We read with great interest the consensus statement from the Spanish Society of Neurology's Headache Study Group on the diagnosis and treatment of trigeminal neuralgia (TN), which was recently published in the journal Neurología.1 The article analyses the scientific literature on the diagnosis and treatment of the disease and establishes practical recommendations with levels of evidence. We would like to add some comments and reflections regarding this consensus document.
One challenge in the study of a safe and effective pharmacological treatment for TN is the relatively small population of patients from which to recruit participants for clinical trials and case series, as well as the limited number of studies reflecting real-world clinical practice, in which combination therapy is frequently used. Thanks to advances in neurology and drug development, a wide range of pharmacological agents are available for the treatment of TN. The guidelines recommend first-line treatment with voltage-dependent sodium channel (Nav) blockers, specifically the subclass of carboxamides, but some patients continue to show resistance to these drugs. In addition, these drugs may cause adverse reactions that may limit treatment. Despite the wide variety of agents available, no optimal pharmacological treatment has been established.
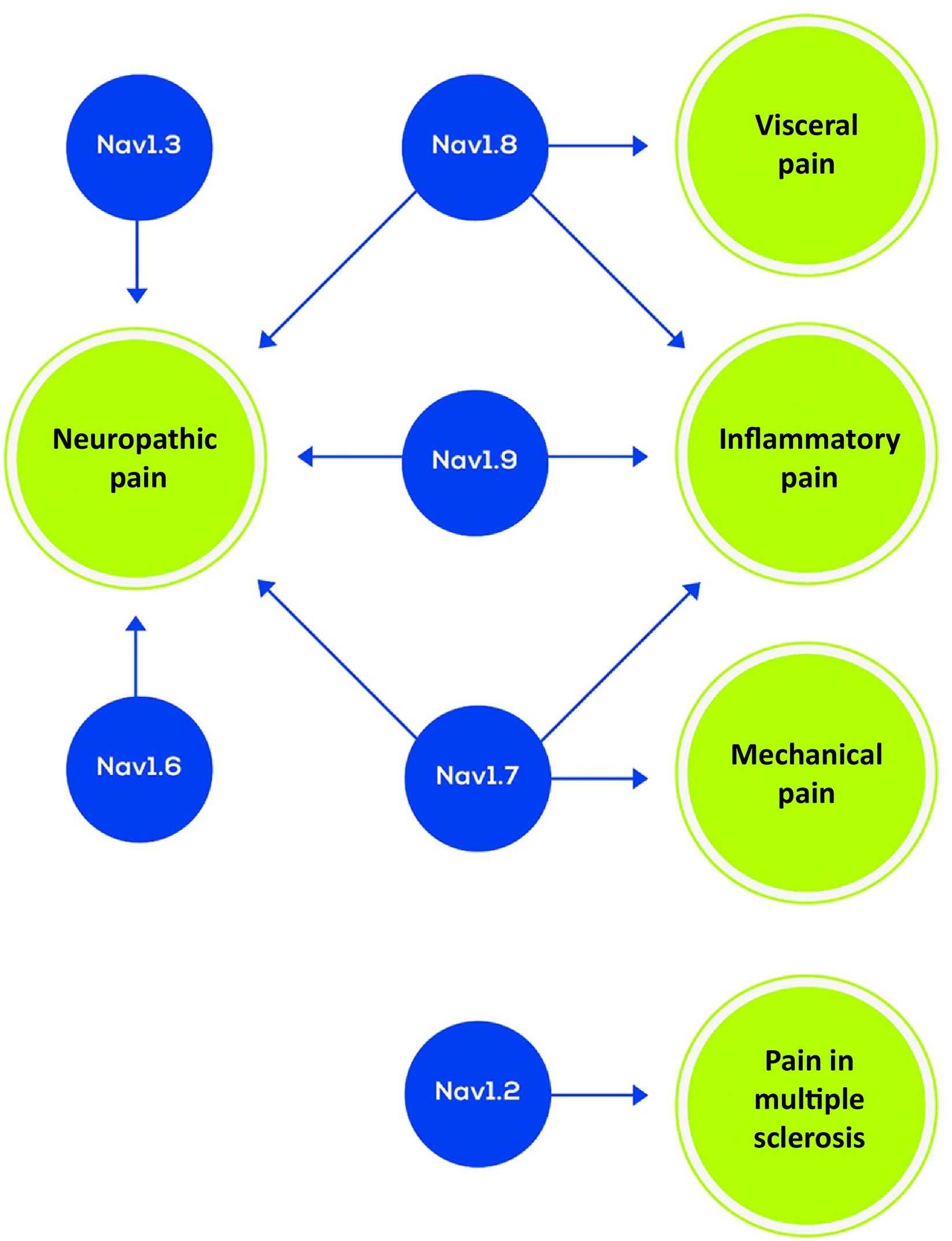
The Nav family has been considered in the development of drugs for neuropathic pain, and there is growing knowledge on the promising role of specific Nav as a therapeutic target for this type of pain. Nine Nav subtypes have been identified (Nav 1.1–1.9) and specific Nav have recently been identified that are associated with specific types of pain; this suggests that targeting treatment at hyperactive channels may constitute an oversimplification of the complex biological processes involved in pain signalling. Selective Nav blockers may represent an effective treatment with fewer adverse reactions.2,3 Further development of vixotrigine (originally named raxatrigine), a broad-spectrum Nav inhibitor, and the development of selective Nav blockers may represent important advances in treatment.4 Nav are crucial in the propagation and generation of action potentials, and the pharmacological interruption of their function may reduce neuronal excitability and impede the transmission of pain signals (Fig. 1). Nav 1.3, 1.7, and 1.8 act on neuropathic pain and Nav 1.9 seems to act on inflammatory syndromes (Fig. 2).2
Carboxamide action mechanism (image courtesy of Carlos Goicoechea García).
Cav: voltage-dependent calcium channel; CBZ: carbamazepine; ESL: eslicarbazepine acetate; GluR: glutamate receptor; Nav: voltage-dependent sodium channel; OXC: oxcarbazepine.
These sodium channel blockers are able to inhibit the entry of sodium and, consequently, the depolarisation of the neuron. This hyperpolarisation inhibits the entry of calcium and therefore the fusion of the glutamate vesicle to the synaptic membrane, slowing the transmission of the nociceptive impulse. Carboxamides bind both to the activated and to the inactivated conformation of sodium channels.
Voltage-dependent sodium channel subtypes and associated pain syndromes (adapted from Álcantara Montero et al.2).
Multimodal analgesic regimens have been beneficial for many disorders and may be especially valuable in the treatment of TN. Using a combination of 2 or more agents with complementary action mechanisms may reduce adverse effects without compromising the analgesic benefit.5,6 Some combinations of drugs present additive or synergistic benefits. For example, baclofen has been reported to have synergistic benefits when administered simultaneously with carbamazepine or phenytoin.7 However, drug–drug interactions have also been reported for the combined administration of carbamazepine or phenytoin with baclofen, leading to an exacerbation of adverse reactions, including dizziness and mental confusion.8 Despite this, there is little evidence to support the decision to prescribe combination therapy in the medical treatment of TN.9
The consensus document presents certain limitations. Although its review of pharmacological options is extensive, it does not address all the drugs used. Topical agents may be used, such as lidocaine 5% medicated plasters and capsaicin 8% patches, which have shown benefits in short case series.10 This wide range of drugs demonstrates that the search for an optimal treatment is ongoing. Complementary and alternative medicine is also used to treat TN, probably due to the lack of an optimal agent.11 Another challenge in finding optimal pharmacological regimens is the heterogeneous aetiology of TN, as well as the genetic polymorphisms that may also have an influence.12
In conclusion, although its prevalence is relatively low, TN may affect quality of life. Several pharmacological treatments, used either in monotherapy or in combination therapy, may alleviate symptoms; however, the first-line agents carbamazepine and oxcarbazepine are associated with adverse reactions that may limit treatment, although both drugs are highly effective in most patients. Such innovative approaches as the use of botulinum toxin type A infiltrations, topical lidocaine and capsaicin, or synergistic combinations of drugs may be effective. TN managed with pharmacological treatment is, on many occasions, a chronic condition; therefore, the optimal pharmacological treatment must be well tolerated. Selective and broad-spectrum inhibition with Nav blockers seems to provide promising solutions, as do combination treatments. However, combination therapy for TN is an important area for further research.
AutoríaHemos tenido en cuenta las instrucciones, las responsabilidades éticas, cumplimos los requisitos de autoría y declaramos la no existencia de conflicto de intereses.
FinanciaciónEste trabajo no ha sido presentado en la Reunión Anual de la SEN o en otras reuniones o congresos, y no ha recibido financiación por un organismo público ni privado.