Dear Editor:
Metformin (biguanide) is a widely used drug for treating diabetes mellitus type 2. It has been used for 60 years and is a highly effective anti-hyperglycaemic drug.1 Extensive research has been conducted on the drug’s action mechanism in order to explain its effects on gluconeogenesis, protein metabolism, fatty acid oxidation, oxidative stress, glucose uptake, autophagia, and pain, among other processes. Some mechanisms were not identified until the late 1990s and early 2000s. Metformin induces beneficial effects in patients with diabetes by activating adenosine monophosphate-activated protein kinase (AMPK). Two kinases are responsible for AMPK activation: liver kinase B1 (LKB1) and calcium/calmodulin-dependent protein kinase II (CaMKII). Once activated, AMPK inhibits the mechanistic target of rapamycin complex 1 (mTORC1), which includes the mammalian target of rapamycin (mTOR). The TOR protein family has pleiotropic functions and participates in the regulation of mRNA transcription and translation in response to intracellular concentrations of amino acids and other essential nutrients.2
AMPK is an enzyme that includes three subunits: a catalytic subunit (α) and two non-catalytic subunits (β and γ). It participates in cellular homeostasis and maintains cell energy levels by regulating the production and consumption of adenosine triphosphate (ATP). When cells are affected by such stressors as hypoxia, hypoglycaemia, or chemical insult, ATP levels decrease, which activates AMPK to restore balance.3,4
AMPK activation may be achieved by several mechanisms. Direct allosteric activators protect the kinase from dephosphorylation through the β- or γ-subunits.3,4 Indirect activators increase phosphorylation in several regions, thereby increasing enzymatic activity. Two activators of AMPK are metformin and O304. Metformin acts non-specifically as an indirect AMPK activator in several intracellular areas and can cross the blood–brain barrier. However, O304 cannot cross the blood–brain barrier, and is a specific AMPK activator that decreases phosphorylation at the Thr172 site on the α-subunit kinase through protein phosphatase 2C, without suppressing the activating effects of AMPK.3,4
As metformin is a disease-modifying drug for diabetes mellitus type 2 that reduces mTORC1 signalling in order to induce its effects on neuronal plasticity, it has been suggested that these mechanisms may also explain the drug’s antinociceptive effect in several models of chronic pain (Fig. 1).5,6 Thus, studies in mouse models have shown that AMPK activation may reduce allodynia and peripheral nerve injury within 7 days after the lesion.7 AMPK activators have also been shown to achieve complete resolution of extensive nerve injury within 60 days of the lesion. A study analysing metformin and O304 in a mouse model of post-operative pain reported decreased sensitivity to mechanical pain with both drugs compared to vehicle. This study also showed the presence of an even greater synergistic effect when both drugs were jointly administered.7
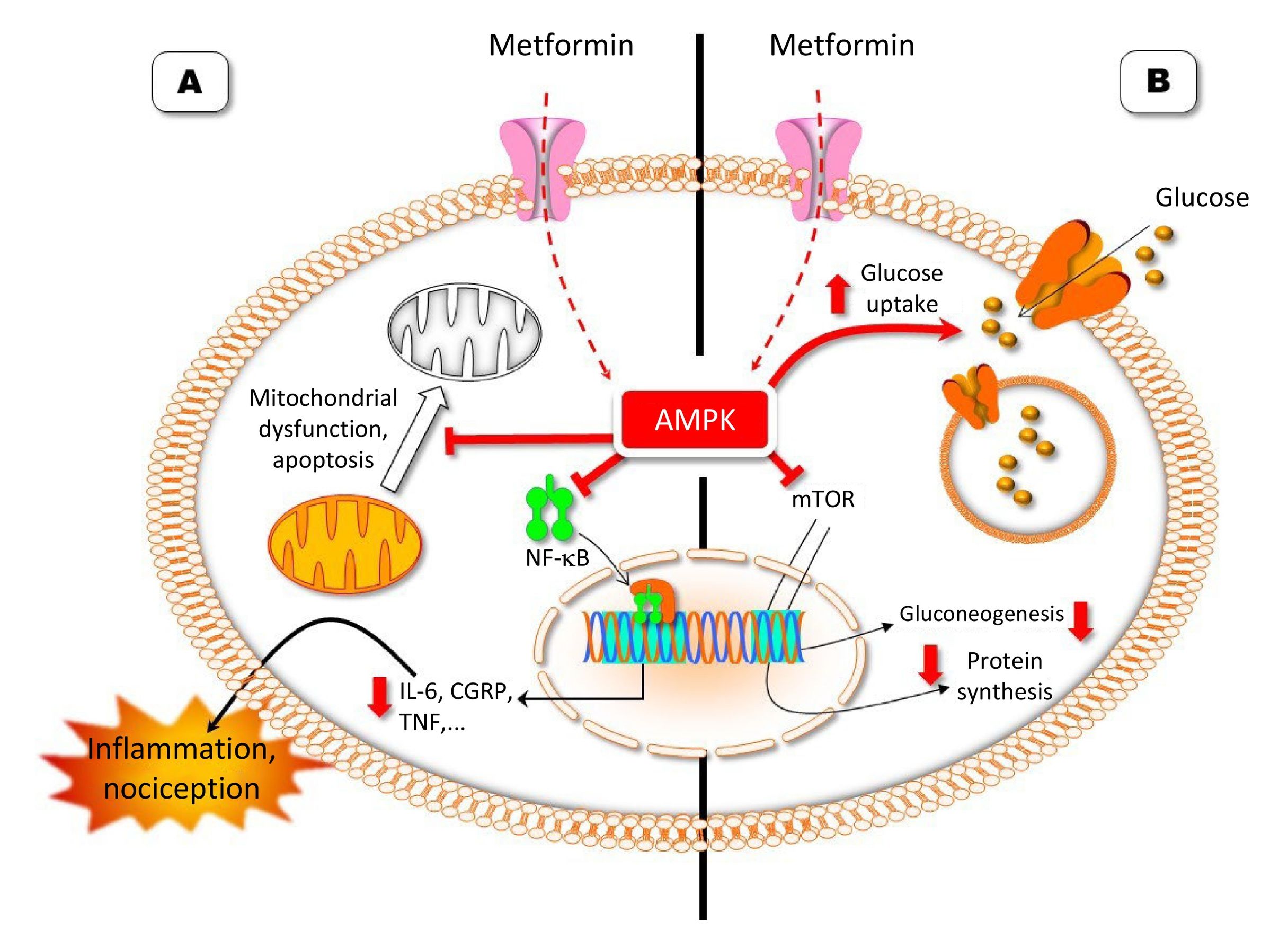
The action mechanism of metformin as an anti-hyperglycaemic drug (B) and its possible analgesic mechanism (A) (Image courtesy of Carlos Goicoechea García).
Metformin indirectly promotes adenosine monophosphate-activated protein kinase (AMPK) activation. Activation of this enzyme promotes the action of the glucose transporter and blocks the action of the mammalian target of rapamycin (mTOR). Inhibition of mTOR decreases gluconeogenesis and protein synthesis. The analgesic mechanism of metformin may be related to an inhibitory effect on nuclear factor kappa B (NF-κB),5 which would lead to a decrease in the synthesis of proinflammatory and pronociceptive proteins, such as interleukin-6 (IL-6), calcitonin gene-related peptide (CGRP), and tumour necrosis factor (TNF). Another possible action mechanism, which may complement the previously mentioned mechanism, may involve alterations in apoptosis.6 Metformin would inhibit processes related to chronic pain, such as mitochondrial degradation, autophagy, and apoptosis.
This is an interesting area of research and advancement. Further studies on metformin may be designed to evaluate treatment for neuropathic and post-operative pain, possibly as part of a preventive perioperative regime, such as a protocol to improve recovery after surgery. Another possibility is the development and exhaustive study of other allosteric activators of AMPK, such as A769662 and OSU-53, for clinical use.3,4 These drugs are 100 times more potent than metformin and activate AMPK by targeting specific γ(2) subunits to achieve strong activation without adverse effects.3 As specific allosteric drugs, they also present a lower dispersion effect as compared with other non-specific activators. These types of drugs probably represent a more robust line of treatment than the drugs currently under study.
Metformin has been shown to have neuroprotective effects in mouse models of neurodegenerative diseases,8,9 and decreases neuropathic pain induced by spinal nerve ligation and spared nerve injury in rats and mice.6,10 Similarly, treatment with metformin alleviates hyperalgesia and allodynia in a rat model of diabetic neuropathy.11 Furthermore, in a mouse model of cisplatin-induced neuropathy, metformin considerably reduced the loss of tactile sensitivity and the onset of mechanical hypersensitivity.12 Another preclinical study, using a rat model of oxaliplatin-induced peripheral neuropathy (OIPN), showed that metformin largely protects against intraepidermal nerve fibre degeneration induced by oxaliplatin.13 In that study, metformin was able to prevent mechanical and cold hypersensitivity induced by chemotherapy. Overall, these data open new paths for the development of treatments to prevent OIPN in human patients.
In summary, there is evidence indicating that AMPK activation signalling underlies the effects of metformin in several conditions, including insulin resistance, diabetes, and chronic pain. However, well-designed placebo-controlled clinical trials are needed to support the putative effect of metformin in preclinical trials, especially for chronic pain and its comorbidities.
Conflicts of interestThe authors have no conflicts of interest to declare.