Coronavirus disease 2019 (COVID-19) has spread rapidly, giving rise to a pandemic, causing significant morbidity and mortality. In this context, many vaccines have emerged to try to deal with this disease.
ObjectiveTo review the reported cases of neurological manifestations after the application of COVID-19 vaccines, describing clinical, analytical and neuroimaging findings and health outcomes.
MethodsWe carried out a review through bibliographic searches in PubMed.
ResultsWe found 86 articles, including 13 809 patients with a wide spectrum of neurological manifestations temporally associated with COVID-19 vaccination. Most occurred in women (63.89%), with a median age of 50 years. The most frequently reported adverse events were Bell’s palsy 4936/13 809 (35.7%), headache (4067/13 809), cerebrovascular events 2412/13 809 (17.47%), Guillain-Barré syndrome 868/13 809 (6.28%), central nervous system demyelination 258/13 809 (1.86%) and functional neurological disorder 398/13 809 (2.88%). Most of the published cases occurred in temporal association with the Pfizer vaccine (BNT162b2), followed by the AstraZeneca vaccine (ChAdOX1-S).
ConclusionsIt is not possible to establish a causal relationship between these adverse events and COVID-19 vaccines with the currently existing data, nor to calculate the frequency of appearance of these disorders. However, it is necessary for health professionals to be familiar with these events, facilitating their early diagnosis and treatment. Large controlled epidemiological studies are necessary to establish a possible causal relationship between vaccination against COVID-19 and neurological adverse events.
La enfermedad por coronavirus 2019 (COVID-19) se ha propagado de forma rápida, dando lugar a una situación de pandemia, con importante morbilidad y mortalidad. En este contexto, han surgido un amplio número de vacunas para tratar de hacer frente a la enfermedad.
ObjetivoRevisar los casos reportados de manifestaciones neurológicas tras la aplicación de las vacunas contra COVID-19, describiendo los hallazgos clínicos, analíticos, de neuroimagen y los resultados de salud.
MétodosRevisión bibliográfica estructurada en la base de datos PubMed.
ResultadosEncontramos 86 artículos, que incluyeron 13.809 pacientes con un amplio espectro de manifestaciones neurológicas asociadas temporalmente con la vacunación contra COVID-19. La mayoría ocurrieron en mujeres (63,89%), con una mediana de edad de 50 años. Los eventos adversos publicados con más frecuencia fueron parálisis facial de Bell 4936/13809 (35,7%), cefalea (4067/13809), eventos vasculares cerebrales 2412/13809 (17,47%), síndrome de Guillain-Barré 868/13809 (6,28%), desmielinización del sistema nervioso central 258/13809 (1,86%) y trastorno neurológico funcional 398/13809 (2,88%). La mayoría de casos publicados se produjeron en asociación temporal con la vacuna Pfizer (BNT162b2), seguida de la vacuna de AstraZeneca (ChAdOX1 nCoV-19).
ConclusionesCon los datos existentes actualmente no es posible establecer una relación de causalidad entre estos eventos adversos y las vacunas contra COVID-19, ni calcular la frecuencia de aparición de estos trastornos. Sin embargo, es necesario que los profesionales de la salud estén familiarizados con estos eventos, facilitando su diagnóstico y tratamiento tempranos. Son necesarios grandes estudios epidemiológicos controlados para establecer una posible relación causal entre la vacunación contra COVID-19 y los eventos adversos neurológicos.
SARS-CoV-2, which causes coronavirus disease (COVID-19), has spread rapidly, causing a pandemic with major healthcare, economic, and social consequences worldwide.1,2 In this context, unprecedented international efforts have been made to develop effective and safe vaccines against COVID-19. Several vaccines have been authorised for emergency use in many countries.1,3
BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna) are mRNA-based vaccines, whereas ChAdOx1-S (Oxford-AstraZeneca), Ad26.COV2.S (Johnson & Johnson), Convidecia, and Sputnik V are non-replicating adenoviral vector–based vaccines, and CoronaVac (Sinovac) and BBIBP-CorV (Sinopharm) are inactivated virus vaccines. The only recombinant spike protein subunit vaccine currently available is NVX-CoV2373 (Novavax).1,4,5
The COVID-19 vaccination programme has raised hopes in the response to the pandemic. However, vaccines are not free from adverse reactions; identifying and quantifying the associated risk is therefore essential. The accelerated development of COVID-19 vaccines has raised concerns about their safety among the general public.4 Randomised clinical trials of SARS-CoV-2 vaccines have shown encouraging efficacy and safety results, but had insufficient power to detect very rare adverse reactions; a wide range of neurological complications have been reported since their authorisation.6
Randomised clinical trials of the Oxford-AstraZeneca ChAdOx1-S vaccine reported 3 cases of transverse myelitis in the vaccine group, among 11 636 participants.7 Only one of the 3 cases was finally considered to be linked to vaccination. Trials of the mRNA-based vaccines developed by Moderna and Pfizer-BioNTech reported 7 cases of Bell’s palsy among 37 000 vaccinated individuals.8,9 The clinical trial of the Janssen Ad26.COV2.S vaccine10 reported 3 cases of Bell’s palsy in the vaccine group, one case of Guillain-Barré syndrome in the vaccine group and another in the control group, and one case of brachial radiculitis in the vaccine group. Venous thromboembolic events were more frequent in the vaccine group, with one cerebral venous embolic event associated with thrombocytopenia.3,11
Most severe neurological adverse reactions to COVID-19 vaccines are anecdotal reports, and their real incidence is unknown.12 The purpose of this study was to define the spectrum of neurological adverse reactions to COVID-19 vaccines, describing the manifestations reported in the literature.
Material and methodsOur review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.13 We performed literature searches on PubMed between 8 January 2022 and 21 January 2022, using the following keywords, combined with the Boolean operators AND and OR: “COVID-19 vaccine,” “COVID-19 vaccines,” “SARS-CoV-2 vaccine,” “SARS-CoV-2 vaccines,” “neurological manifestations,” “neurologic manifestations,” “neurological symptoms,” “neurologic symptoms,” and “neurology.” The complete search strategy is shown in Supplementary Material Table 1S. We examined the titles and abstracts of the articles gathered in the literature search. Duplicate articles were excluded.
The inclusion criteria were as follows: 1) reporting at least one case of any neurological manifestation showing a temporal association with human SARS-CoV-2 vaccination and not explained by any other aetiology; 2) including individuals vaccinated with any COVID-19 vaccine; and 3) published in English. We read the full texts of the selected articles, and excluded review articles and letters to the editor or brief communications, except those reporting cases of neurological disease after COVID-19 vaccination.
The following data were gathered: number of patients presenting neurological complications, age, sex, neurological complications, type of vaccine, dose, country, time elapsed between vaccination and onset of the neurological complication, clinical presentation, neuroimaging findings, laboratory findings, and outcomes. Qualitative data are expressed as numbers and percentages, and quantitative data as median and range.
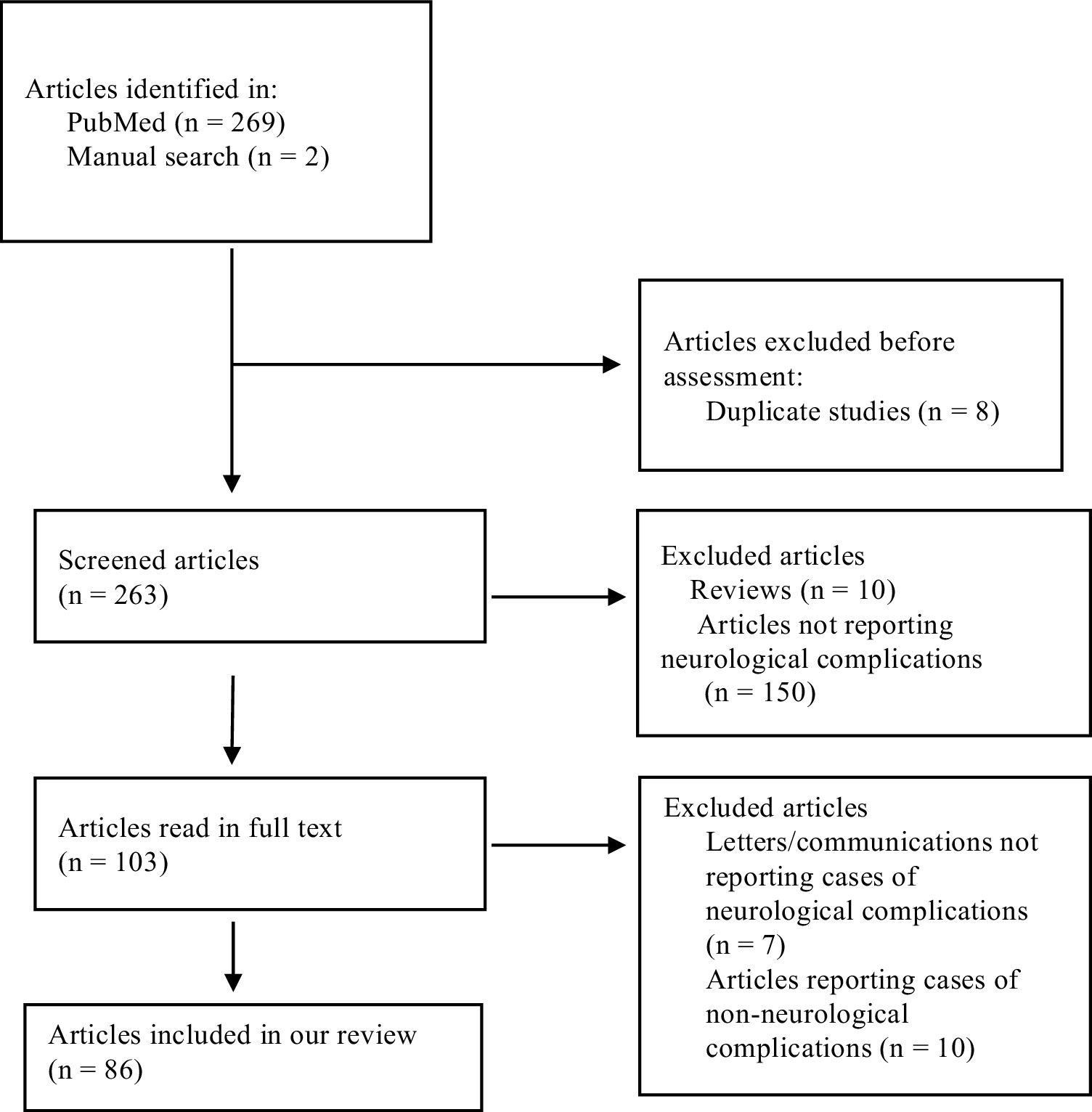
ResultsOur literature search identified 271 articles, 8 of which were duplicate studies. After reviewing the titles and abstracts of the remaining 263 studies, we excluded 10 review articles and 150 articles not reporting cases of neurological complications. The remaining 103 articles were read in full text; of these, we excluded 7 letters/communications not reporting cases of neurological complications, and another 10 articles that reported cases of non-neurological complications. A total of 86 articles met the inclusion criteria and none of the exclusion criteria, and were therefore included in our review. The flow chart in Fig. 1 describes the study selection process.
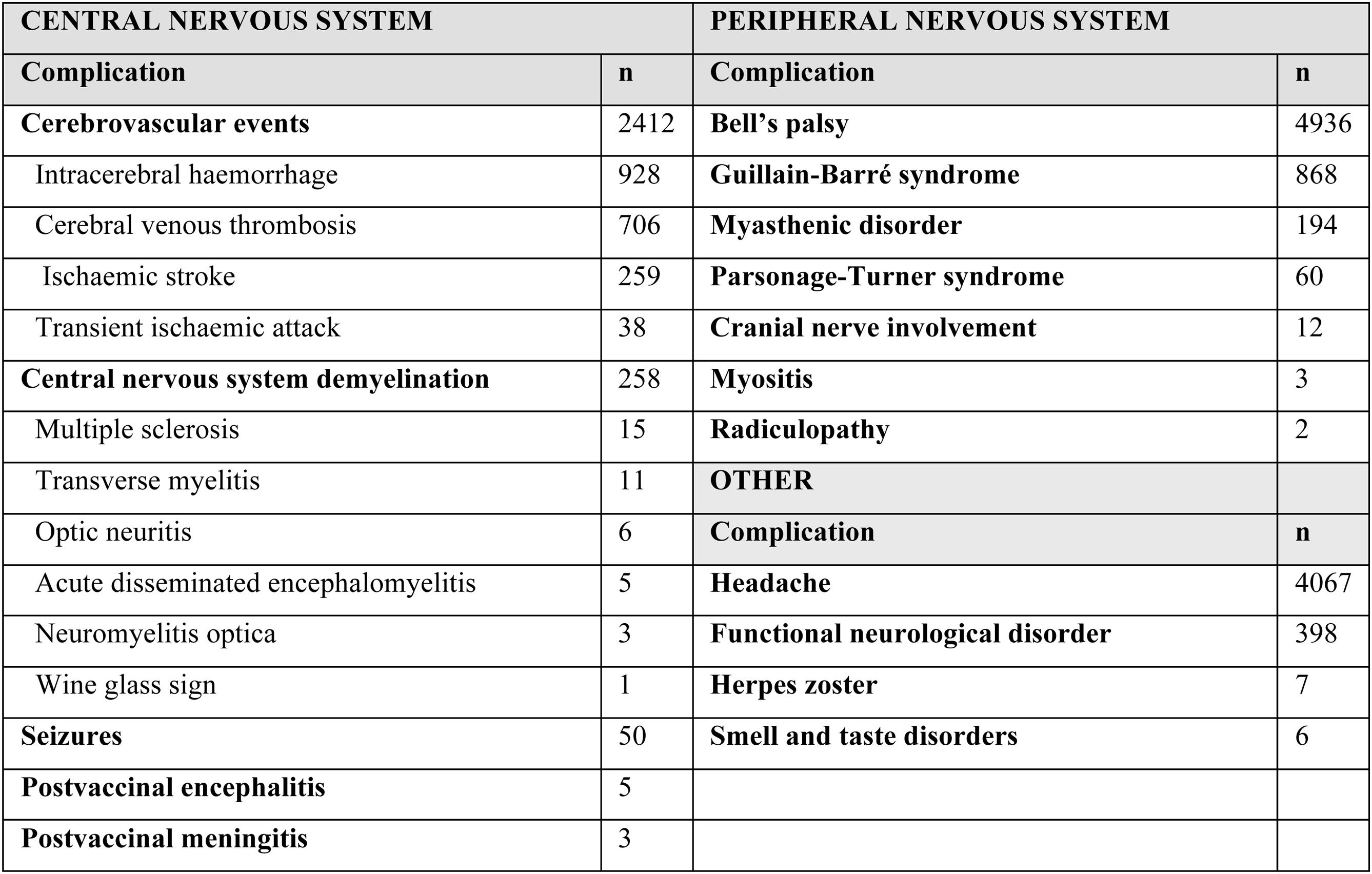
The 86 articles included a total of 13 809 patients presenting any type of neurological complication. Table 1 summarises the neurological adverse events observed after administration of COVID-19 vaccines in the studies included in our review. These adverse events were more frequent in women (63.89%): of the 12 379 cases for which sex was reported, 7910 were women and 4469 were men. The median age was 50 years (range, 19-97).
The most frequently reported neurological complications were Bell’s palsy (4936, 35.7%), headache (4067, 29.4%), cerebrovascular events (2412, 17.47%), Guillain-Barré syndrome (GBS) (868, 6.28%), functional neurological disorder (398, 2.88%), and central nervous system (CNS) demyelination (258, 1.86%). Other less frequently reported complications were myasthenic disorder (194), Parsonage-Turner syndrome (60), seizures (50), cranial nerve involvement (12), herpes zoster (7), smell and taste disorders (6), encephalitis (5), meningitis (3), myositis (3), and radiculopathy (2).
In most cases, complications occurred after vaccination with BNT162b2 (n = 8820). A total of 3561 cases of neurological complications were reported after vaccination with ChAdOx1-S, 1256 cases with mRNA-1273, 251 cases with Ad26.COV2.S, 2 cases with Sputnik V, one case with CoronaVac, one case with BBIBP-CorV, and one case with Convidecia. Most neurological adverse events occurred after the first dose (2991 of the 3216 cases for which this information was available; 93%), whereas only 225 occurred after the second dose. Neurological complications occurred a median of 9 days after vaccination (range, 1–32).
Cerebral venous thrombosis (CVT) was more frequently reported after vaccination with adenoviral vector vaccines. The same is true for CNS demyelination, although a more detailed analysis by subtype revealed that neuromyelitis optica, acute disseminated encephalomyelitis (ADEM), and multiple sclerosis (MS) were more frequently reported after vaccination with mRNA-based vaccines. Peripheral nervous system disease was also more frequently reported after administration of viral vector vaccines, with the exception of Bell’s palsy, which most commonly occurred after administration of mRNA-based vaccines.
The studies included can be classified into those reporting different types of neurological complications associated with COVID-19 vaccination, mainly those including data from pharmacovigilance databases and multicentre studies (Supplementary Material Table 2S), and those focusing on a single neurological complication, mainly case reports and case series. Thus, we included studies describing cases recorded in such pharmacovigilance databases as VigiBase (the World Health Organization’s global database of suspected adverse drug reactions),5 EudraVigilance (the European Medicines Agency’s pharmacovigilance system),14,15 VAERS (the US national vaccine safety monitoring system),16 NIMS (a management system for the influenza and COVID-19 vaccination programmes in England),6 and SAEFVIC (the vaccine safety surveillance service of Victoria, Australia), in addition to studies including data from the Directorate General of Epidemiology of the Mexican Ministry of Health17–19 and the United States Centers for Disease Control and Prevention.20 Our review also included multicentre prospective studies conducted in several countries (Singapore,21 United Kingdom,22 Germany23). The remaining articles were case reports or case series.
Central nervous systemCerebrovascular eventsThe studies reporting cases of cerebrovascular events are included in Supplementary Material Table 3S. A total of 2412 cases were reported. Of the 1615 cases for whom sex was reported, 946 were women (58.57%) and 669 were men (41.42%). The median age was 46 years (range, 29-67). The median time from vaccination to the cerebrovascular event was 8 days. Most events occurred after vaccination with non-replicating adenoviral vector–based vaccines (1527/2294, 66.5%). A total of 767/2294 cerebrovascular events were reported after vaccination with mRNA-based vaccines (33.4%). Most cerebrovascular events occurring after vaccination with viral vector–based vaccines were associated with thrombocytopenia (182/265), elevated D-dimer levels (72/78), and anti-PF4 antibodies (73/78). However, this was not observed in individuals receiving mRNA-based vaccines. At least 65.6% (1222/1863) of patients died.
Cerebral venous thrombosisCVT was the most frequently reported cerebrovascular event, in 706 individuals (217/290 women [74.8%] vs 73/290 men [25.17%]), with a median age of 40 years. Most cases showed a temporal association with vaccination with viral vector–based vaccines, with 402/511 cases reported after vaccination with ChAdOx1-S, 29/511 cases after vaccination with Ad26.COV2.S, and 48/511 cases after vaccination with mRNA-based vaccines. Thrombocytopenia was reported in the majority (182/264, 68.9%) of cases of CVT following vaccination with viral vector–based vaccines for which laboratory data were available.
Arterial eventsWe found 259 cases of ischaemic stroke, 928 cases of intracerebral haemorrhage, and 38 cases of transient ischaemic attack. Arterial events occurring in the context of COVID-19 vaccination were also frequently associated with vaccine-induced immune thrombotic thrombocytopenia (VITT). At least 807/929 patients died (86.8%).
Central nervous system demyelinationWe found several cases of neuromyelitis optica, transverse myelitis, optic neuritis, newly-diagnosed MS, MS relapses, and ADEM showing a temporal association with COVID-19 vaccination. We identified 258 cases of CNS demyelination (96/245 men [39%] and 149/245 women [58.6%]), with a median time from vaccination to the initial manifestation of CNS demyelination of 9 days (range, 1-30) (Supplementary Material Table 4S). The median age was 43 years (range, 19-88). Most cases occurred after vaccination with the ChAdOx1-S vaccine (153/236); 78/236 cases were reported after vaccination with BNT162b2, 7/236 after vaccination with mRNA-1273, 2/236 after CoronaVac, 1/236 after vaccination with Ad26.COV2.S, and 1/236 after BBIBP-CorV.
Transverse myelitisWe found 11 cases of transverse myelitis (6/9 men [66.7%] and 3/9 women [33.33%]), with symptom onset occurring within 3 weeks of vaccination. Transverse myelitis occurred most frequently after administration of ChAdOx1-S (6/11), followed by BNT162b2 (3/11), BBIBP-CorV (1/11), and CoronaVac (1/11). Five cases were reported as longitudinally extensive transverse myelitis. CSF analysis revealed pleocytosis in 3 patients and elevated protein levels in 7. Five patients tested negative for anti-AQP4 and anti-MOG antibodies.
Acute disseminated encephalomyelitisA total of 5 cases of ADEM were reported (4 women and 1 man), with a median age of 56 years. In all cases, symptoms presented within a month of vaccination. Three cases occurred following the administration of mRNA-based vaccines, one case after CoronaVac, and the remaining one after ChAdOx1-S. Three patients underwent anti-AQP4 and anti-MOG antibody determination, with negative results in all 3; oligoclonal banding also yielded negative results. CSF analysis revealed pleocytosis in 2/4 patients and elevated protein levels in one.
Multiple sclerosisSymptoms of MS were reported in 13 patients, 5 of whom had previously been diagnosed with the condition. Patients were predominantly women (9 [70%], vs 4 men [30%]). The median age was 41 years (range, 24-64). Symptoms appeared between one day and one month after vaccination. In all cases, symptoms occurred after administration of mRNA-based vaccines. MRI revealed new brain lesions in all 13 patients, and new spinal cord lesions in 7. CSF analysis detected pleocytosis in 8/9 patients and oligoclonal bands in 7/9.
Postvaccinal encephalitisA total of 5 cases of postvaccinal encephalitis were reported (3 women and 2 men); all cases occurred within a week of vaccination (4 after administration of ChAdOx1-S and one after mRNA-1273) (Supplementary material Table 5S). The reported symptoms include headache, confusion, visual and tactile hallucinations, concentration and attentional difficulties, seizures, opsoclonus-myoclonus syndrome, and aphasia. All patients presented normal neuroimaging findings and presence of lymphocytic pleocytosis.
Postvaccinal meningitisOur literature review found 3 cases of aseptic meningitis, in middle-aged women in all cases, following administration of the BNT162b2 vaccine (Supplementary Material Table 5S). All 3 patients presented lymphocytic pleocytosis and elevated CSF protein levels.
Peripheral nervous systemGuillain-Barré syndromeGuillain-Barré syndrome was a relatively frequent neurological complication (Supplementary Material Table 6S), with 868 reported cases: 507 cases occurred after vaccination with ChAdOx1-S, 212 after BNT162b2, 77 after mRNA-1273, 59 after Ad26.COV2.S, 4 after CoronaVac, and one after Sputnik V. Patients with GBS were predominantly men (474/861 men [55%] vs 387/861 women [44.94%]). Symptoms appeared after a median of 12 days. The median age was 61 years (range, 25-86).
Interestingly, GBS frequently presented with unusual phenotypes. The clinical form of GBS was indicated in 41 cases: 12 presented the classical form, 11 presented a variant associated with facial diplegia, 3 showed unilateral facial paralysis, 3 had tetraparesis, 2 had sensory GBS, 2 had Miller-Fisher syndrome, and one presented an axonal variant. CSF analysis revealed albuminocytological dissociation in 15 patients and elevated protein levels in 11. Most patients showed a partial, progressive response to treatment.
Bell’s palsyBell’s palsy was the most frequently reported neurological complication, with 4936 cases (Supplementary material Table 7S). It was more frequent among women (1994 women [60%] vs 1326 men [40%]). In most cases, Bell’s palsy was reported after the administration of mRNA-based vaccines: 2538 cases after BNT162b2, 1136 after mRNA-1273, 1107 after ChAdOx1-S, 162 after Ad26.COV2.S, 13 after CoronaVac, one after Sputnik V, and one after Convidecia. Most patients recovered completely.
Amyotrophic neuralgia/Parsonage-Turner syndromeWe found 60 cases of Parsonage-Turner syndrome, 41 in women and 17 in men (Supplementary material Table 8S). The clinical presentation was described in 3 cases: 2 cases manifesting with pain and proximal muscle weakness of the upper limbs and one presenting paralysis and acute-onset paraesthesia in the lower limbs. Neuroimaging studies and laboratory tests yielded normal results.
Smell and taste disordersAll cases of smell and taste disorders were reported by Lechien et al.24 (Supplementary material Table 9S), with 5 cases of olfactory dysfunction and one case of gustatory dysfunction occurring between 2 and 24 days after vaccination with ChAdOx1-S or BNT162b2; patients were 5 women and one man, with ages between 25 and 52 years. Symptoms resolved in all cases.
Herpes zosterAll cases of herpes zoster occurred after vaccination with BNT162b2. A total of 7 cases have been reported, occurring predominantly in women (6/7) between the first and third weeks after vaccination (Supplementary material Table 9S).
Functional neurological disorderCases of functional neurological disorder are presented in Supplementary material Table 9S. García-Grimshaw et al.18 conducted a study of 10 929 patients reporting adverse reactions to the first dose of the BNT162b2 vaccine in Mexico, 354 of whom experienced transient sensory symptoms (3.4%).
SeizuresWe found 50 cases of seizures. Ghosh et al.25 reported the case of a 68-year-old man who, 4 days after the first dose of ChAdOx1-S, presented disconnection from his surroundings and behavioural alterations. He was diagnosed with focal-onset non-motor seizures with altered consciousness and transient, episodic behavioural alterations.
DiscussionRandomised clinical trials of COVID-19 vaccines report a very low frequency of severe neurological complications. However, they did not have sufficient power to detect very rare adverse reactions, due to the small sample size and the limited study period; this underscores the importance of monitoring adverse reactions to COVID-19 vaccines through pharmacovigilance monitoring systems.3
Despite the devastating consequences of the pandemic and the availability of efficacious vaccines, public fear of the vaccines continues to be widespread.3 The neurological adverse events associated with COVID-19 vaccines may result in significant morbidity and mortality, and constitute a cause for concern.26 However, the temporal association between vaccination and adverse events does not necessarily imply causation. Patients presenting neurological symptoms after vaccination should undergo a thorough differential diagnosis, but vaccination against COVID-19 should always be considered a possible trigger factor.
The frequency of severe neurological complications associated with COVID-19 vaccines seems to be very low compared to the impact of COVID-19 itself; according to the information currently available, the benefits of these vaccines outweigh the potential risks.1,12,27,28 The high number of reported cases of COVID-19 and deaths due to the disease underscore the importance of vaccination programmes, which have been found to be highly efficacious.
The age at presentation and female predominance observed in our review are in line with the findings of previous studies.1,2,4,27 The time between vaccination and symptom onset is also similar to that reported in previous studies, with most adverse events occurring between weeks 1 and 4 post-vaccination.2,29 The vaccine most frequently associated with neurological complications was BNT162b2, which may partly be due to the large number of patients who received this vaccine; the study by García-Grimshaw et al.18 alone included 4258 patients receiving this vaccine.
Patone et al.6 analysed hospital admissions due to neurological complications occurring after the first dose of the ChAdOx1-S (n = 20 417 752) and BNT162b2 vaccines (n = 12 134 782), and after positive SARS-CoV-2 screening test results (n = 2 005 280) in the vaccinated population. Greater risk of hospital admission due to haemorrhagic stroke was observed among individuals receiving the BNT162b2 vaccine, with greater risk of admission due to GBS, Bell’s palsy, and myasthenic disorders in those vaccinated with ChAdOx1-S. Although the risk of neurological complications was greater among vaccinated individuals, the authors observed a much greater increase in the risk of any neurological complication after a positive SARS-CoV-2 screening test.
A prospective study of 704 003 first-dose recipients of BNT162b2 in Mexico found that 65.1% of complications were neurological.17 Non-severe neurological events were observed in less than 1% of the cases, and severe neurological events in only 0.005%. However, 52% of all severe adverse events were neurological. Most patients recovered completely within days or weeks, presenting no sequelae, which points to an acute, transient, inflammatory trigger factor. A recent study, published after the period analysed in our review, reports 30% more adverse events.30
One of the complications of COVID-19 vaccines raising the greatest concern is cerebrovascular events, which are mainly reported in women of childbearing age after the administration of viral vector–based vaccines.1 Cerebrovascular events reported after vaccination against COVID-19 are frequently associated with VITT. VITT shares characteristics with heparin-induced thrombocytopenia, with presence of anti–platelet factor 4 (PF4) antibodies.12,20,28
Cerebral venous sinus thrombosis secondary to COVID-19 vaccination may either occur in the context of VITT, in individuals receiving non-replicating adenoviral vector–based vaccines, or present without characteristics of VITT, in individuals receiving any type of vaccine. Patients with VITT present anti-PF4 antibodies, which trigger platelet activation.12,20,28
In line with our results, CVST associated with VITT frequently presents within 2 weeks of the first dose of an adenoviral vector–based vaccine. Typical laboratory findings include low platelet count (<100 × 109/L), elevated D-dimer levels, and presence of anti-PF4 antibodies.27,28,31
Krzywicka et al.14 analysed clinical data from 212 cases of CVST after COVID-19 vaccination reported by the European Medicines Agency and compared them against a control group of patients presenting CVST before the COVID-19 pandemic. The study showed that CVST occurring after vaccination with ChAdOx1-S displays a distinct clinical profile: it presented with thrombocytopenia and anti-PF4 antibodies, was frequently accompanied by other venous thrombotic events, and was associated with high mortality rates. The reported cases of CVST after administration of mRNA-based vaccines were similar to those reported before the pandemic.
The specificity of the clinical characteristics of CVST associated with viral vector–based vaccines, the identification of a pathophysiological mechanism, and the coherent temporal sequence support a causal association. Furthermore, the high risk of mortality associated with this complication underscores the importance of early diagnosis and treatment.15,20,28
Vaccination against COVID-19 may trigger an immune-mediated inflammatory response, leading to autoimmune processes.32,29 Demyelination frequently has an underlying autoimmune cause. The exact mechanism of post-vaccination acute demyelination is still poorly understood, but several hypotheses have been proposed. One of these revolves around the phenomenon of molecular mimicry. Another hypothesis focuses on the immunogenic role of some vaccine adjuvants.2,33 A third possible mechanism may be linked to T and B cell responses and bystander activation of latent autoreactive lymphocytes.2
According to our review, 60% of cases of demyelination occurred in women, with a median age of 42 years. Most cases occurred after administration of ChAdOx1-S. A systematic review of cases of CNS demyelination reported after vaccination against COVID-19 found similar results to our own.2 This review gathered 32 cases, predominantly in women (68.8%), with a median age of 44 years. However, mRNA-based vaccines were associated with a greater number of demyelinating syndromes, followed by viral vector–based vaccines.
We identified cases of neuromyelitis optica, transverse myelitis, optic neuritis, MS, and ADEM showing a temporal association with vaccination against COVID-19. These are relatively infrequent conditions, with post-vaccination cases being even rarer. These patients’ good response to immunosuppressive treatment suggests a transient immune reaction.34 It remains to be clarified whether COVID-19 vaccines are the direct cause of autoimmune complications, or rather a trigger factor in patients with previously asymptomatic autoimmune diseases.29,35
We found a considerable number of cases of GBS showing a temporal association with vaccination against COVID-19, especially with viral vector–based vaccines. Cases have been published of GBS after administration of other types of vaccines; however, only vaccination against influenza has shown a statistically significant (though weak) correlation with risk of GBS.36–39 Particularly interestingly is the fact that many of the cases of GBS published after COVID-19 vaccination presented with bilateral facial weakness. This presentation seems to be characteristic of post–COVID-19 vaccination GBS, which, together with the frequent observation of other rare variants, supports the hypothesis of a causal association. COVID-19 vaccine–related GBS may present atypical laboratory findings, such as absence of antiganglioside antibodies.40,41
In Australia, Osowicki et al.42 note an excess of reported cases of GBS after vaccination with ChAdOx1-S and BNT162b2 as compared to the expected number of cases. Min et al.43 reviewed the reported cases of GBS after COVID-19 vaccination. All but one presented rare phenotypes, such as distal paraesthesia with facial diplegia or quadriparesis. Patients tested negative for antiganglioside antibodies.
Bell’s palsy was the most frequently reported neurological complication, especially after administration of mRNA-based vaccines, and was more common in women. One of the largest studies included in our review was that by Noseda et al.,5 which reported 3320 cases of Bell’s palsy.
A study analysing the frequency of Parsonage-Turner syndrome, Bell’s palsy, and GBS in VigiBase found that the incidence of post-vaccination amyotrophic neuralgia and GBS was similar to that previously observed for other viral vaccines, whereas Bell’s palsy was reported at a disproportionately higher frequency in individuals receiving COVID-19 vaccines as compared to other viral vaccines, especially among men older than 75 years.5 In contrast, according to a previous study by Sato et al.,16 reporting of cases of Bell’s palsy in the VAERS database was significantly high for BNT162b2 and mRNA-1273, although numbers were comparable to those reported following influenza vaccination before the COVID-19 pandemic.
Our review has several limitations. Most of the available studies are case reports and case series lacking a control group, which may have resulted in underreporting of adverse events or selective reporting of the most severe cases. Therefore, as most of the evidence is from isolated case reports and case series, our results should be interpreted with caution, as the temporal association observed does not imply causality, but rather only biological plausibility. All articles were gathered from a single database; data on the frequency of adverse events after each vaccine are only available from pharmacovigilance studies, which do report the adverse events observed and the number of vaccinated individuals.
Furthermore, the type of vaccine after which neurological complications were reported probably reflects the frequency of administration in each region and patient profile. Complications following administration of the Pfizer-BioNTech vaccine are probably the most frequently reported because this is the most frequently administered vaccine. Therefore, data on neurological complications reported after each vaccine are not comparable.
Headache may have not been reported as a neurological complication of vaccination: while it was found to be one of the most frequent neurological events in randomised clinical trials of COVID-19 vaccines, our study only found isolated reports of headache in pharmacovigilance studies.7–11
ConclusionsA wide range of neurological complications have shown a temporal association with COVID-19 vaccination. The most frequent complications were Bell’s palsy, headache, cerebrovascular events, GBS, CNS demyelination, and functional neurological disorder. However, a causal association cannot be established, nor can we estimate the frequency of these disorders based on our results.
It is vital for healthcare professionals to be familiar with these rare but potentially severe complications, given that early diagnosis and treatment may help to achieve more favourable outcomes. We wish to underscore the importance of pharmacovigilance following the marketing authorisation of COVID-19 vaccines, with a view to identifying these rare adverse events and ensuring the safety of these products. Prospective, controlled studies with larger samples are needed to confirm or refute our results and to evaluate whether there is a causal association between COVID-19 vaccines and neurological adverse events. If a causal association is confirmed, future research should aim to weigh the risks and benefits of COVID-19 vaccines.
Conflict of interestThe authors have no conflicts of interest to declare and have received no funding.
- Descargar PDF
- Bibliografía
- Material adicional