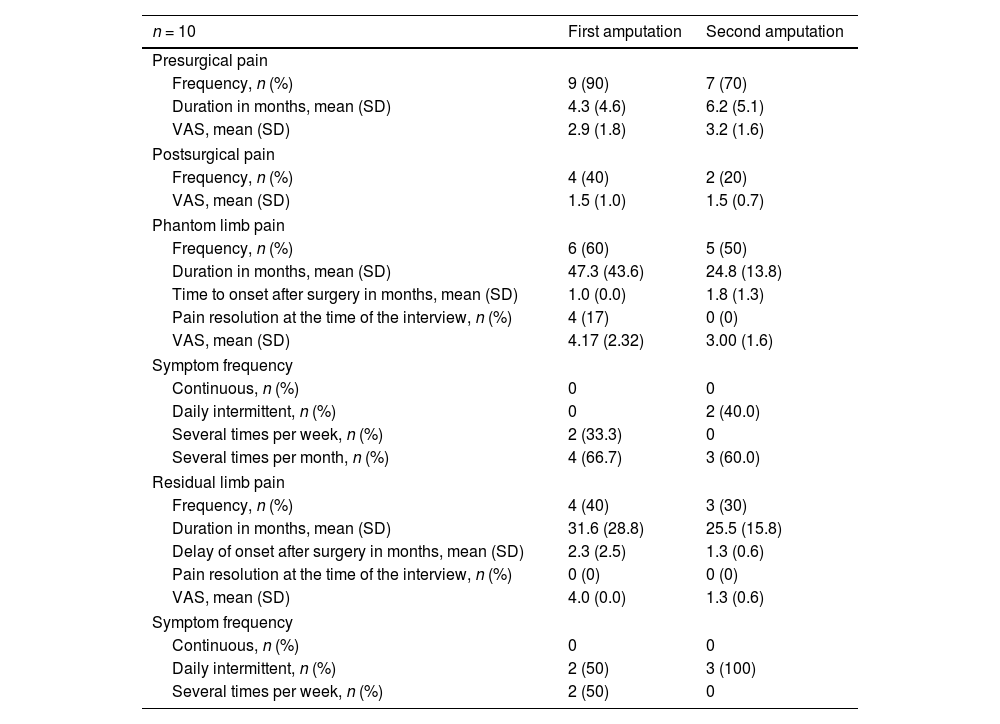
ResultsWe identified 380 patients with lower limb amputations. Fig. 1 describes the patient selection process. Our study included 43 patients, accounting for 53 amputations (10 patients had bilateral amputation). Bilateral amputation was performed in separate surgical procedures in all cases. Table 1 summarises the clinical data related to the first amputation in the 43 patients. All patients presented some type of abnormal sensation related to the amputation, whether painful or non-painful. PLP was reported by 26 patients (60%), of whom only 3 (12%) reported complete resolution of PLP. Table 2 presents the clinical characteristics of symptoms associated with amputation. Nine of the 10 patients with bilateral amputation were men. Mean time between the first and second amputation was 25.7 (30.2) months. Table 3 presents the clinical characteristics of the first and second amputations in the patients with bilateral amputation. Three patients with bilateral amputation underwent the first procedure before 2015. Fig. 2 shows the frequencies of each component of PLS, by level of amputation.
Clinical characteristics of the first amputation in our cohort.
| Epidemiological data | Medical history | Amputation | |||
|---|---|---|---|---|---|
| Age in years, mean (SD) | 63.2 (10.6) | Drug habits, n (%) | Cause of amputation, n (%) | ||
| Age at amputation in years, mean (SD) | 60.7 (11.8) | Non-smoker | 8 (19) | Ischaemic | 30 (70) |
| Time since amputation in months, mean (SD) | 27.8 (22) | Former smoker | 20 (46) | Traumatic | 9 (21) |
| Sex, n (%) | Active smoker | 15 (35) | Tumour | 3 (7) | |
| Men | 34 (79) | Non-drinker | 13 (30) | Infectious | 1 (2) |
| Women | 9 (21) | Former drinker | 8 (19) | ||
| Living arrangement, n (%) | Drinker | 22 (51) | Unilateral amputation, n (%) | 33 (77) | |
| Living alone without caregiver support | 3 (7) | Risk factors, n (%) | Bilateral amputation, n (%) | 10 (23) | |
| Living alone with caregiver support | 8 (19) | AHT | 22 (51) | ||
| Living with family, no caregiving responsibilitiesa | 29 (67) | DM | 26 (60) | Use of prosthetics, n (%) | 26 (60) |
| Living with family, with caregiving responsibilitiesa | 1 (2) | Dyslipidaemia | 22 (51) | ||
| Institutionalised | 2 (5) | Previous diagnoses, n (%) | Level of amputation, n (%) | ||
| Education level, n (%) | Peripheral neuropathy | 7 (16) | Transtibial amputation | 21 (49) | |
| Primary education | 3 (7) | Cardiovascular disease | 6 (13) | Transfemoral amputation | 14 (32) |
| Lower secondary education (compulsory secondary education) | 15 (35) | Anxiety and/or depression | 8 (19) | Ankle disarticulation | 2 (5) |
| Upper secondary education (baccalaureate) | 4 (9) | Knee disarticulation | 3 (7) | ||
| Vocational training | 8 (19) | Hip disarticulation | 3 (7) | ||
| Higher vocational training | 4 (9) | ||||
| University education | 9 (21) | ||||
AHT: arterial hypertension; DM: diabetes mellitus; SD: standard deviation.
Clinical characteristics of the symptoms associated with first amputations in our sample.
| Non-painful phantom sensations | Phantom limb pain | Residual limb pain | Presurgical pain | Postsurgical pain | |
|---|---|---|---|---|---|
| Frequency (n = 43), n (%) | 39 (91) | 26 (60) | 19 (44) | 27 (63) | 15 (35) |
| VAS, mean (SD) | – | 3.9 (1.9) | 2.9 (1.6) | 3.1 (1.9) | 2.7 (1.5) |
| Duration in months, mean (SD) | 25.4 (22.8) | 27.1 (22.8) | 24.0 (18.0) | 17.9 (36.7) | – |
| Time to onset after surgery in months, mean (SD) | 1.2 (0.7) | 1 (0) | 1.4 (1.1) | – | – |
| Resolution at the time of the interview, n (%) | 1 (5) | 3 (12) | 18 (95) | – | – |
| Symptom frequency, n (%) | |||||
| Continuous | 15 (37) | 6 (23) | 0 | – | – |
| Daily intermittent | 5 (13) | 9 (35) | 12 (60) | – | – |
| Several times per week | 13 (33) | 5 (19) | 6 (30) | – | – |
| Several times per month | 7 (17) | 6 (23) | 2 (10) | – | – |
SD: standard deviation; VAS: visual analogue scale.
Clinical characteristics of painful sensations associated with first and second amputations in patients with bilateral amputation.
| n = 10 | First amputation | Second amputation |
|---|---|---|
| Presurgical pain | ||
| Frequency, n (%) | 9 (90) | 7 (70) |
| Duration in months, mean (SD) | 4.3 (4.6) | 6.2 (5.1) |
| VAS, mean (SD) | 2.9 (1.8) | 3.2 (1.6) |
| Postsurgical pain | ||
| Frequency, n (%) | 4 (40) | 2 (20) |
| VAS, mean (SD) | 1.5 (1.0) | 1.5 (0.7) |
| Phantom limb pain | ||
| Frequency, n (%) | 6 (60) | 5 (50) |
| Duration in months, mean (SD) | 47.3 (43.6) | 24.8 (13.8) |
| Time to onset after surgery in months, mean (SD) | 1.0 (0.0) | 1.8 (1.3) |
| Pain resolution at the time of the interview, n (%) | 4 (17) | 0 (0) |
| VAS, mean (SD) | 4.17 (2.32) | 3.00 (1.6) |
| Symptom frequency | ||
| Continuous, n (%) | 0 | 0 |
| Daily intermittent, n (%) | 0 | 2 (40.0) |
| Several times per week, n (%) | 2 (33.3) | 0 |
| Several times per month, n (%) | 4 (66.7) | 3 (60.0) |
| Residual limb pain | ||
| Frequency, n (%) | 4 (40) | 3 (30) |
| Duration in months, mean (SD) | 31.6 (28.8) | 25.5 (15.8) |
| Delay of onset after surgery in months, mean (SD) | 2.3 (2.5) | 1.3 (0.6) |
| Pain resolution at the time of the interview, n (%) | 0 (0) | 0 (0) |
| VAS, mean (SD) | 4.0 (0.0) | 1.3 (0.6) |
| Symptom frequency | ||
| Continuous, n (%) | 0 | 0 |
| Daily intermittent, n (%) | 2 (50) | 3 (100) |
| Several times per week, n (%) | 2 (50) | 0 |
SD: standard deviation; VAS: visual analogue scale.
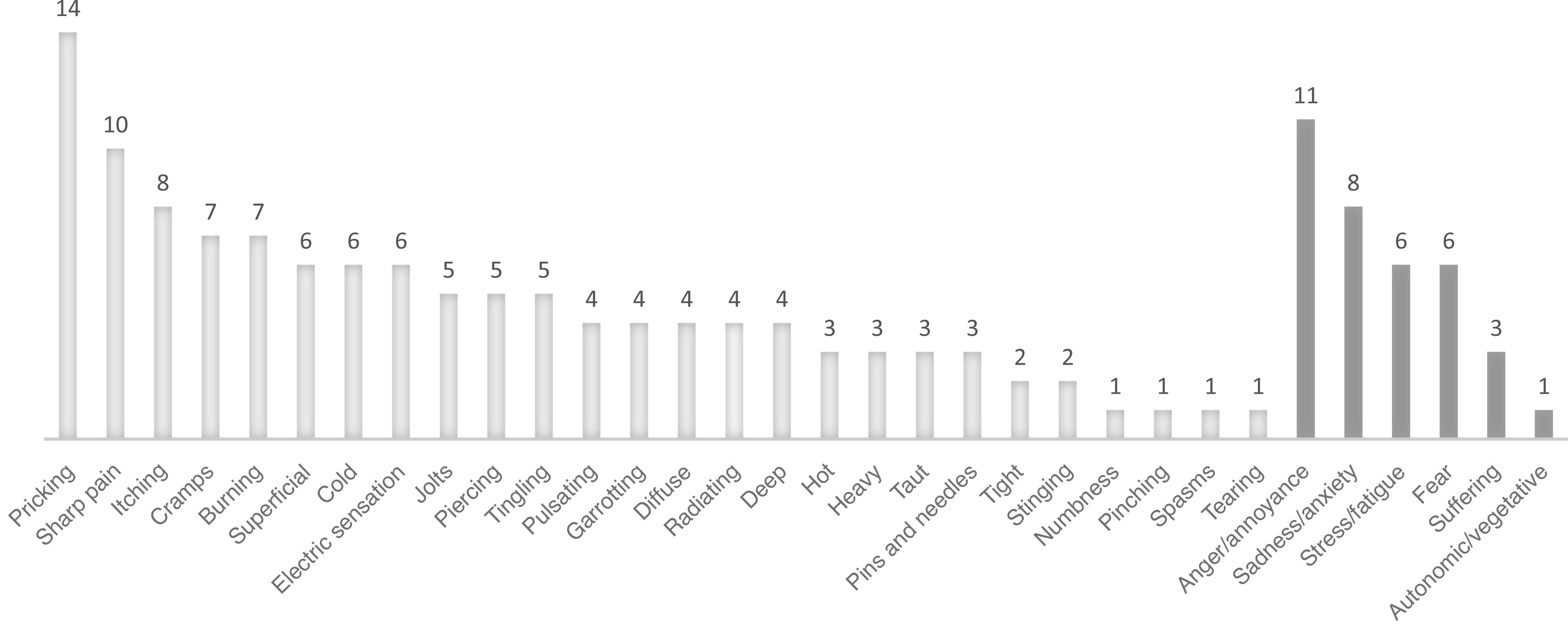
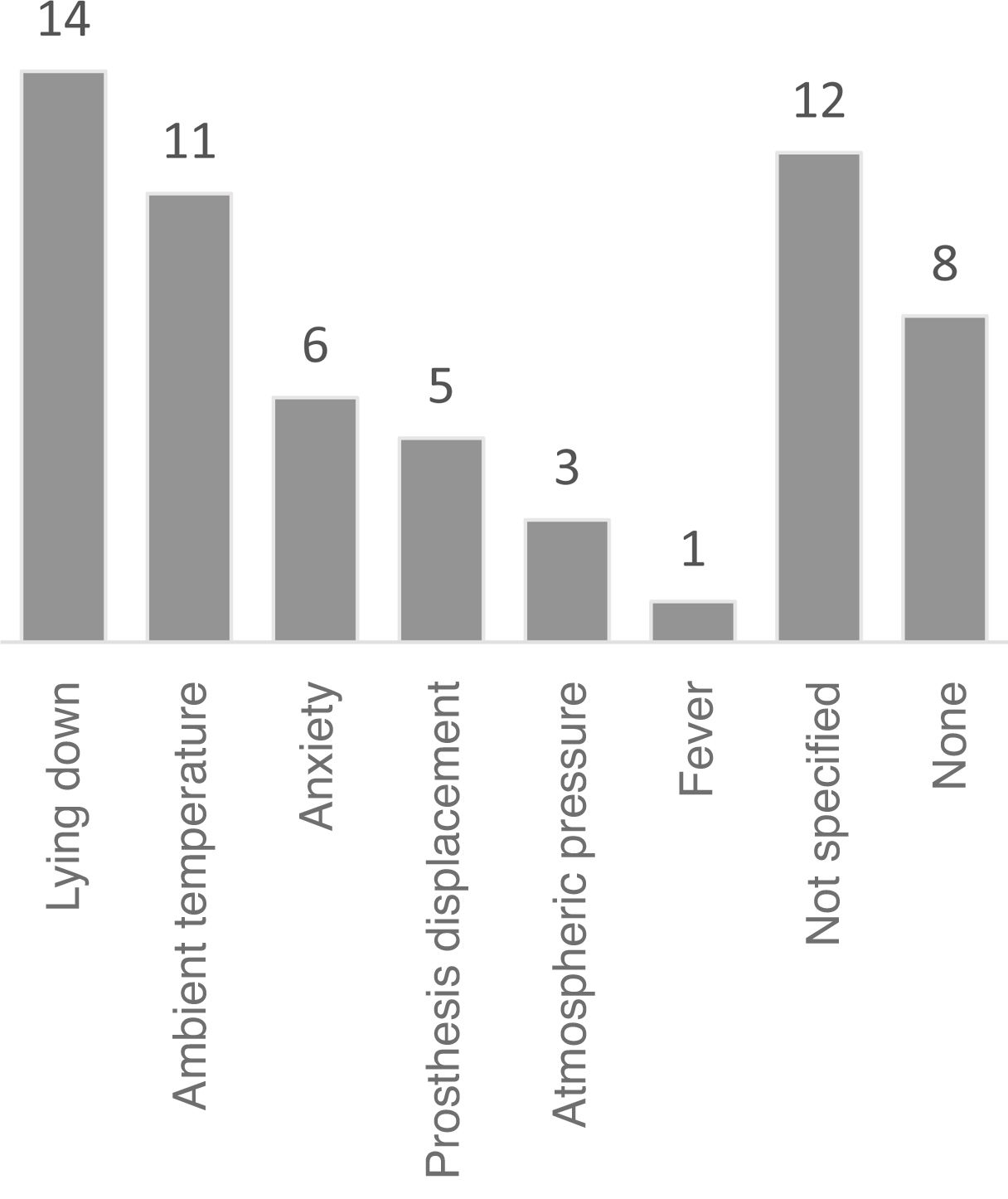
Fig. 3 presents the frequencies of each clinical characteristic of PLP, according to the Spanish-language Pain Questionnaire, by dimension (sensory vs affective/emotional). Fig. 4 presents the most frequent trigger factors for PLP.
Depression was observed in 9 patients (21%) and anxiety in one (2%). Depression and anxiety symptoms were mild in all cases.
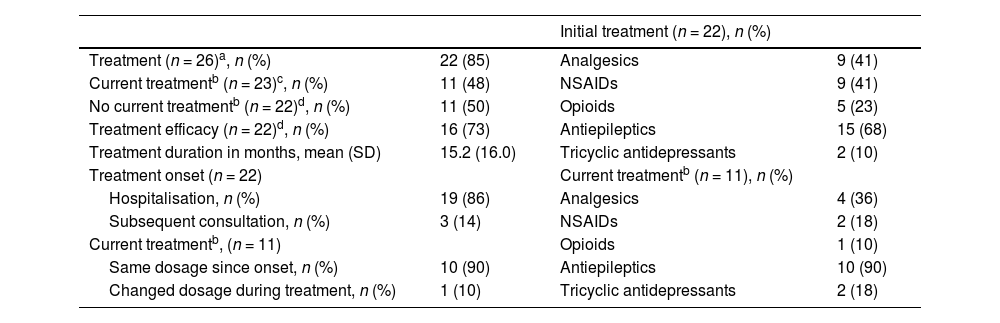
Table 4 summarises the symptomatic treatments used for PLP.
Pharmacological treatment for phantom limb pain.
| Initial treatment (n = 22), n (%) | |||
|---|---|---|---|
| Treatment (n = 26)a, n (%) | 22 (85) | Analgesics | 9 (41) |
| Current treatmentb (n = 23)c, n (%) | 11 (48) | NSAIDs | 9 (41) |
| No current treatmentb (n = 22)d, n (%) | 11 (50) | Opioids | 5 (23) |
| Treatment efficacy (n = 22)d, n (%) | 16 (73) | Antiepileptics | 15 (68) |
| Treatment duration in months, mean (SD) | 15.2 (16.0) | Tricyclic antidepressants | 2 (10) |
| Treatment onset (n = 22) | Current treatmentb (n = 11), n (%) | ||
| Hospitalisation, n (%) | 19 (86) | Analgesics | 4 (36) |
| Subsequent consultation, n (%) | 3 (14) | NSAIDs | 2 (18) |
| Current treatmentb, (n = 11) | Opioids | 1 (10) | |
| Same dosage since onset, n (%) | 10 (90) | Antiepileptics | 10 (90) |
| Changed dosage during treatment, n (%) | 1 (10) | Tricyclic antidepressants | 2 (18) |
NSAID: non-steroidal anti-inflammatory drug; SD: standard deviation.
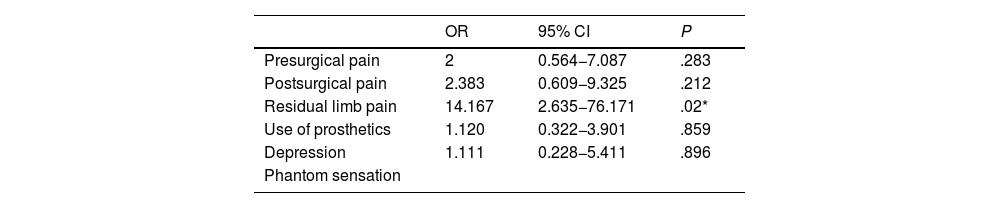
All variables were evaluated as potential risk or protective factors for PLP. The only statistically significant association identified was between RLP and higher frequency of PLP (Table 5). The relationship between phantom sensation and PLP could not be assessed with a logistic regression model due to the lack of patients presenting neither phantom sensation nor PLP. No statistically significant differences were observed between the clinical characteristics of the first and the second amputation.
Univariate logistic regression models analysing the relationship between presence of phantom limb pain and other factors related to the amputation.
| OR | 95% CI | P | |
|---|---|---|---|
| Presurgical pain | 2 | 0.564−7.087 | .283 |
| Postsurgical pain | 2.383 | 0.609−9.325 | .212 |
| Residual limb pain | 14.167 | 2.635−76.171 | .02* |
| Use of prosthetics | 1.120 | 0.322−3.901 | .859 |
| Depression | 1.111 | 0.228−5.411 | .896 |
| Phantom sensation |
The interest of neurology researchers in chronic pain in general, and in neuropathic pain in particular, has increased in recent years; this has led to an active participation of this specialty in the diagnosis and treatment of patients with neuropathic pain, aiming to improve these patients’ quality of life.22
PLP, the focus of this study, is a component of PLS; the latter term encompasses an array of sensations affecting the amputated limb.2,12,14 PLP is frequently classified as neuropathic, since these patients usually report characteristics typically associated with neuropathic pain, such as burning, stabbing, or tingling sensations; however, they also identify such nociceptive features as pressure on the foot or toes.4 This highly prevalent, chronic condition represents one of the main reasons for seeking healthcare among people with amputations, given its significant impact on quality of life.4,23,24
The underlying pathogenic mechanisms are not fully understood. The most widely accepted explanation is maladaptive neuroplasticity25; according to this theory, after amputation, the cortical area previously responsible for the processing of sensorimotor information from the missing limb undergoes neuronal reorganisation. Due to the deprivation of sensory input, this cortical area loses its normal function and undergoes neuronal reorganisation in favour of other body parts. Thus, pre-amputation engrams and control processes may coexist with new conscious and unconscious patterns, involving visual, sensory, and motor afferent projections. In the event of inappropriate reorganisation, the cortical area becomes overactive, leading to the abnormal sensations involved in PLS, and particularly PLP.25–27 Nociplastic pain, first described in 2016, represents a distinct type of chronic pain, separate from nociceptive and neuropathic pain. It is associated with dysfunction of pain-related sensory pathways in the central and/or peripheral nervous system, promoting central sensitisation. Future studies should aim to determine whether PLP has a nociplastic component.28
This study presents a series of patients with lower limb amputations who developed PLS. To our knowledge, this is the first study on PLP conducted in Spain. Unlike previous studies, which report prevalence rates of PLS ranging from 60% to 80%,9,29 all patients in our sample presented amputation-related symptoms. In fact, PLP was present in 60%, a rate similar to those reported in the literature, suggesting that PLP is frequent in these patients. However, the prevalence rates reported vary considerably (2%-85%) depending on age, cause, level of amputation, or associated comorbidities.2,9,18,30 PLP starts early after surgery, manifesting as chronic pain. In fact, PLP persisted at 2 years after surgery in nearly 90% of our patients. This finding stands in contrast with most studies, which report progressive improvement of PLP over time.29–31 Another interesting finding from our study is the fact that over half of our sample described continuous or daily pain. Among all post-amputation pain syndromes, PLP was the most intense. In our sample, pain was of mild-to-moderate intensity (VAS = 4), whereas in other studies, up to 40% of patients report severe pain intensity.31 Given all of the above, PLP is one of the main reasons for which patients with amputations seek specialised care.
Our sample was very homogeneous: most patients were men in the seventh decade of life, with cardiovascular risk factors and history of toxic substance use. Ischaemic disease was the most frequent cause of amputation; according to the literature, this is the most frequent cause of amputation in developing countries, whereas trauma remains the leading cause worldwide.30,32 Chronic lower limb ischaemia explains the higher prevalence of patients with bilateral amputation in our sample, as compared to other studies including younger patients with non-vascular causes of amputation.33 Other less frequent causes in our study were trauma and tumours. Several studies have shown that tumour- and trauma-related amputations in young populations are more frequently associated with PLP and higher pain intensity.30,34,35 The reason for this increased frequency of PLP is unclear. One hypothesis suggests that a lower prevalence of such cardiovascular risk factors as diabetes mellitus may alter pain transmission pathways, and consequently pain perception in patients with vascular-related amputations.10,36 Although it was not the focus of our study, we also observed a higher frequency of PLP among patients with upper limb amputations, which may be related to greater representation of the upper limbs in the somatosensory cortex.25
Presurgical and postsurgical pain, RLP, and NPPS have been identified as the main factors associated with development of PLP.3,9,30 Other researchers have observed an association between PLP and cardiovascular risk factors35; however, this was not the case in our series. The literature also describes greater frequency of PLP among patients experiencing severe pain in the limb before amputation.3,9,30 In our study, however, although 70% of patients reported presurgical pain, no association was observed with PLP. Notably, only RLP was identified as a risk factor for PLP; this finding is in line with previous evidence.30 PLP and RLP have some common pathogenic mechanisms, involving peripheral nervous system damage to the nerve terminals of the amputated limb and maladaptive cortical adaptation to severe painful stimuli following amputation.25 It is therefore unsurprising that factors that exacerbate RLP may also worsen PLP.8,30 Conversely, the application of such stump management strategies as massages, heat, or compression bandages may improve phantom pain.37,38 In contrast, no relationship was observed between presence of PLP and prosthetic use, in spite of the direct compression exerted by the device over the stump.39 Knowledge of the factors that promote the development of PLP has clinical implications, as early management of these factors may help to reduce the frequency and intensity of this type of pain.
Supporting the theory of cortical reorganisation, current research is focused on strategies to prevent PLP, with the use of analgesics before surgery to decrease presurgical and postsurgical pain, as well as RLP. It has been hypothesised that a decrease in painful stimuli from the limb to be amputated, and subsequently from the stump, may prevent maladaptive sensory cortical reorganisation, avoiding the amplification of pain signals from the amputated body part.37 Administration of preventive analgesics in the days preceding surgery is empirically regarded as relevant, although evidence on their efficacy in reducing the incidence of PLP is inconsistent. In other types of pain, however, administration of analgesic treatment before surgery has been shown to decrease acute and chronic postsurgical pain.3 Regarding non-painful sensations, nearly all patients in our cohort experienced both PLP and NPPS, but no significant association was observed between them. Other studies have observed higher prevalence of PLP among patients with NPPS, which may be explained by a common pathogenesis involving maladaptive neuroplasticity.12
In patients with bilateral amputation, no differences were found in the clinical characteristics of PLS between the 2 amputated limbs; the frequency and intensity of PLP in these patients were comparable to those observed in patients with unilateral amputations. This suggests that the development and persistence of phantom pain may be determined by individual susceptibility, and does not change after the first amputation.31 Quantitative sensory testing may be helpful in identifying individuals with susceptibility to PLP after amputation.40
The mental health of patients with amputations should be prioritised, given that limb loss can result in great emotional distress. According to the literature, 20% to 40% of patients with amputations present symptoms of depression.4,24 In our study, however, the incidence of depression was surprisingly low, and no association with PLP was observed. Although this finding is difficult to explain, older age and high incidence of comorbidities may have resulted in better psychological adaptation to amputation than in younger populations with other causes of amputation.2,24 However, further research is needed to confirm this hypothesis.
Regarding treatment, the need for analgesic treatment for PLP is high across studies, with 60% to 70% of the patients requiring medication.18 Use of symptomatic treatment in our sample was optimal in early stages, but progressively decreased as PLP became chronic. Another noteworthy finding of our study is the low rate of treatment changes and high rate of treatment discontinuation, which may be due to limited symptomatic efficacy, given the mild-to-moderate intensity of PLP, and the short clinical follow-up. According to the literature, the most commonly prescribed drugs for PLP treatment are antiepileptics and tricyclic antidepressants, despite discrepancies regarding their efficacy.9,18,37,41 Our study, to the contrary, found high rates of analgesic and non-steroidal anti-inflammatory drug use for the treatment of both acute and chronic PLP; these drugs are not recommended for neuropathic pain.36 Notably, in our sample, opioids were more frequently used for analgesia at the onset of PLP; this is consistent with the hypothesis that these drugs may alter somatosensory cortical reorganisation, and their use decreased in the long term to prevent abuse, given the current opioid crisis, particularly in older adults.42 Other parenteral treatments frequently used at pain units for neuropathic pain or central sensitisation, such as intravenous lidocaine or ketamine, are indicated for severe, refractory PLP. Few patients with PLP are eligible for these fourth-line treatments as, according to our results, most patients present pain of moderate intensity.31
In our sample, none of the patients underwent new non-pharmacological interventions, which have shown efficacy in PLP, as is the case with mirror therapy.43 This therapy promotes correct neuronal plasticity and remapping of the somatosensory cortex responsible for movement in the amputated limb through movement and physiotherapy targeting the contralateral limb observed in a mirror, to create the illusion that the missing limb is moving.44 Given the poor symptomatic control of PLP, more rigorous clinical follow-up of these patients is needed; furthermore, progression of PLP should be closely monitored and managed on an outpatient basis.
Our study presents some limitations, mainly its retrospective design, the lack of a control group, and the limitations arising from our patients’ cultural characteristics. The physical disability of patients with amputations limited participation in the study. The items included in our interview were based on previous studies, and considered the clinical characteristics and risk factors for PLP; however, the questionnaire was designed ad hoc and was not validated prior to application. No specific instrument is currently available for the assessment of PLP. Although the McGill Pain Questionnaire is widely used in studies into neuropathic pain, it is not validated for PLP.
ConclusionsPLP is highly prevalent among patients with amputations and is frequently poorly controlled. A multidisciplinary approach is essential, with neurologists playing an active, central role. Future research should aim to deepen our knowledge of the risk factors for PLP; this would enable earlier implementation of more targeted preventive treatments.
CRediT authorship contribution statementAll authors made substantial contributions to:
- 1)
study design and conception, data acquisition, or data analysis and interpretation;
- 2)
manuscript drafting or critical review of the intellectual content; and
- 3)
approval of the final version.
No funding was received for this study.