International criteria are used for the diagnosis of multiple sclerosis (MS) and Isolated Clinical Syndrome (ACS), and Neuromyelitis Optica (NMO). PEVs are not included in any of them. We intend to analyse the sensitivity and specificity of visual evoked potentials in the initial diagnosis of MS, ACS, and NMO.
Patients and methodsDescriptive, observational, retrospective study of patients treated for a first episode of RR-EM, NMO, or ACS. VEPs are performed during the study of the first episode, with and without ocular symptoms, between October 2012 and March 2019.
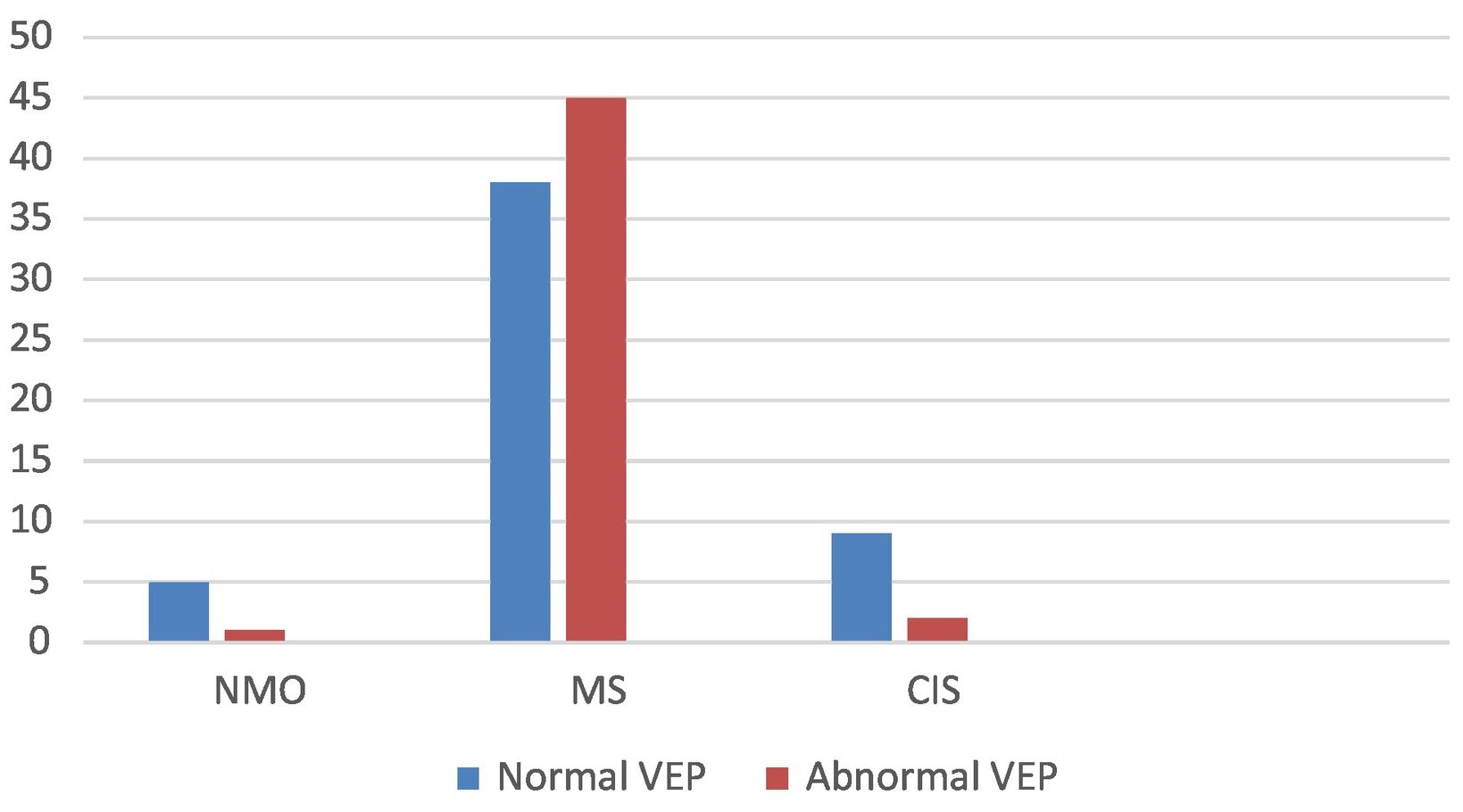
Results142 patients with suspected demyelinating disease, 100 with disease. Of them, 61 women (61%), mean age: 38.82 years ± SD 4.6. Of the total, 83 with RREM, 6 NMO, 11 ACS. Of the 6 patients with NMO, 1 (16.66%) had altered VEPs; of 11 with ACS, 2 (18.18%) with altered VEP; Of the 83 patients with RR-MS, 45 patients (54.21%) had altered VEP. The sensitivity and specificity of visual evoked potentials in MS was 92.86% and 84.74%, respectively. In NMO, the sensitivity was 16.66%, and the specificity was 93.34%. In ACS, the sensitivity was 18.18%, and the specificity was 96.94%.
ConclusionThe sensitivity of VEP in our series is slightly higher than other series published in optic neuritis in RR-MS, being very low in patients with NMO and ACS. However, the very high specificity of the test in these variants can help its diagnosis. These data should be corroborated in larger series.
Para el diagnóstico de esclerosis múltiple (EM) y síndrome Clínico Aislado (SCA) y Neuromielitis óptica (NMO) se utilizan los criterios internacionales. En ninguno de ellos no se incluyen los PEV. Nos proponemos analizar la sensibilidad y especificidad de los potenciales evocados visuales en el diagnóstico inicial de EM, SCA y NMO.
Pacientes y métodosEstudio descriptivo, observacional, retrospectivo, de pacientes atendidos por primer brote de EM-RR, NMO ó SCA. Se realizan los PEV durante el estudio del primer episodio, con y sin clínica ocular, entre Octubre 2012 y Marzo 2019.
Resultados142 pacientes con sospecha de enfermedad desmielinizante, 100 con enfermedad. De ellos, 61 mujeres (61%), media de edad: 38,82 años +/-DE 4.6. Del total, 83 con EM-RR, 6 NMO, 11 SCA. De los 6 pacientes con NMO, 1 (16,66%) tenía los PEV alterados; de 11 con SCA, 2 (18,18%) con PEV alterados; de los 83 pacientes con EM-RR, 45 pacientes (54,21%) con PEV alterados. La sensibilidad y especificidad de los potenciales evocados visuales en EM fue de 92,86% y 84,74% respectivamente. En NMO la sensibilidad fue de 16,66%, y especificidad de 93,34%. En SCA, la sensibilidad fue de 18,18%, y especificidad de 96,94%.
ConclusionLa sensibilidad de los PEV en nuestra serie es discretamente superior a otras series publicadas en neuritis óptica en EM-RR, siendo muy baja en pacientes con NMO y SCA. Sin embargo, la altísima especificidad del test en estas variantes puede ayudar a su diagnóstico. Estos datos deberían ser corroborados en series más amplias.
Optic neuritis (ON) is a self-limiting condition caused by acute inflammatory demyelination, characterised by loss of visual acuity (unilateral involvement in 90% of cases) and ocular pain that worsens with movement.1,2 It typically presents with central scotoma, and may also be associated with photopsia, dyschromatopsia, altitudinal field defects, or hemianopia. ON is very frequently associated with multiple sclerosis (MS), with 15%–20% of patients presenting ON as the initial manifestation and 50% developing it over the course of the disease. However, it may also present in patients with other diseases (e.g., ischaemic optic neuritis, diabetes mellitus, giant cell arteritis, neuromyelitis optica [NMO], encephalomyelitis, sarcoidosis, lupus erythematosus, clinically isolated syndrome, etc).3–6 It is more frequent in women (66%), and usually manifests between the ages of 20 and 40 years. Its annual incidence rate is 1–5 cases per 100 000 population. Patients with ON develop perivascular and perineural oedema, potentially leading to secondary axonal damage when myelin is affected.
Visual evoked potentials (VEP) are electrical signals generated in the central nervous system through peripheral nerve stimulation (light stimulation of the eye with a checkerboard pattern or with orange LED goggles).7–10 VEP may help establish the anatomical location of a lesion to the visual pathway (pre or post chiasm) that cannot be determined by imaging techniques (e.g., MRI) and provide data on the type of lesion affecting the optic nerve (demyelinating, axonal, or both). After an acute episode of ON, VEP alterations may persist despite recovery of visual function (especially demyelination, which persists for 2 years in 80% of patients).
MS is a chronic autoimmune, inflammatory, degenerative disease of the central nervous system. Its prevalence varies with latitude; Spain is one of the countries with the highest prevalence rates, ranging from 80 to 180 cases per 100 000 population.11,12 Clinically isolated syndrome (CIS) is a phenotype of MS characterised by an isolated clinical episode (mainly ON, although it may also present in the form of motor or sensory deficits) lasting over 24 h and associated with radiological alterations suggestive of demyelinating disease13–16 but not meeting all diagnostic criteria for MS. The prevalence of CIS is estimated at 20–30 cases per 100 000 population.
NMO belongs to a spectrum of inflammatory, demyelinating diseases of the central nervous system that mainly affect the optic nerve and spinal cord and are mediated by anti–aquaporin-4 antibodies.17–22 Its worldwide prevalence is estimated at 0.5–4.4 cases per 100 000 population.19–21
Diagnosis of MS and CIS is established according to the 2017 McDonald criteria, based on follow-up data from clinical assessment scales, MRI studies, and CSF analysis.15 Diagnostic criteria for MS and CIS do not consider VEP results, as is also the case for NMO.18
In this study, we analyse the sensitivity and specificity of VEP for the initial assessment of demyelinating diseases in our department.
MethodsWe conducted a descriptive, analytic, observational, retrospective study of patients attended due to a first episode of relapsing–remitting MS (RRMS), NMO, or CIS. VEP was performed during the assessment of the first episode associated with visual symptoms, between October 2012 and March 2019. We gathered the following data: age, sex, symptoms of the first episode, subsequent diagnosis (RRMS, NMO, CIS), VEP results, type of involvement (axonal or demyelinating), and severity.
The patients included in our study belong to a healthcare district including a population of 325 000 (in 2019). We included patients older than 18 years diagnosed with RRMS, NMO, or CIS according to international diagnostic criteria and who had undergone a VEP study. We excluded patients with an uncertain diagnosis; patients not undergoing a complete diagnostic assessment including brain MRI, CSF analysis, complete blood analysis with serology and immune tests; patients lost to follow-up; and those with missing data. We did not include data from any subsequent follow-up VEP studies for additional episodes of ON in patients already included in the study.
VEP was performed with a checkerboard pattern (pattern VEP) or orange LED goggles (goggle VEP). VEP is a simple, non-invasive method for studying the function of the visual pathway, and is available in most hospitals. All patients in our series were preferentially assessed with the pattern PEV technique; goggle VEP was used when visual deficits were particularly marked or in uncooperative patients.
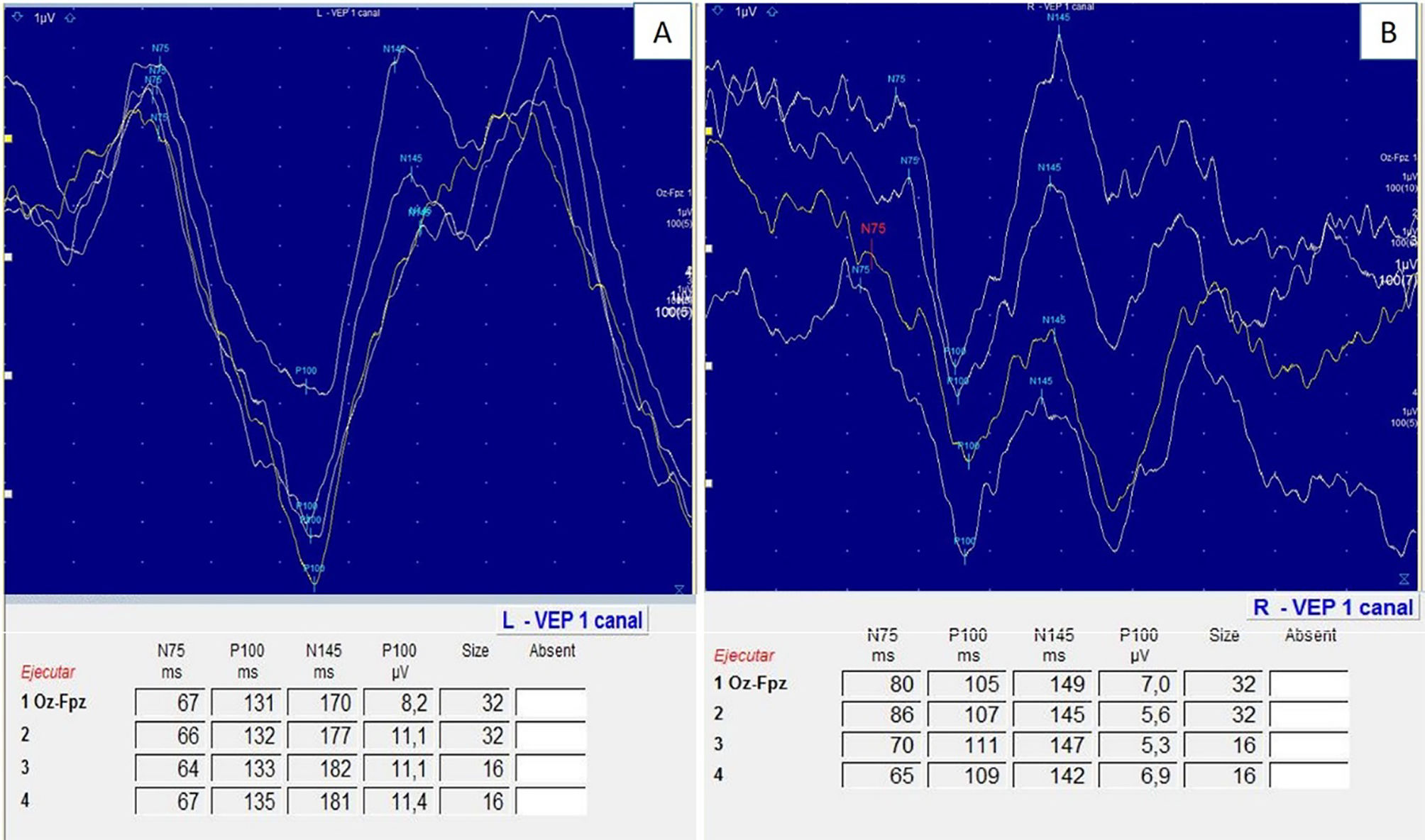
Pattern VEP performs monocular stimulation of each eye using a checkerboard pattern, with checks alternating black/white to white/black (check size of 60′ and 15′), with the patient at a distance of 100 cm from the screen. The aim of this technique is to evoke P100, N75, and N145 waves. P100 wave latency of 110 ± 3.9 ms and amplitude of 10 ± 4 μV in pattern VEP were regarded as normal.19,20 In goggle VEP, the normal values used were 100 ± 5 ms latency and 12.3 ± 4.4 μV amplitude.19,20
Qualitative variables are expressed as relative frequencies (%) and quantitative variables as either mean and standard deviation (SD) or median and percentiles 25 and 75 (p25–p75), depending on the normality of data distribution (Kolmogorov–Smirnov test). ROC curve analysis was performed to determine the cut-off point with the best discriminative ability for diagnosing the condition. Data were processed with the IBM SPSS statistics software package (version 25) using an anonymised database; data were treated in groups. The required sample size for a statistical significance threshold of p < .05, a confidence interval of 95%, and a 5% precision level was 73 patients.
Among the limitations of our study, our sample size is smaller than desired for 2 main reasons: (1) we excluded patients not meeting all diagnostic criteria for the diseases analysed, and (2) our hospital is not a reference centre for the management of these conditions; consequently, some patients were lost to follow-up due to transfer to other centres.
Patient data management complied with Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data.
This study was approved by our centre's research committee in May, 2021.
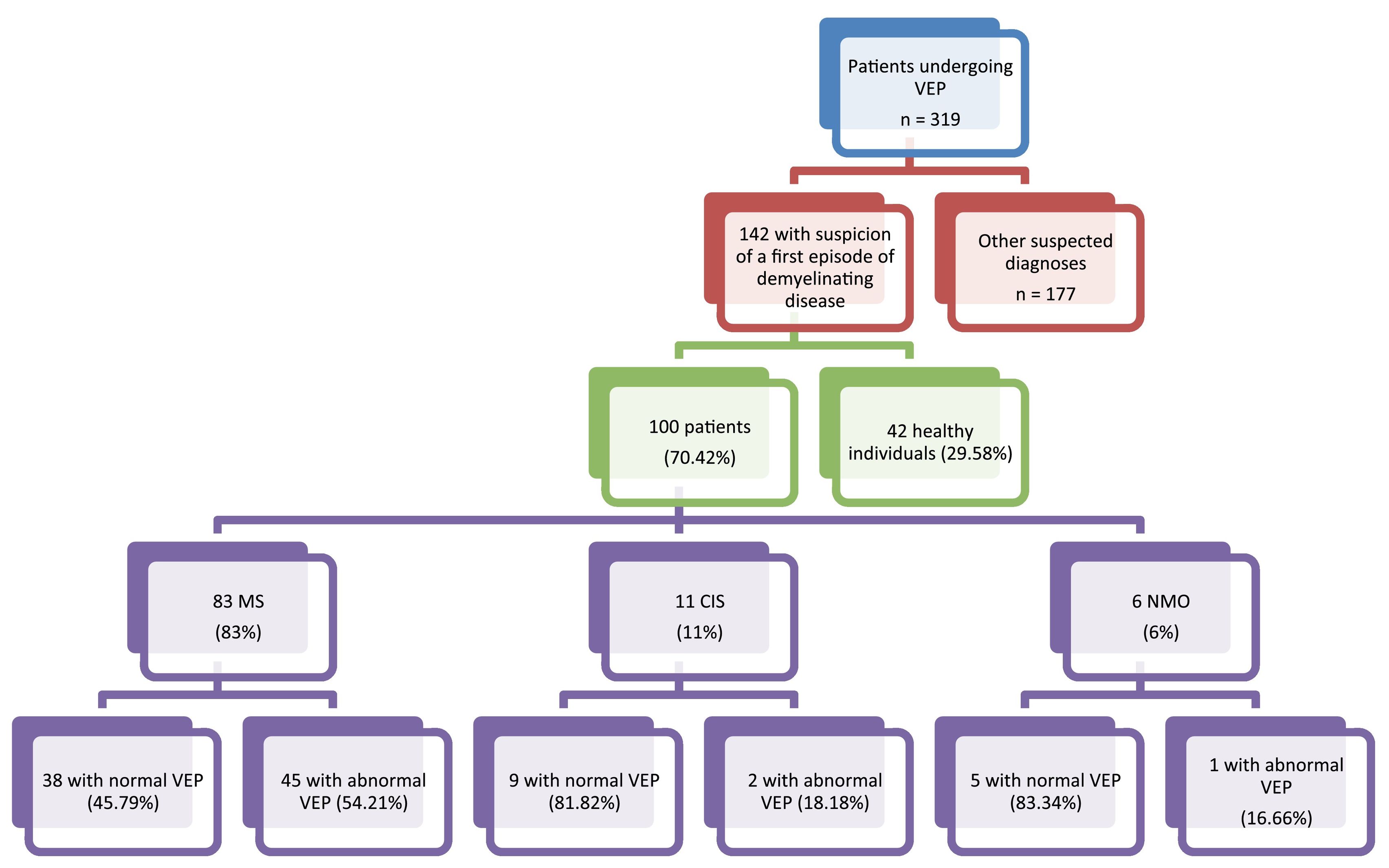
ResultsOf a total of 319 patients undergoing VEP testing at our centre for different reasons between 2012 and 2019, we selected 142 patients in whom demyelinating disease was initially suspected; 100 of these were eventually diagnosed with MS, NMO, or CIS (61 women and 39 men). Mean age (SD) in our sample was 38.82 (4.6) years (range, 17–66). Eighty-three were diagnosed with RRMS, 11 with CIS, and 6 with NMO. Fig. 1.
Abnormal VEP results were obtained for 48 patients (48%=: demyelination was observed in 41 patients (mean P100 wave latency of 126 ±[8] ms) and axonal involvement in 35 (mean amplitude of 4 ±[2.8] ms).
By disease, VEP alterations were observed in 1 patient with NMO (16.66%), 2 with CIS (18.18%), and 45 patients with RRMS (54.21%). Visual symptoms (ON) were observed in 28 patients, 26 (92.86%) of whom showed abnormal VEP results. Fig. 2. Sensory symptoms were found in 46 patients, with 24 (52.17%) showing abnormal VEP results. Twenty patients presented motor deficits, 11 (55%) of whom showed abnormal VEP results. Of the 6 patients presenting other symptoms (trigeminal neuralgia, facial palsy, vertigo), 1 (16.66%) showed abnormal VEP results.
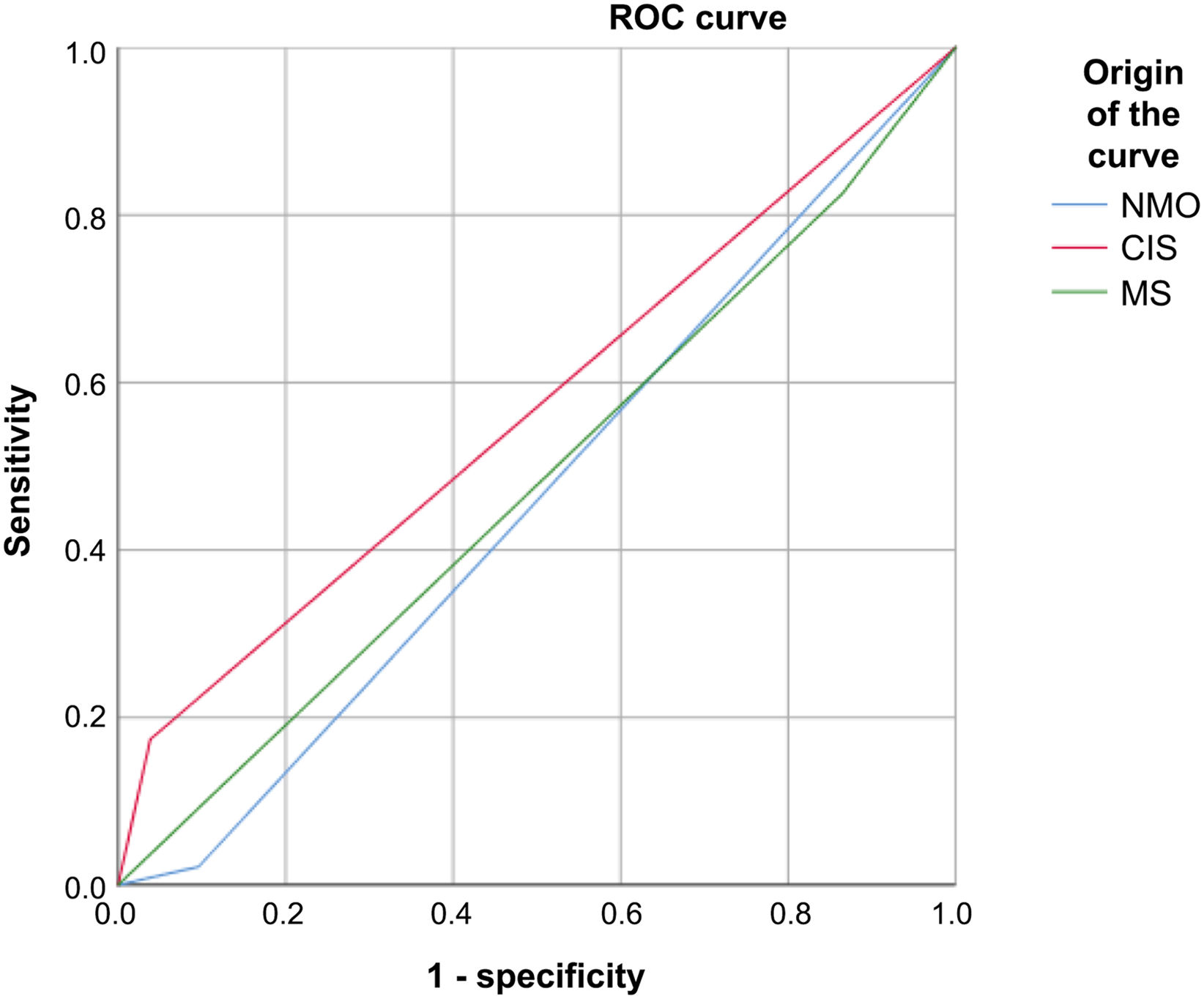
VEP presented 92.86% sensitivity and 84.74% specificity for MS, 16.66% sensitivity and 93.34% specificity for NMO, and 18.18% sensitivity and 96.94% specificity for CIS. Fig. 3
Receiver operating characteristic curve of sensitivity and specificity of visual evoked potentials in patients with multiple sclerosis, neuromyelitis optica, and clinically isolated syndrome.
CIS: clinically isolated syndrome; MS: multiple sclerosis; NMO: neuromyelitis optica; ROC: receiver operating characteristic.
At our centre, VEP is routinely performed in the diagnostic evaluation of patients with a wide range of visual alterations due to its availability, innocuity, and ease of use. Although VEP alterations are not included among the diagnostic criteria for MS, they do support diagnosis and the technique is always performed in patients with a first episode of ON when demyelinating disease is suspected. Therefore, we deemed it interesting to estimate the real value of VEP in the diagnosis of ON in the most frequent demyelinating diseases, despite the fact that ON is not included in the diagnostic criteria for any of the 3 diseases analysed (MS, NMO, and CIS). Furthermore, few studies have analysed VEP in patients with NMO and CIS.
At our hospital, VEP may be performed using the checkerboard pattern technique or the LED goggle technique; the second is the most frequently used and is available at most centres (Fig. 4). Multifocal VEP is more sensitive and specific for ON; however, this technique is not available at most centres, and is therefore not performed routinely.6–8 All patients in our series underwent pattern VEP as the first option, as standardised values for this technique have been widely validated for the diagnosis of ON; the study had to be completed with goggle VEP in 10% of patients, normally due to severe eye pain that prevented patients from seeing the checkerboard.
Compared to other studies, our series is small, probably due to the small size of our centre, a secondary-level hospital serving a population of 325 000, with 260 beds and a specialised demyelinating diseases unit.
The demographic characteristics of our sample (age and sex) are similar to those of other series.21–26 The type of VEP alterations (mainly demyelinating involvement in the acute phase of an episode of ON) is also consistent with the published evidence.27–29
The sensitivity of VEP for detecting the 3 diseases analysed is clearly linked to the number of cases: it was found to be higher in MS, due to the higher prevalence of the disease, and lower in NMO and CIS, which are less prevalent.30–36
The sensitivity of VEP for detecting MS is very high in nearly all published series (75%–90%). In our study, sensitivity was found to be slightly higher (92.86%). In the case of NMO and CIS, VEP presented very low sensitivity (16.66% and 18.18%, respectively), which we believe is linked to the small size of the sample.
The specificity of VEP for diagnosing MS in our series presents similar values to those reported in most published series (84.74%).4,5 However, VEP is highly specific for diagnosing NMO (93.34%) and CIS (96.94%). Although these values should be interpreted with caution due to the small number of patients with CIS and NMO included in our study, they are very significant and seem to show a clear trend.
We feel that the possibility of reliably ruling out NMO or CIS in patients with a first episode of ON is relevant, particularly considering the challenge of diagnosing these 2 entities.
ConclusionsIn our series, VEP presented slightly higher sensitivity than in other published series of ON in patients with RRMS; in patients with NMO and CIS, sensitivity values were very low. However, we found an extremely high specificity for NMO and CIS, which we believe is relevant for diagnosis. Our results should be confirmed in studies with larger samples.
Conflict of InterestThe authors have no conflicts of interest to declare. This study received no public or private funding. The results of our study were partially presented at the 2019 Annual Meeting of the Spanish Society of Neurology and the 2019 Annual Congress of the Madrid Association of Neurology.