Alzheimer's disease is a neurodegenerative disease characterized by progressive deterioration of superior brain functions especially memory which generally affects older adults. Animal models of Alzheimer's are partially representative of the disease conditions under study since there is no model that fully incorporates all the characteristics that are present in humans, so similar conditions to AD in animal models should not be actually called Alzheimer's disease; however, these models are very useful for the study of several alterations involved in the pathophysiology of AD, including mainly the deterioration of memory capacity. The objective of this article is to determine the diversity of animal models of Alzheimer's disease, biological plausibility and the main affective disorders and behavior studied.
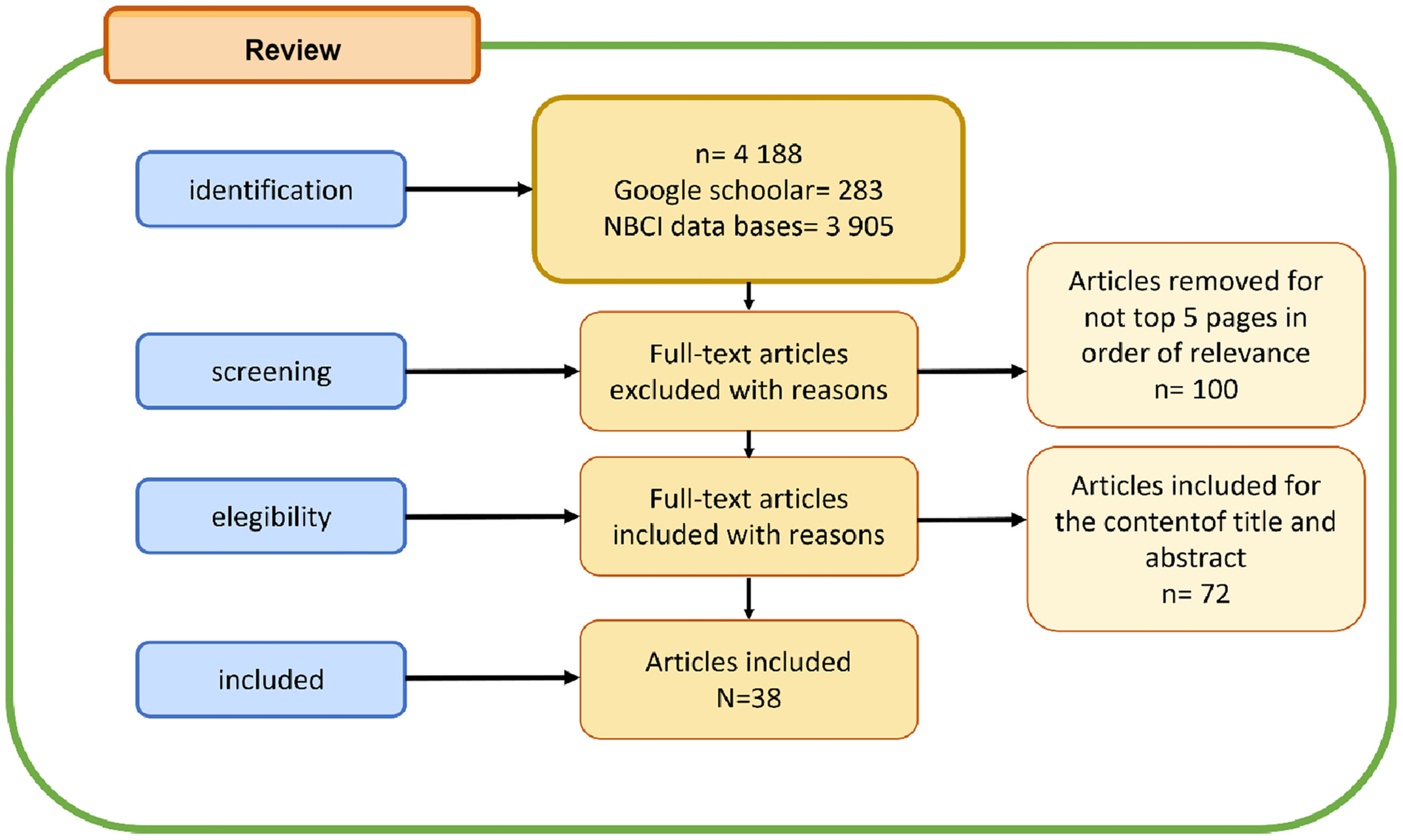
MethodThe keywords “animal models of Alzheimer's disease + mood/affective disorders” were placed in the Google scholar and NCBI databases, the first 5 pages of the search were considered.
Results7 animal models were found that are used to model the disease and 5 for affective disorders.
ConclusionAmong the animal models found, the rat model has been the most studied in terms of mood/affective disorders. We believe that more studies are needed where molecular changes are correlated with animal behavior of AD. The mood disorders studied are depression, anxiety, dysthymia, bipolar disorder, and drug-induced affective disorders.
La enfermedad de Alzheimer es una enfermedad neurodegenerativa caracterizada por el deterioro progresivo de las funciones cerebrales superiores, especialmente la memoria, que generalmente afecta a los adultos mayores. Los modelos animales de la enfermedad de Alzheimer son parcialmente representativos de las condiciones de la enfermedad en estudio, ya que no existe un modelo que incorpore completamente todas las características que están presentes en los humanos, por lo que condiciones similares a la EA en modelos animales no deberían llamarse enfermedad de Alzheimer; sin embargo, estos modelos son de gran utilidad para el estudio de diversas alteraciones involucradas en la fisiopatología de la EA, incluyendo principalmente el deterioro de la capacidad de memoria. El objetivo de este artículo es determinar la diversidad de modelos animales de la enfermedad de Alzheimer, la plausibilidad biológica y los principales trastornos afectivos y de conducta estudiados.
MétodoLas palabras clave “modelos animales de la enfermedad de Alzheimer + trastornos del estado de ánimo/afectivo” se colocaron en las bases de datos de Google academic y NCBI, se consideraron las primeras 5 páginas de la búsqueda.
ResultadosSe encontraron 7 modelos animales que sirven para modelar la enfermedad y 5 para los trastornos afectivos.
ConclusiónEntre los modelos animales encontrados, el modelo de rata ha sido el más estudiado en términos de trastornos del estado de ánimo/afectivo. Creemos que se necesitan más estudios donde los cambios moleculares se correlacionen con el comportamiento animal de la EA. Los trastornos del estado de ánimo estudiados son la depresión, la ansiedad, la distimia, el trastorno bipolar y los trastornos afectivos inducidos por fármacos.
Alzheimer's Disease (AD) is a neurodegenerative disease characterized by progressive deterioration of superior brain functions, mainly memory, with a higher incidence of this disease in older adults.1,2 AD is the most common group entity of dementia worldwide. A duration of 20 to 30 years is estimated of the preclinical period characterized by the development of progressive molecular alterations that are responsible for the development of AD and its manifestations in later phases.3–5
There are two types of histopathological lesions in the brain tissue that are characteristic of AD; amyloid plaques, also called senile plaques, consisting of the amyloid-beta (Aß) peptide, located in the interstitium and the neurofibrillary tangles or skeins located inside the cells, mainly in the somatodendritic compartment. The neurofibrillary tangles are composed of the Tau protein in its abnormal hyperphosphorylated form which conditions destabilization of the microtubules of the cytoskeleton and thus promotes cellular apoptosis by interference in the transport of organelles and nutrients through the scaffolding system that represent such microtubules.4–7
Neuronal loss leads to deficits in synaptic transmission systems in conjunction with significant alteration in the generation and release of neurotransmitters, which implies deficiency in certain connectomes and a “compensatory” pattern in others, progressively altering the concentration of neurotransmitters; the hippocampus being the area initially affected in AD, whereby these phenomena lead to the prodromal phase of AD, characterized by mild cognitive impairment (MCI).8,9 In particular, the cognitive impairment and decreased memory capacity observed in patients with AD correlates more with synaptic pathology than with the presence of senile plaques per se.10,11
The presumptive diagnóstico of AD is essentially clinical, according to the DSM IV, CIE-10 and Clinical Practice Guide, it is divided into 3 stages, where the first one is characterized by decreased memory capacity that may be accompanied in a diverse manner by cognitive alterations such as dysphasia, apraxia or agnosia and neuropsychiatric symptoms such as sleep disturbances, depression and apathy and secondarily the presence of alterations such as difficulty in starting a conversation, demotivation and visual–spatial alteration, the second stage is characterized by neuropsychiatric syndromes such as psychotic and hyperactive disorders; the latter includes neuropsychiatric symptoms of disinhibition and aggressiveness, the third stage is characterized by neuromotor alterations mainly manifested as urinary incontinence and alterations in the capacity of making facial expressions, eating disorders and lastly loss of autonomy. To conduct the differential diagnóstico (since it is also a rule-out diagnóstico), reversible causes of the observed manifestations such as vascular diseases, metabolic diseases (vitamin B12 deficiency, alcoholism), endocrine diseases (hypothyroidism, hypocalcaemia) and infectious diseases (HIV, Neurosyphilis) must be considered, among others.12,13
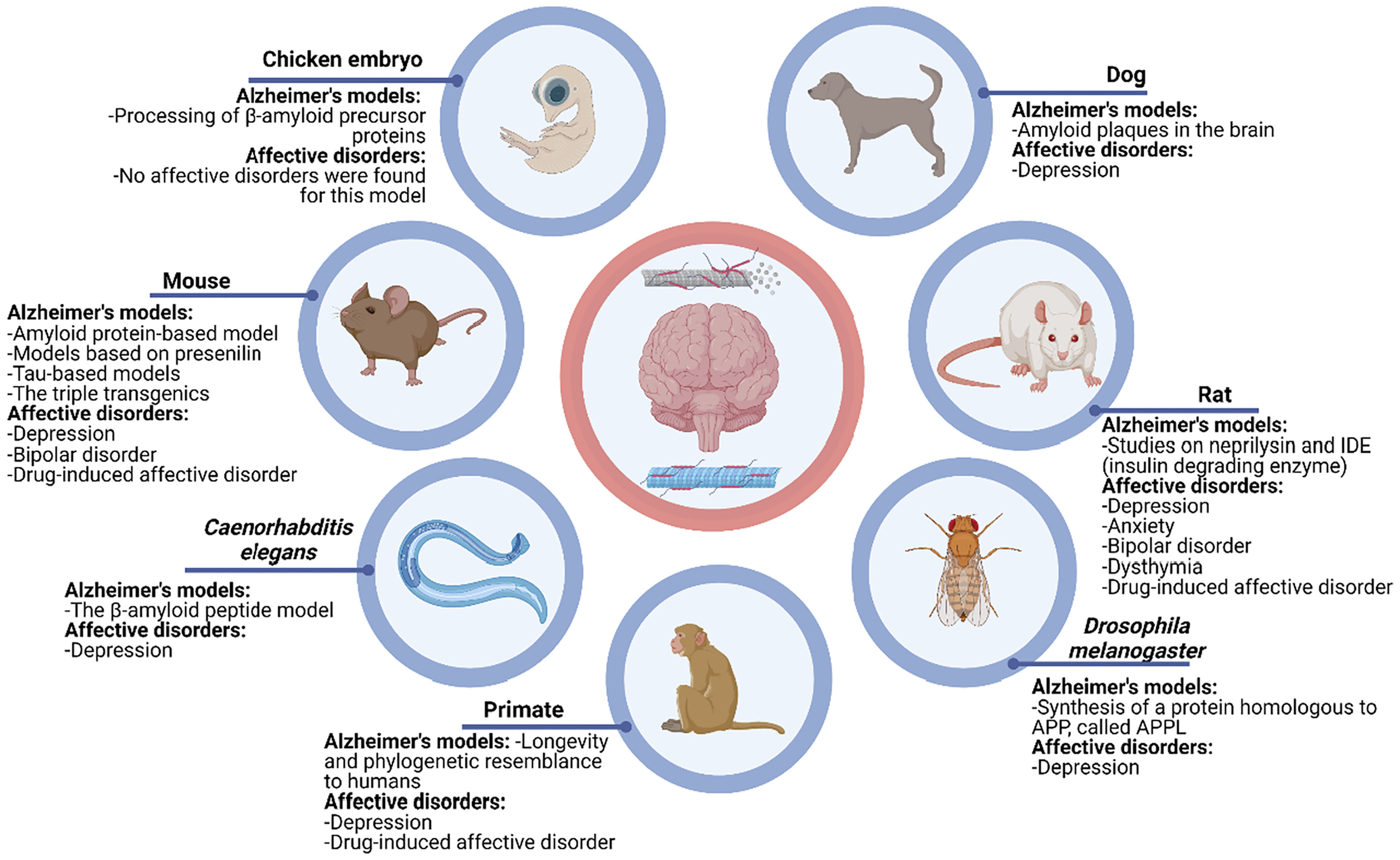
The AD study of animal models does not exclude the fact that they are partially representative of the pathophysiological events under study since no animal model incorporates all the characteristics present in humans and the diagnostic/clinical criteria of AD, thus the conditions analogous or similar to the disease that are induced in these models are not referred to as Alzheimer's Disease; however, these models are very useful for the study of various alterations involved in the pathophysiology of AD, mainly including the alteration of memory capacity due to the similarity and analogy of manifestations, so this research paper aims to compare the different animal models of AD (Fig. 1).
The animals that stand out for having a histopathological pattern like that of humans, are elderly monkeys, dogs, cats and mice, so these species are considered advantageous in terms of eligibility for AD models, however, they are not very practical.10,11 Therefore, various species have been used that are more practical and appropriate for the study of AD, as mentioned below:
Mice modelModel based on APP generationThere are models that consist of transgenic mice that express the amyloid precursor protein (APP) similar to that generated in humans, whereby this protein is a key piece in Aß generation since this derives from the proteolytic cleavage of APP, in AD this process is considered to be increased which leads to excessive Aß generation and its accumulation in the interstitium in the form of amyloid plates, and in conjunction with the neurotoxic nature of Aß lead to alterations such as interference in the synaptic communication of neurons, atrophy of the cellular processes of neurons (axons and dendrites), glial activation, alterations of the cellular metabolism of glucose and neuronal apoptosis, whereby cholinergic neurons are the main ones affected followed by serotonergic, noradrenergic, gabaergic and glutamaergic neurons, initially in the hippocampus region and also progressively in limbic system areas, which represent structures that are significantly linked with the memory process.7,13,14
This coincides with observations in the PDAPP mice model, which exhibits extracellular deposits of Aß and neuritic plaques and microgliosis between 6–9 months of age. Most APP mice do not develop NFT and no neuronal loss is observed in the entorhinal cortex, CA1 or cingulate cortex.14 To generate this model, the platelet-derived growth factor promoter region is isolated, and it is used to express the cDNA (an APP minigene) with the V717F mutation, allowing it to express the 3 human APP isoforms (APP695, 751 and 770).15 Although this model meets important molecular characteristics of AD such as the increased Aß generation and the presence of amyloid plaques.
In this review were found some studies behavioral and psychological symptoms of dementia related with AD, in mouse models. Some patients of AD present these symptons, that includes depression, anxiety, lost sociability.58 Among the mouse models, the APP/PS1 is a doble transgenic mouse model where overexpresses the amyloid precursor protein and presenilin 1,59 Tg2576,60 3xTg,11 presented genotype effects in a anxiety test but different according to behavioral test (e.g. the open-field, elevated plus maze and light dark box). These results are not consistent with another behavioral studies.
Models based on presenilin (PS) and ß-secretasePresenilin 1 (PS-1) is a 467 amino acid protein encoded by the S 182 gene located on chromosome 14. Genetic variants of PS-1 as well as those of presenilin 2 (PS-2) increase the proteolysis of APP by the γ-secretase, protein complexes composed of PS-1, PS-2, nicastrin and APH-1, which leads to increased Aß generation. These variants have been particularly associated with the development of early-onset familial Alzheimer's Disease (EOFAD).16–18 The process of Aß generation requires the proteolysis of APP by both γ-secretases and ß-secretases, these are transmembrane proteins, whose main subtype associated with AD is BACE1.19
The cross breeding of APP and PS transgenic mice presents acceleration of Aß deposition. On the other hand, the APP and BACE transgenic model has an increased turnover in serotonin and a higher aggressive behavior than in the control groups.20,21 In the research by Hartmann et al. of this model, it was shown that “OPEN FIELD” tests do not show significant differences compared to the control group, in terms of acetylcholine release;21 however, in this review, no reports of clinical manifestations additional to the molecular findings present in these models were found.
Models based on TauTau is a protein that belongs to the family of microtubule-associated proteins (MAP) given that it has the ability to interact with tubulin thus favoring the polymerization of microtubules, for which it undergoes a dynamic process of phosphorylation and dephosphorylation, in its phosphorylated state it cannot bind to microtubules which allows the reorganization of the cytoskeleton, which is crucial in processes such as cell division and neuroplasticity.22 In AD, the process of phosphorylation and dephosphorylation is presumed to be altered, generating the abnormal hyperphosphorylated form of Tau protein, which tends to self-aggregate, eventually forming the pathognomonic neurofibrillary tangles of AD.23,24 There are 6 isoforms of this protein in humans, 3 that possess 3 repeats of the microtubule interaction domains (group 3R) and 3 that possess 4 repeats of the microtubule interaction domains (group 4R), the mice possess only the 4R group isoforms. The combination of the mice model that expresses the human tau protein (P301L)-JNPL3- which causes neurofibrillar degeneration with Tg2576 mice that overexpresses APP, exposes NFT production in the limbic system and olfactory cortex, in conjunction with Aß deposits. As for behavioral alterations of this model, a significant decrease in spatial memory retention has been reported, through the “water maze” test.25,26
Triple-transgenic modelThe term triple-transgenic alludes to the triple mutation present in these models, highlighting those that exhibit the APP/PS/Tau mutation; in which Aß deposits are observed with a heterogeneous distribution in brain regions, whereby the neocortex and hippocampus are more pronounced, and correlated with age; in addition, it was observed that the increased generation of Aß preceded the generation of pTau and decrease in long-term potentiation (LTP), a mechanism involved in the memory and learning process. Although the triple mutation that is characteristic of this animal model does not occur in humans, it allows for the study of events related to Aß deposition, speculation regarding the temporal relationship between the generation of Aß and the generation of pTau and association of these molecular phenomena with alterations of synaptic transmission.11
Chicken embryo modelIt is considered a “natural model” for APP processing anomalies and Aß generation, it can present alterations of these mechanisms spontaneously (not induced by drugs or interventions of any other nature). However, such alterations can also be induced by cloning the major isoforms of the amyloid-beta precursor protein in the chicken embryo, until obtaining high homologation with the human sequences and the identical C-terminal sequence, including the Aß domain. From this model, it can be highlighted that it expresses genes of the main proteolytic proteases involved in the production of Aß, including BACE-1, BACE-2, presenilin-1, presenilin-2, nicastrin, and the disintegrin and metalloprotease 17 (ADAM-17) enzymes, a protease involved in the non-amyloidogenic processing of APP. The characteristics of this model allow us to consider it suitable for AD study with a molecular approach with regards to Aß generation and pharmacological therapies aimed at these mechanisms.27
Drosophila MelanogasterDrosophila Melanogaster is one of the most used models for genetic research, due to the vast information of its genome, and additionally it is considered a relatively inexpensive model, easy to handle given its biological characteristics, including specifically this model's size and short life cycle of 10 days, allowing for the rapid reproduction of the specimens and the rapid evaluation of the progressive impact of aging, as compared to other models with longer life cycles. 70% of human genes that are related to our species' diseases have homologation with the Drosophila genome.28,29
The use of Drosophila to study Alzheimer's disease has been reported in the Aβ model, APP/BACE1 and based on the Tau model.61 The Aβ model consists in producing transgenic flies (E.G. Aβ42 transgenic Drosophila model) with Aβ peptide-induced amyloid formation and neurodegeneration using the GAL4/UAS system.61 In this model, it expresses a single homologous protein of the human APP, called APPL (Amyloid-precursor like protein), and the functional substitution capacity of this species must be considered, since, when inserting a transgene targeted to APPL or with human APP it is able of recovering the behavioral defects, which indicates that the APP functions are preserved in Drosophila. This model also allows in vivo analysis of the differential effect of Aβ variants. One of the studies showed that the expression of Aβ42 in photoreceptors caused a disorganization phenotype that worsened with age and the progressive accumulation of said peptide, on the other hand, the expression of this peptide in the brain reduces the survival of adult flies.28,30 Although a decrease in longevity and neurodegeneration has been reported in this model, no behavioral test reports associated with the degree of neurodegeneration were found in this review.
The model APP/BACE1 refers the deposits of Aβ were generated sequential proteolytic in the amyloidogenic pathway by APP, where they participate two enzymes: β-secretase (β-site APP cleaving enzyme or BACE-1) and γ-secretase. So, the Aβ cleaving enzyme (BACE-1) is a target for AD treatments.63 It was reported in Drosophila larvae, they expressed human APP and BACE transgenes, it was reported that expression of human APP and BACE in motor neurons affects mitochondria. This model showed the loss of synaptic and behavioral defects. It correlated with there showed the loss of locomotion with fewer synaptic connections to neuromuscular junction.64,65
In addition, the Tau model with flies focus on the role of hyperphosphorylation in tau toxicity, pathways related with Wnt, JNK, TOR were presented in humans.61,62 The fruit fly has a tau homolog and it expression induces learning and memory deficits.
Caenorhabditis ElegansSimilarly to Drosophila, the Caenorhabditis elegans genome is widely characterized and it is also considered a practical model due to its easy handling associated with the relatively small size of this species and its inexpensive maintenance, the life cycle of this model is 3 days and it reproduces on a large scale compared to other species; where a peculiarity of this model is that its nervous system is composed of 302 neurons, favoring the understanding of some aspects of neurodegenerative pathologies. From the Caenorhabditis elegans species, two AD models stand out; one focused on APP and another focused on pTau.31,66,67 The first model has the APL-1 gene (APP ortholog), which expresses the PAL-1 protein which lacks the Aβ domain, additionally this model encodes 3 presenilin orthologs (sel-12, hop-1 and spe-4) and nicastrin whose gene products combine to form the γ-secretase complex. However, the C. elegans genome does not encode a β-secretase (BACE ortholog). As for the pTau C. elegans model, it has a single tau ortholog, called ptl-1; which is expressed non-exclusively in the mechanosensory neurons of C. elegans; the ptl-1 alteration decreases the number of viable progenies, and these animals are discreetly less sensitive to body touch. Given that the loss of ptl-1 function does not recapitulate tau pathology, the transgenic C. elegans model expressing human tau has been chosen. Pan-neuronal (aex-3 promoter) expression of the most abundant human brain tau isoform (4R1N) and mutant tau (V 337 M and P 301 L) resulted in uncoordinated locomotion.32,33
The rat modelAmong AD rat models, those focused on neprisilin and the insulin-degrading enzyme (IDE) stand out as the main proteases involved in degradation of Aβ. Rat models have also been used to study the expression kinetics of APP isoforms in response to motor neuron axotomy and APP expression in embryogenesis. One of the aspects that have been pointed out as a disadvantage of the rat model is the longevity and mainly the great difference in the mechanisms of APP processing when compared to the mechanisms in humans.34,35
Although it is considered that the generation of transgenic rat models has advanced more slowly than transgenic mice models, the homozygous App knock-in rat model also called App NL-GF has the ability to express human APP with the advantage that it does not overexpress it as it occurs with other models, in terms of the similarities with the alterations exhibited by AD subjects, whereby the App NL-GF model showed there was an increase in the production and accumulation of Aβ and the formation of amyloid plaques in the brain but not in the cerebellum, gliosis, atrophy of cellular processes (axons and neurites) in the hippocampus, entorrineal cortex and prefrontal cortex, neuronal loss in the hippocampus and cortex, increase in the generation of pTau associated with age, although it differs in the lack of neurofibrillary tangles generation, deficiencies in the memory of spatial learning and discrete increase in anxiety-type behavior.36
The dog modelAlthough a great advantage in achieving large specimen volumes in a short time is due to the short life cycle, longevity, does not match those models with regards to the human AD model, this is not the case of the dog. On top of that, as old age is reached, amyloid plaques form in the brain that are similar to those in humans and some long-lived breeds (such as teckel, yorkshire and poodle) suffer from symptoms that are similar to those of AD such as cognitive impairment related to age and over time they can reach canine dementia even more similar to AD with short- and long-term memory loss, behavioral changes, incontinence, spatial memory loss and even amnesia.37 On the other hand, behavioral assessment is easier in dogs than in some other models. Due to its size, other types of tests can also be conducted to complement the diagnóstico such as CAT (computed axial tomography), EEG (electroencephalogram), general laboratory and cerebrospinal fluid tests that allow for the evaluation of multiple parameters including amyloid peptides. In addition, canine APP isoforms have been cloned and sequenced and they have been found to be virtually identical to humans, including the amyloid peptide sequence, which makes the dog one of the best models in order to study AD.34
Non-human primate modelIn practically all animal models of neurodegenerative diseases, the non-human primate model must be kept in mind, due to longevity and phylogenetic similarity with humans. Unfortunately, they are difficult to preserve and handle and they require a large investment of financial resources for their upkeep.38
Among the presently established animal models, non-human primates share the closest relationship with humans, and their neural anatomy and neurobiology share highly similar characteristics with those of humans. Thus, there is no doubt that these play an irreplaceable role in AD research.
In this studies, multiples non human primate were included, rhesus monkeys, chimpanzees, crab-eating macaques, stump-tailed macaques, lemurs, vervet monkeys, marmosets and baboons, each one with characteristics of weight and height, life expectancy, age of maturity and old age.
Models induced by Aβ injectionAβ1–40 was injected into the prefrontal cortex of old rhesus monkeys, while the control groups were injected with nontoxic peptides, peptide fragments (CA4) and antisense Aβ.1–40 The results revealed that the Aβ1–40 group developed cerebral cortex injury, which was dose-dependent and obviously larger than that in the control group. Furthermore, young monkeys did not develop visible changes of neuronal bodies or axons. These experiments corroborate the neurotoxic effect of Aβ in the brain tissues of non-human primates and reveal that the cytoskeletal response to Aβ is specific and age-related.
Nevertheless, these types of models also have certain disadvantages, the success or failure of the model establishment is influenced by many factors, such as the Aβ form, its purity, and the vehicle form and components, and the requirement for aged animals also somewhat constrains related research.55,56
Streptozotocin models of ADStreptozotocin (STZ) is a methyl nitrosourea sugar compound with a molecular weight of 265 Da, and antimicrobial and antitumor effects.
The intracerebroventricular injection of STZ (ICV-STZ) can disturb the phosphorylation of the insulin receptor in the central nervous system, which blocks the insulin signaling pathway. This leads to reduction in function of the cholinergic nervous system, at the same time, the dysfunctional expression of insulin degrading enzyme prevents the timely and efficient clearance of Aβ, leading to its accumulation. A blockage in the insulin signaling pathway leads to increase in the activity of its downstream target GSK-3β, and induces tau hyperphosphorylation, which is a classical feature of AD.
Yeo et al. used an ICV-STZ to establish an AD model in crab-eating macaques. The results revealed that ICV-STZ induced severe ventricular enlargement and substantial brain atrophy, accompanied by Aβ deposition, loss of neurons in the hippocampus, tau hyperphosphorylation, loss of ependymal cells and astrocytes, and the activation of microglia. These changes were all also observed in the brains of elderly or AD patients.
Park et al. used an ICV-STZ AD model in crab-eating macaques to quantitate the expression of genes related to APP and tau phosphorylation. The results revealed similar gene expression levels in the ICV-STZ and control groups, but there were significant differences in gene expression patterns between these two groups.
Although STZ has been used to establish AD models in rodents in increasing numbers of studies, corresponding research in non-human primates remains limited. It is also possible to adopt the advances made in rodent models, and use the intravenous injection of STZ for the establishment of a non-human primate model of DE, in order to study the mechanistic relationships between age-related dementia, AD and the specific mechanism of IR. These models can also be used to screen and confirm neuroprotective drugs.55
Based on genetic engineeringA Chinese research team produced the first genetically modified model of Parkinson's disease in monkeys in 2015. Genes closely correlated to AD pathogenesis (such as APP, PSEN1, PSEN2, APOE, etc) can be used as potential target genes for gene modification for modeling. Meanwhile, tau protein-related genes can also be regarded as an important target for the modeling of gene modification methods ethical issues.51,53,55
Sato et al. introduced a pathogenic mutation in the marmoset PSEN1 gene because the majority of familial AD-causing mutations reside in the PSEN1 gene35. Typically, deletion mutations in exon 91–5 or point mutations at the 3′ splice site (acceptor site) of exon 9 in the PSEN1 gene cause dominantly inherited familial AD. They set out to generate a marmoset model of AD in which exon 9 of PSEN1 gene product is deleted using TALEN to produce AD in these animals.53
Fibroblasts obtained from newborn marmosets exhibited uncleaved full-length presenilin 1 protein (PS1) caused by the perturbation of PS1 endoproteolysis as well as an increased ratio of Aß42/Aß40 production, a signature of familial AD pathogenesis. This is the first nonhuman primate model of familial AD.53
Hyperphosphorylated tau modelTargeting the vulnerable entorhinal cortex (ERC), rhesus monkeys received two injections of an adeno-associated virus expressing a double tau mutation (AAV-P301L/S320F) in the left hemisphere. Among the markers analyzed for neuronal damage, neurofilament light (NfL) had the highest increase in CSF and plasma in injected monkey, as well as total tau in the CSF. There was also a temporal reduction of BDNF levels. Observed a high increase of p-tau levels in three different epitopes related to NFT pathogenesis in the CSF of treated monkeys and AD controls: tau phosphorylation at Ser199, at Thr 231 and at Ser 396. All these sites of phosphorylation are known to cause a structural impact in tau interaction with microtubules and are reflections of the disease's progression.54
Mood disorders models in animalsMood disorders (also called affective disorders) are psychiatric diseases that affect emotions, energy and motivation in a diverse and simultaneous manner, aspects that are also affected by neuropsychiatric symptoms and syndromes, which is why they are often poorly diagnosed and confused with each other; however, an important difference between the two is that neuropsychiatric symptoms and syndromes derive from a disease that may or may not be of a psychiatric nature and they are modified depending on its own progress, for example diabetes or Alzheimer's disease; with regards to mood disorders, the main indicator of these diseases are major depression disorder and bipolar disorder.39
Depressive affective disorders include clinical depression, chronic depressive disorder (dysthymia), bipolar disorder, mood disruptive dysregulation disorder, dysphoric premenstrual disorder, affective disorder due to a general medical condition, and drug-induced affective disorder.40 Thanks to multiple TAD models carried out in animals, greater understanding has been obtained about the behavioral and molecular implications of these disorders; where the main specimen of these models are rodents, given that in both mice and rats, depression, bipolar disorders, dysthymia, and drug induced affective disorder models have been conducted. In primates, ADD models have also been carried out in depression and drug-induced affective disorder. On the other hand, in some other less common models such as with the dog, Drosophila melanogaster and Caenorhabditis elegans, genes associated with behaviors such as fear and anhedonia for ADD models have been studied.41–50
DiscussionThe purpose of this article was to describe the different animal models that exist on AD, biological plausibility of the models and finally to mention the different mood disorders of each model (Table 1).
Summary about advantages and disadvantages in AD animal models.
| Experimental models | Advantages | Disadvantages |
|---|---|---|
| Mice (Mus musculus) | APP generation: emphasis on molecular characteristics as increase Aß generation, presence of amyloid plaques, tau pathology and neurodegeneration process.Transgenic model: it was observed that the increased generation of Aß preceded the generation of pTau as hallmarks lesion of AD36,57 | APP generation: there is a discrepancy between studies according to the behavioral test behaviors similar anxiety in models: APP/PS1, 3xTg-AD, Tg2576, 5xFAD, APP2358Transgenic model had contributed to the study of AD pathways but in the human clinic it is not possible to see it11 |
| Rat (Rattus novergicus) | It is also useful this model to study the expression kinetics of APP isoforms in response to motor neuron axotomy and APP expression in embryogenesis. | The longevity and mainly the great difference in the mechanisms of APP (amyloid precursor protein) processing when compared to the mechanisms in humans.34,35 Generation of transgenic rat models has advanced more slowly in comparison than mice models |
| Fruit fly (Drosophila Melanogaster) | The Aβ, APP/BACE1 and the Tau are models to allow understanding pathogenetic processes occurring in the AD brain and biological pathways.61 | Although it shares a large number of genes with humans, it does not have a great homology with the structures of the brain, and it is not comparable with tests for the evaluation of hippocampal cognitive functions.66 |
| C-Elegans (Caenorhabditis Elegans) | This model has a known number of neurons and its average lifespan is 3 days, which makes it possible to follow up due to a process of neurodegeneration in long-lived stages.312 models: APP and pTau.31 APP model: APL-1 has 71% sequence similarity to the intracellular domain of APP66pTau model: the loss of ptl-1 no encompasses tau pathology, but a transgenic model express human tau. It allows neurobehavioral analysis: the toxicity of Aβ is assayed by parameters and tests as chemotaxis, odorant preference, motility, locomotion, lifespan, and egg-laying67 | The C. elegans genome lacks a β-secretase ortholog and an ortholog of APP that would be processed to yield Aβ peptides. APL-1 is an ortholog of the proteins APLP1 y APLP2 (human amyloid beta precursor-like proteins 1 and 2) and has a lack of Aβ domain66 |
| Chicken embryo (Gallus gallus domesticus) | It allow to study molecular characteristics about Aß generation and pharmacological therapies. The chicken APP gene is identical to humans, and the Aβ sequence is the same27,69 | It is considered that for the study of adult or long-lived chickens there is not enough evidence of amyloid plaques in the brain or any neurobehavioral syndrome related to dementia but the expression of APP gen is related with memory consolidation in an imprinting task, in early stages of AD70 |
| Dog (Canis lupus familiaris) | It can study behavioral aspects, it's easier or similarity in comparison with another models. By its size, other types of tests can also be conducted to complement the diagnóstico such as CAT (computed axial tomography), EEG, general laboratory and cerebrospinal fluid tests.37 | It cannot have numerous samples that reach long-lived stages (e.g. a big n sample in control studies).68 |
| Non-human primate model | Aβ injection: There is evidence that corroborates the presence of a neurotoxic effect of Aβ in brain tissue, correlating with the fact that the response of the cytoskeleton to Aβ is specific and related to age. | Aβ injection: Exist some factors that defined the success of the model such as the Aβ form, its purity and the vehicle form and components, and the requirement for aged animals also somewhat constrains related research.55,56 |
Biological plausibility is one of the Bradford Hill criteria and it makes reference to the pathophysiology of the disease and whether the association studied is plausible, if it makes sense or if it is credible; in other words, whether it can be characterized within the knowledge that is available in this regard. In certain associations there is no scientific evidence at the time of discovery; however, after a while it can be understood52.
The main limitation of this section was that a quantitative means of measuring this section (biological plausibility) was not found, so the research and writing ended up being qualitative, which is no less important for the reader.
These animal models to study Alzheimer's disease are important due to their usefulness for pathophysiology and possible pharmacotherapeutic targets in preclinical studies, but as in any animal model, it is always limited to cover all the components of the disease.
In this review, 7 animal models and a model based on cell culture were described, each with its advantages and disadvantages, both due to the similarity with humans and their behavior, as well as the ability of their reproduction in each models. As described in this article, models for AD are mainly based on presenilin (PS) and ß-secretase, pTau, where the researcher's question of a specific aspect of the disease allows the animal model to be chosen. As for the mouse models, in the transgenic ones that express the amyloid precursor protein (APP), advantage was found that it brings together important molecular characteristics of AD, such as the increased generation of Aß and the presence of amyloid plaques. In the one based on presenilin (PS) and ß-secretase, pathological changes in serotonin levels and acceleration of Aß deposition were seen, without additional clinical manifestations to the molecular findings present in these models. And in mouse tau model, there are 6 isoforms of this protein in humans, 3 possessing 3 repeats of the microtubule interaction domains (3R group) and 3 possessing 4 repeats of the microtubule interaction domains (4R group), mice possess only the isoforms of the 4R group, which could be taken as disadvantage. Regarding the behavioral alterations of this model, a significant decrease in spatial memory retention has been reported, through the “water maze” test. The murine models offer other types of advantages, such as observing excessive Aß generation in transgenic lines and their respective interaction with other neurotransmission systems or signaling pathways, alteration of cellular metabolism, apoptosis, etc. Likewise, depending on the specific model, there is a discrepancy between studies according to the behavioral test behaviors similar anxiety in models: APP/PS1, 3xTg-AD, Tg2576, 5xFAD, APP23. It is known that many patients with AD may present psychological and behavioral symptoms of dementia, including anxiety, apathy, agitation, aggressiveness and even reduced socialization.58
Rat models generally focus on neprisilin and insulin-degrading enzyme (IDE), major proteases involved in Aß degradation. The homozygous APP knock-in rat model can express human APP with the advantage that it does not overexpress it. Unlike other models, in terms of similarities with the alterations presented by subjects with AD, it differs in the lack of generation of neurofibrillary tangles, deficiencies in spatial learning memory and discrete increase in anxiety-type behaviors.
The chicken embryo model can be considered suitable for the study of AD with a molecular approach with regard to the generation of Aß and pharmacological therapies aimed at these mechanisms, due to great similarity in the expression of isothermal patterns, however, the question arises whether the aging adult chick is a good experimental model for Alzheimer's research, since aging is a fundamental epigenetic factor for Alzheimer's disease. To date it is not known whether during aging the chicken embryo is able to form the characteristic cerebral amyloid plaques and may suffer from age-related dementia.
In the models of the fruit fly and C-Elegans, invertebrate animals, the characteristic of being generous models prevails, due to their manipulation due to their development cycle and short lifespan, in terms of the objective of longevity and evaluation of aspects related to dementia at a level of chemical-genetic analysis. To these two models, the chicken embryo can be added, because it also allows modeling molecular aspects, for example the chicken APP gene is identical to humans, and the Aβ sequence is the same. Drosophila Melanogaster is one of the most used models for genetic research, due to its genome, cost, reproduction of specimens and rapid evaluation of the progressive impact of aging, focused on APPL (amyloid precursor type protein) and Aß, but lacking in terms of behavior associated with the degree of neurodegeneration. As for Caenorhabditis Elegans, it has a shorter life cycle than Drosophila Melanogaster, with a nervous system composed of 302 neurons, favoring the understanding of some aspects of neurodegenerative pathologies. Include two models, the APL-1 gene and pTau C. elegans, however, not very clear in their results, genetic techniques can be applied to identify genes that modify the tau-induced phenotype and in mammals, due to the complexity of the nervous system and the lack of sensitive behavioral measures, it can be extremely difficult to establish cause-and-effect relationships for pathogenesis. Perhaps the main disadvantage of these models is the comparable neurobehavioral evaluation with mammals. Such evaluation is important to further understand aspects of impaired locomotion and cognition in late adulthood.
To mice and rats, we must add that the model of the dog and non-human primates, exhibit advantages of presenting more similarities in the behaviors related to Alzheimer's disease in a long stage of life. Nonhuman primates especially share with humans a phylogenetic similarity that allows comparisons with homologous structures. However, the accessibility and high cost of maintaining a significant sample in controlled studies makes research centers rethink their use. The dog model could be considered superior due to the advantages of the formation of amyloid plaques in the brain like humans and some long-lived breeds who suffer symptoms like AD, the ease of behavior evaluation and the performance of tests to complement the diagnóstico. Among their main limitations is the shortage of screening tests for use in domestic dogs to predict amyloid accumulation and/or response to therapy. The non-human primate, we describe those based on induced by injection of Aß, Streptozotocin models, hyperphosphorylated tau and based on genetic engineering. With similar results in the pathophysiological changes of AD, longevity and phylogenetic similarity with humans. Unfortunately, they are difficult to conserve and manage, they also require a large investment of economic resources for their maintenance, their multiple species each with different lineage, life expectancy, size, weight, which represent important limitations to consider.
Regarding the cell culture-based model, as mentioned, three-dimensional (3D) models of in vitro AD using induced pluripotent stem cells (iPSC) - derived neurons show potential advantages in providing better physiology relevant information and more predictive data for in vivo tests. Being the main weakness, insufficient maturation and aging of neural cells and the lack of functional tests such as behavioral evaluations.
Currently, there is a wide range of options in using animal AD models, however, the behavioral component is rarely assessed in these models and therefore they are not optimally characterized; behavioral assessment of the models is a key piece in understanding the phenomena involved in AD and it is a fundamental pillar in human diagnóstico, the results obtained from this review point out the need to supplement molecular assessments with behavioral assessments in animal AD models, thus improving the understanding of said disease. The mood disorders that have been studied are depression, anxiety, dysthymia, bipolar disorder, and drug-induced affective disorders, whereby the animal AD model in rats is the most used for assessments focused on mood/affective disorders related to AD. Furthermore, there are multiple pathophysiological models of AD, in different animals and in the last decade, models of cell differentiation from pluripotent hematopoietic cells. All this to establish the pathophysiological pathways of the disease, and therefore, seek therapeutic targets with better clinical responses and consequently, decrease the progression and limitations of the progression of AD.
On the other hand, to avoid making this article too lengthy we opted for one section only which makes general mention of the different mood disorders that were found in each animal model, and it was classified in Fig. 2; however, if the reader wishes to read more in depth about the topic, the references may be approached to do so.
Alzheimer's models and affective disorder models.41–50
These 7 animal models of AD were found, among them the rat model has been the most studied in terms of mood/affective disorders. We believe that more studies are needed where molecular changes are correlated with the animal behavior of AD. The mood disorders that have been studied are depression, anxiety, dysthymia, bipolar disorder, and drug-induced affective disorders.
FundingNone.
Patient consent (Informed consent)This form does not apply due the type of article (review).
Ethics in publishing1. Does your research involve experimentation on animals?:
No
2. Does your study include human subjects?:
No
3. Does your study include a clinical trial?:
No
4. Are all data shown in the figures and tables also shown in the text of the Results section and discussed in the Conclusions?:
Yes
The author G.M. of this paper want to thank Secretaria de Educación, Ciencia, Tecnología e Innovación de la Ciudad de México (SECTEI) for totally support granted to carry out this research. Figures were created with BioRender.com.