Alzheimer's disease (AD) is characterised by a progressive decline in cognitive abilities, especially learning and memory. To validate the zebrafish as a suitable model organism for AD, the study examined the effects of 2 neurotoxin agents, aluminium chloride (AlCl3) and okadaic acid (OKA). In the full experimental design, both neurotoxins were administered intraperitoneally at 3 distinct doses (low, medium, and high) twice weekly for 21 days. At 3 time-points, behavioural tasks were conducted on day 7 (short duration), day 14 (moderate duration), and day 21 (long duration). The behavioural tasks consisted of a novel tank test lasting 6 min, followed by a T-maze tank test lasting 5 min.
MethodsIn this article, the T-maze tank test was discussed in detail to evaluate which neurotoxins and their optimal dosages are impactful in developing a zebrafish AD model towards learning and memory functions. This evaluation measured four parameters: the amount of time spent in the wrong arm, the total distance travelled in the deeper chamber, and the 3-h and 24-h inflexion ratios.
ResultsIn summary, a 100 nM dosage of OKA with a maximum of 21 days of evaluation resulted in significant (P<.05) outcomes in all parameters evaluated. The longest duration was spent in the wrong arm, accompanied by a reduction in the total distance travelled in the deeper chamber and a decreasing pattern in the 3-h and 24-h inflexion ratios.
ConclusionThese observations suggest that OKA is the optimal choice of neurotoxin for a validated and optimised zebrafish AD model.
Enfermedad de Alzheimer (EA) se caracteriza por un deterioro progresivo de las capacidades cognitivas, especialmente en el aprendizaje y la memoria. Para validar el pez cebra como un organismo modelo adecuado para la EA, el estudio examinó los efectos de dos agentes neurotóxicos, el cloruro de aluminio (AlCl3) y el ácido okadáico (OKA). En el diseño experimental completo, ambos neurotóxicos se administraron por vía intraperitoneal en tres dosis distintas (baja, media y alta) dos veces por semana durante 21 días. En tres momentos diferentes, se llevaron a cabo tareas de comportamiento en el día 7 (duración corta), día 14 (duración moderada) y día 21 (duración larga). Las tareas de comportamiento consistieron en una prueba de tanque novedoso de seis minutos, seguida de una prueba de tanque en forma de T que duró cinco minutos.
MétodosEn este artículo, se discutió en detalle la prueba de tanque en forma de T para evaluar qué neurotóxicos y sus dosis óptimas son impactantes en el desarrollo de un modelo de EA en pez cebra hacia las funciones de aprendizaje y memoria. Esta evaluación midió cuatro parámetros: el tiempo pasado en el brazo incorrecto, la distancia total recorrida en la cámara más profunda y las relaciones de inflexión a las 3 horas y 24 horas.
ResultadosEn resumen, una dosis de 100 nM de OKA con un máximo de 21 días de evaluación resultó en resultados significativos (p < 0.05) en todos los parámetros evaluados. Se pasó la mayor duración en el brazo incorrecto, acompañada de una reducción en la distancia total recorrida en la cámara más profunda y un patrón decreciente en las relaciones de inflexión a las 3 horas y 24 horas.
ConclusiónEstas observaciones sugieren que el OKA es la elección óptima de neurotóxico para un modelo de EA validado y optimizado en pez cebra.
Alzheimer's disease (AD) is a prevalent neurodegenerative disorder affecting the central nervous system in older individuals. It is characterised by persistent and severe cognitive impairment, resulting in an acquired clinical syndrome of intellectual decline. The main clinical feature of AD is a gradual and progressive deterioration of cognitive function.1 Individuals diagnosed with AD experience impairments in memory, emotion, cognition, and spatial recognition, without any accompanying disturbances in consciousness.2 The progression of the disease is typically categorised into 3 stages: early, middle, and late. In the early stages, individuals may have memory impairment and visual–spatial disorientation, among other symptoms. The middle stage is characterised by a loss of independent living ability. In the late stage, patients may exhibit significant mental decline, limb rigidity, and other related symptoms. Ultimately, many patients succumb to the disease due to complications from associated infections. Currently, there is a lack of targeted pharmaceutical interventions to effectively treat AD or halt its progression.3
The T-maze tank is a valuable and widely used tool for conducting behavioural tasks aimed at assessing learning and memory functions in various animal models, including zebrafish.4 It serves as an effective means to investigate the cognitive abilities and memory processes of these organisms. The T-maze tank consists of a T-shaped apparatus with 2 arms and a central stem. The task involves placing the zebrafish, in the starting position at the end of the central stem and allowing it to explore and make choices between the 2 arms. During the T-maze task, the zebrafish learns to associate specific spatial cues or stimuli with particular arm choices.5 The experimental subjects consisted of individual zebrafish that underwent training to successfully navigate and reach the deeper end of the T-maze. Making correct choices led to rewards of spacious and favourable environments, while incorrect choices led to confinement in smaller, congested space.6 The evaluation of memory and learning is predicated on a multitude of parameters. The duration of time spent in the left zone, denoting the time the zebrafish invests in the arm deemed the “incorrect” or non-preferred option, is one of the parameters. This parameter may serve as an indicator of the fish's propensity for erroneous decision-making and its capacity to learn and retain the appropriate arm selection. An additional criterion is the distance covered within the preferred zone, which signifies the fish's inclination towards the arm that is deemed “proper” or preferred. This parameter provides information about the zebrafish's ability to recognise and recall the spatial cues associated with the preferred arm.7 The parameters obtained from the T-maze task help characterise learning and memory function in zebrafish, supporting research on neurological disorders. The task can be combined with genetic manipulations, disease models, or pharmacological interventions to investigate specific factors' impact on zebrafish learning and memory performance.6 In general, the T-maze tank serves as a robust experimental framework for investigating the cognitive processes of learning and memory in zebrafish. This allows scientists to delve into the intricacies of these processes and potentially make valuable contributions to the formulation of therapeutic approaches for disorders associated with impaired learning and memory.
In recent decades, zebrafish have been widely employed in various scientific investigations encompassing developmental biology, toxicology, pharmacology, and behavioural experimentation.8 Zebrafish demonstrate a range of behavioural responses, which can be classified into fundamental motor responses, such as sensorimotor reactions, that can combine to give rise to more intricate reactions associated with learning and memory. Zebrafish may undergo many behavioural tests, including the T Maze, Y Maze, light–dark test, novel tank test (NTT), hole board test, and inhibitory avoidance paradigm.9
The study conducted by Raduan, Ahmed10 investigated the effects of aluminium chloride (AlCl3) and okadaic acid (OKA) on zebrafish behaviour. The findings suggested that the administration of a minimum concentration of 100 nM of OKA resulted in neurotoxicity, as evidenced by the induction of AD-like behaviour in zebrafish throughout the NTT. The study assessed the endpoints associated with anxiety and locomotion following the administration of OKA for a minimum duration of 21 days. In an extension to respective research, a recent study aimed to validate and optimise whether AlCl3 or OKA successively contribute to the development of the zebrafish AD model by assessing their learning and memory performance using the T-maze test as a behavioural task.
MethodologyAnimal care and AD modelThe recent study, being an extension of Raduan, Ahmed,10 replicated the methods employed in terms of animal care and the establishment of the Alzheimer's (AD) model (Fig. 1a). The animal experiments conducted in this research were authorised by the Institutional Animal Care and Use Committee (I-ACUC) at the International Islamic University Malaysia (IIUM) Kuantan Campus, located in Pahang, Malaysia with approval no of IIUM/IACUC-2019(21). The experimental sequence mirrored that of Raduan, Ahmed,10 with the initiation of the novel tank assessment followed by a detailed exploration of the T-Maze in the subsequent stages of the recent study (Fig. 1b).
T Maze test: Learning and memory function testAfter having been exposed to the novel tank for 6 min,10 the zebrafish was then placed in the T-Maze tank for 5 min while being recorded. The T-maze was made up of 1 long arm measuring 45.72 cm and 2 shorter arms measuring 30.48 cm each. A square chamber of greater depth, with dimensions of 15.24×22.86×22.86 cm (width×height×length), was connected to one of the shorter arms. Kundap, Kumari7 have determined that the square chamber is an ideal habitat for zebrafish. According to Panula, Sallinen11 and Yang, Kim12 (Fig. 2a), this area was 1.97 cm deeper than any other location in the maze. In a favourable environment, the chamber exhibits greater depth and width compared to the other arms within the T-maze. Upon locating the chamber, zebrafish tend to allocate a significant portion of their time to it. The experiment involved placing individual zebrafish at the start of the long arm and allowing them to habituate for 3 min. During a 5-min exploration period, the duration of their descent to the deeper chamber was recorded (Fig. 2b). The video was captured with a Canon IXUS 275 HS compact digital camera (UK). The resulting footage was then analysed using Smart V3.0.05 tracking software developed by Pan Lab (Harvard Apparatus, USA). The zebrafish travelling time into the deeper chamber was determined as transfer latency (TL; L0, L1, or L2). Transfer latencies were recorded at 0, 3, and 24 h on the days of the 7th, 14th, and 21st. The TL was expressed as inflexion ratio (IR),13 where IR refers to [(L0-L1)/(L1)] or [(L0-L2)/(L2)]. L0 was the initial latency(s) at 0 h, and L1 and L2 were the latency(s) at 3 and 24 h, respectively. Thus, a 3-h IR and a 24-h IR ratio were calculated. The other recorded endpoints included time spent in the wrong arm (s) and total distance travelled in the deeper chamber (cm). Patterns of locomotion were also recorded.7
Statistical analysisThe research employed a two-way analysis of variance in conjunction with a multiple comparison test to investigate the behaviour of zebrafish. The Turkey post hoc test was employed solely for supplementary analysis. The statistical analysis was performed using GraphPad Prism (version 9).
Results and discussionWhile the OKA-induced AD model does not mimic all aspects of AD pathology, it has been demonstrated to induce abnormal accumulation of Aβ-protein, leading to the formation of toxic oligomers. These oligomers are associated with neuronal and synaptic damage, as well as the overexpression and hyperphosphorylation of tau protein in zebrafish.14 Furthermore, the generation and aggregation of Aβ are necessary for tau hyperphosphorylation, which induces neurodegenerative changes such as synaptic alterations, dendritic simplification, and neuron loss.15 Therefore, although the OKA-induced model may not develop classic amyloid plaques seen in human AD pathology, it still provides a robust system for studying key molecular events such as tau hyperphosphorylation that are relevant to AD progression. This suggests that despite its limitations, the OKA-induced model can still be valuable for assessing drug candidates targeting these specific pathological processes.
The T-Maze is a valuable tool for assessing cognitive functions such as spatial learning and memory, particularly in AD. In rodent models of AD, significant impairments in T-Maze performance reflect the cognitive decline seen in human patients.16 Zebrafish have emerged as a promising model for AD research due to their genetic similarity to humans.17 Using the T-Maze with adult zebrafish allows researchers to evaluate cognitive functions in a simpler and more cost-effective model. In the OKA-induced AD model in zebrafish, abnormal amyloid accumulation and tau phosphorylation lead to cognitive impairments, which can be assessed using the T-Maze. This model provides insights into the cognitive deficits associated with AD and facilitates high-throughput screening of potential therapeutic compounds.17
The T-maze is employed as a means to evaluate the capacity of fish to learn through visual cues. In the T-maze experimental setup, the fish is put into the elongated arm, also known as the base of the T structure. Subsequently, the fish typically exhibits a tendency to swim towards one or both of the shorter arms, referred to as the short arms. Nevertheless, it has been observed that a small proportion, up to 5%, of fish exhibited a tendency to remain stationary in the base arm unless stimulated.4 The desired result is for a fish to exhibit a preference for one arm dependent on the attractiveness of its surroundings (referred to as “spatial preference”). In the recent study, the right arm was connected to the favourable condition, which is a deeper chamber.
Time spent in the wrong armAt the end of the neurotoxin induction period of 21 days, there was a statistically significant difference (P <.05) in the duration of time spent in the wrong arm between the control group and the 300 mg/kg AlCl3 group. This finding is visually represented in Fig. 3a(i). However, zebrafish induced with 300 mg/kg AlCl3 did not show a statistically significant increase (P >.05) in spending more time in the wrong arm between days 7, 14, and 21 (see Fig. 3b(i)).
The information in Fig. 3a(ii) shows that on day 21, the experimental groups induced with 50 and 100 nM of OKA spent more time in the wrong arm than the control group. This difference was statistically significant (P <.05). In the 100 nM OKA-induced dosage-dependent group, there was a statistically significant rising trend (P <.05) in the wrong arm from days 7 to 21 (Fig. 3b(ii)).
According to the findings of the control group, zebrafish demonstrate the ability to effectively acquire knowledge, traverse their environment, and differentiate between the wrong and right arm in order to access the deeper chamber. In contrast, the findings provide unequivocal evidence that zebrafish exposed to OKA exhibit a consistent pattern of becoming disoriented within the T-maze. This observation indicates a decline in spatial memory function, which is associated with AD in zebrafish.
Total distance travelledFig. 4a(i) shows that on day 21 of neurotoxin induction, there were no significant changes (P >.05) in the total distance travelled in the deeper chamber for the AlCl3-induced dosage-dependent groups compared to the control group. Concerning this, as shown in Fig. 4b(i), there was no significant decrease (P >.05) recorded in the total distance travelled in the deeper chamber between the set of times (days 7, 14, and 21) within the AlCl3-induced dosage-dependent groups.
As depicted in Fig. 4a(ii), starting on day 14, the groups induced with 50 and 100 nM OKA-induced dosage-dependently displayed a substantial decrease (P <.05) in the total distance travelled in the deeper chamber compared to the control group. According to Fig. 4b(ii), there was a decreasing pattern (P <.05) observed in the total distance travelled in the deeper chamber between the set of times (days 7, 14, and 21) within the 50 and 100 nM OKA-induced dosage-dependent group.
Locomotor patternFor 21 days, the zebrafish in the AlCl3-induced dependent groups showed the same patterns of movement as the control group, as shown in Table 1a. The observed resemblance aligns with the statistically insignificant results (P >.05) regarding the total distance travelled in the deeper chamber.
Starting on the day 14, it was noted that the zebrafish induced with 50 and 100 nM of OKA exhibited reduced locomotion towards the deeper compartment, as depicted in Table 1b. There was a match between the patterns of movement seen and the results from the particular groups that demonstrated a statistically significant decrease (P <.05) in the total distance travelled in the deeper chamber.
In the context of this iterative learning paradigm, zebrafish were individually instructed to engage in exploration and navigate towards the deeper end of the T-maze. Subjects who made good selections were rewarded with a spacious and favourable setting, whereas those who made bad choices were subjected to a cramped and congested environment.6 Spatial memory is responsible for encoding and storing information pertaining to an individual's surroundings. A fish with deficient spatial memory capabilities may experience a recurring tendency to become disoriented within a maze-like environment.18 In brief, the induction of OKA at as low as 50 nM resulted in the impairment of spatial memory in zebrafish. This was evident through a decrease in tracking activity and total distance travelled, specifically inside the preferred deep chamber region of the T-Maze, starting on day 14.
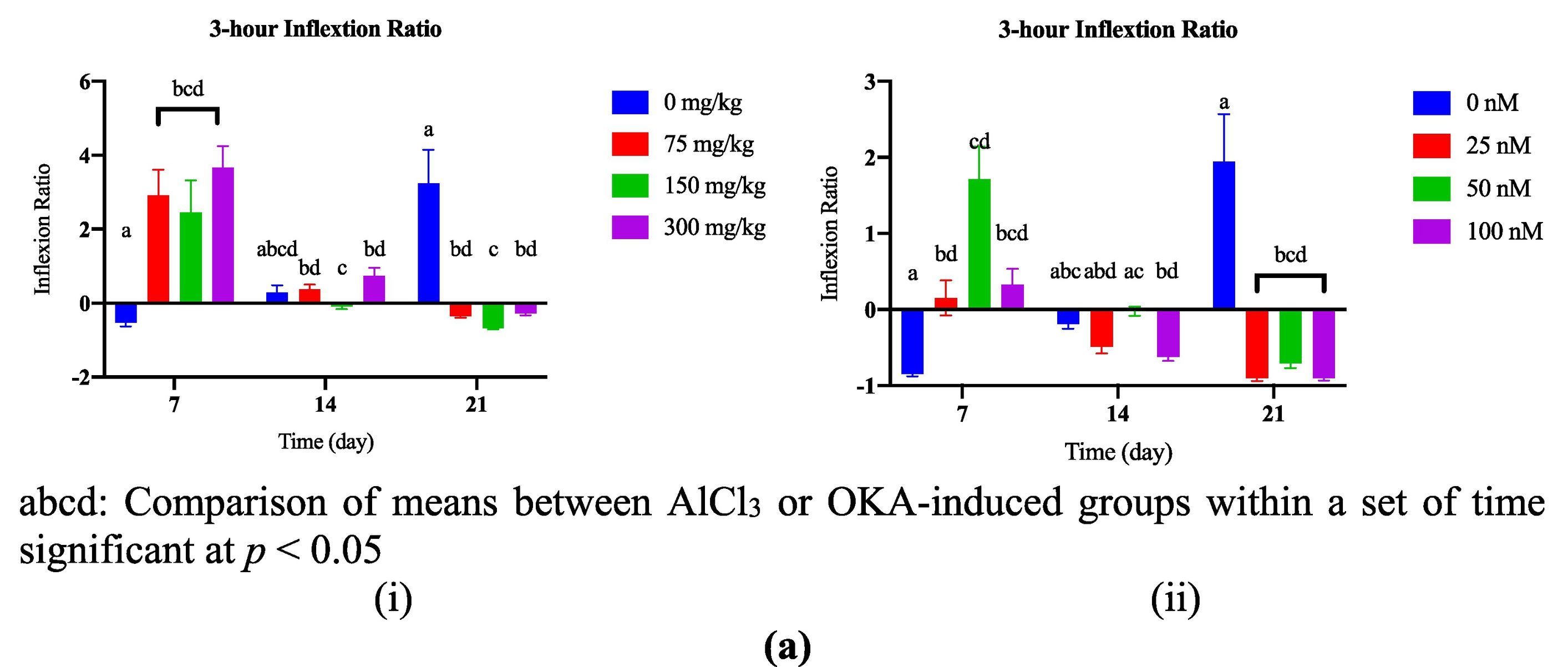
Transfer latency: 3-h inflexion ratioUtilising the T-maze paradigm, a recent study evaluated the cognitive abilities of zebrafish. Three successive trials carried out at intervals of 0, 3, and 24 h served to assess the transfer latency, which is the amount of time it takes for wild-type fish to reach a deeper chamber.
During the 21-day observation period, the 3-h IR changed significantly (P <.05) between the control group and the AlCl3-induced dosage-dependent groups. This can be seen in Fig. 5a(i). The 3-h IR dropped significantly (P <.05) in the groups that were induced to dosage-dependent AlCl3 on days 7, 14, and 21. This can be seen in Fig. 5b(i).
Fig. 5a(ii) shows that on day 21, the 3-h IR was significantly lower (P <.05) in the groups that were given different dosages of OKA compared to the control group. The 3-h IR dropped significantly (P <.05) in the groups that were given 50 and 100 nM of OKA at different times (days 7, 14, and 21). This can be seen in Fig. 5b(ii). Darland and Dowling4 have observed that fish may exhibit bottom-oriented behaviour that is indicative of stress, leading them to swim rapidly and without visual guidance into the reservoir, resulting in 0 h. The immediate stress response initially made it difficult to study learning in these “fast” fish because it led to slower, more relaxed swimming behaviour in subsequent trials.
Transfer latency: 24-h inflexion ratioSimilar to other organisms, zebrafish exhibit an enhanced behavioural repertoire through the process of learning from previous experiences.13 The expeditious learning and retention of valuable skills in the long-term memory of zebrafish brains is of paramount importance in assessing the IR.
There were major differences (P <.05) in the 24-h IR between the control group and the AlCl3-induced dosage-dependent groups on day 21 of the observation period. These differences can be seen in Fig. 6a(i). Fig. 6b(i) shows that on days 7, 14, and 21, the 24-h IR dropped significantly (P <.05) in the groups that were exposed to AlCl3.
Fig. 6a(ii) shows that on day 21, the 24-h IR was significantly lower in the groups that were given OKA compared to the control group (P <.05). From days 7 to 21, the 24-h IR went down (P <.05) in the OKA-induced dosage-dependent groups (see Fig. 6b(ii)).
ConclusionThe T-maze is used as an evaluative instrument that offers a concise depiction of the cognitive condition inside a zebrafish model. The T-maze is a commonly employed behavioural paradigm in academic research for investigating the complex aspects of spatial working memory.19 The behavioural effects of 2 neurotoxins, AlCl3 and OKA, were investigated using a T-maze test. It was assumed that the administration of either one of these neurotoxins would result in a decrease in the distance travelled to the deeper chamber by zebrafish, as well as an increasing effect on transfer latency and the time spent in the wrong arm. However, when the results were looked at based on the specific parameters, it was found that the 100 nm OKA (particularly by day 21) met the requirements as a notable neurotoxin that could make zebrafish behave in a way that would be suitable for studying AD.
FundingThis work was supported by The Sarawak Research Development Council (SRDC) (grant no: RDCRG/02/RIF/2020/_33) and Sarawak Biodiversity Centre (SBC) (research permit: SBC-2018-RDP-15-SZR). The authors are also extending their appreciation to the Faculty of Medicine and Health Sciences (FMHS) at UNIMAS, Sarawak, Malaysia; the Kulliyyah of Pharmacy at IIUM Kuantan Campus, Pahang, Malaysia; and the Jeffrey Cheah School of Medicine and Health Sciences at Monash University Malaysia, Selangor, Malaysia, for their valuable administrative and technical assistance.
Patient consentNo patient involved.
Ethical considerationsThis research involved experimentation on animals. Below are the details of the committee approving these experiments and the registration number:
Institutional Animal Care and Use Committee (I-ACUC) at the International Islamic University Malaysia (IIUM) Kuantan Campus, Pahang, Malaysia. Registration No: IIUM/IACUC-2019(21).