The aim of the study was to characterize sleep architecture and self-reported sleep questionnaires in patients with frontal brain tumor.
MethodsEight patients with frontal brain tumor underwent polysomnographic testing and completed self-report questionnaires on sleep quality, insomnia, drowsiness and risk of obstructive sleep apnea, in addition to depression and anxiety inventories.
ResultsPatients with frontal brain tumor exhibited a disruption of the sleep architecture, characterized by changes in the pattern and in the time they spend asleep. It was found that objective values (polysomnographic variables) do not necessarily correspond to the perception of alteration through subjective methods (self-reported questionnaires).
ConclusionsChanges in the architecture of sleep in patients with frontal tumors allow us to understand the organization of the brain during this state in the presence of this type of pathology, which causes structural and functional changes.
El objetivo del estudio fue caracterizar la arquitectura del sueño y los cuestionarios de auto-informe de sueño en pacientes con tumor cerebral frontal.
MétodoA ocho pacientes con tumor cerebral frontal se le realizó un registro polisomnográfico y completaron cuestionarios de auto-informe sobre calidad del sueño, insomnio, somnolencia y riesgo de apnea obstructiva del sueño, además de inventarios de depresión y ansiedad.
ResultadosLos pacientes con tumor cerebral frontal presentaron una alteración de la arquitectura del sueño, caracterizada por cambios en el patrón y en el tiempo que permanecen dormidos. Se encontró que los valores objetivos (variables polisomnográficas) no necesariamente corresponden a la percepción de alteración a través de métodos subjetivos (cuestionarios de auto-informe).
ConclusionesLos cambios en la arquitectura del sueño en pacientes con tumores frontales nos permiten comprender la organización del cerebro durante este estado en presencia de este tipo de patología, que provoca cambios estructurales y funcionales.
Normal sleep is a complex physiological activity. It is characterized by discrete neurological patterns that represent different stages of sleep1; such as sleep of Rapid Eye Movements (REM sleep) and sleep of Non-Rapid Eye Movements (NREM sleep), and the latter is divided into three stages; stage 1 and 2 or light sleep and stage 3 or Slow Wave Sleep (SWS).2 The succession of these stages forms a unique sleep architecture and its study can be carried out in the sleep laboratory, by means of polysomnography (PSG).
The registration of these sleep patterns in brain pathologies has made it possible to know the participation of delimited regions. Such is the case of the frontal lobes, that previously have been associated in the regulation of eye movements3–5 and with the cognitive changes during the dreams due to deactivation of this frontal areas during REM sleep stage in healthy people.6–9 Through a study of patients with tumor in this frontal region, changes in sleep architecture are observed, characterized by a deficiency of the second stage of sleep and an increase representation of SWS.10 Likewise, studies carried out in various space-occupying pathologies with some impact on the frontal lobe, refer to significant changes in the PSG parameters, for instance; in patients with cerebral infarction in the territory of the middle cerebral artery, a shorter time of REM sleep, lower ratio of REM sleep to NREM sleep and a lower rate of sleep efficiency were found. Additionally, hemispheric differences were observed, since patients who suffered from a stroke of the right hemisphere, show a decrease in REM sleep, on the contrary, SWS was found decreased by damage to the left hemisphere.11 On the other hand, Montplaisir et al.,12 also report changes in sleep architecture in patients with progressive supranuclear palsy, which mainly affects frontal regions of the brain, as well as some subcortical regions such as the basal ganglia, cerebellum and brainstem. They report a decrease in sleep efficiency, total sleep time and REM sleep, furthermore an increase in wakefulness and stage 1 sleep.
On the other hand, the study of sleep can be carried out through subjective methods, such as scales and self-reports, mainly to know aspects of the quality of sleep and the presence of symptoms that guide the diagnosis of some sleep disturbance. Thanks to these evaluation methods, it is known that sleep disorders such as insomnia,13,14 sleepiness,15,16 narcolepsy and respiratory disorders13 have been associated with patients with brain tumors. Likewise, patients report poor sleep quality through the Pittsburgh Sleep Quality Index (PSQI) and impaired sleep patterns.17 These sleep disturbances have been reported to be a prevalent and disabling problem for these patients and said complaints are often associated with reports of depression, fatigue, anxiety and pain,18 and can be present throughout the course of the disease, especially during oncological treatment and during survival.19,20
Although interest has previously been generated in this relationship between changes in the sleep–wake cycle and brain pathologies involving the frontal lobes, there are few published studies, despite the fact that these lobes serve important functions for various processes during sleep. There are even fewer studies with pathologies like tumors, which compared to other, cause specific damage and allow to know the participation of a delimited area. A last important aspect is the lack of comparison of polysomnographic variables (sleep architecture) with adequate normative criteria. In addition to know the type of relationship between these objective methods with subjective methods (self-reported questionnaires) of sleep evaluation. Therefore, the aim of this study was to characterize sleep architecture and the perception of sleep quality and disturbances in patients with frontal brain tumor.
Materials and methodsParticipantsPatients with brain tumor in the frontal lobe and glial histological lineage were recruited in the consultations of the National Cancer Institute in Mexico. They were in the absence of treatment (surgery, chemotherapy or radiotherapy) and with aged between 18 and 59 years. Without neurological, psychiatric or autoimmune comorbid. All participants were free of drug abuse and without ingesting of caffeine or other stimulating drinks two days before the PSG record. It was controlled use of medications known to cause effects to sleep, however, some patients were under treatment with antiepileptics, to control the seizures caused by the tumor.
To determine the diagnosis, location and lesion size of the tumors, brain images were analyzed by structural Magnetic Resonance Imaging (MRI) using 8-channel Magnetic Resonance GE Signa Excite II, Tesla 3 equipment. For the anatomical mapping of lesions, the study had the support of expert neuroradiologists who were blind to our objectives.
ProcedureA cross-sectional study was carried out, during the period from 2018 to 2020. This was approved by the ethic committees of the Master's and PhD Program in Psychology of the UNAM and the National Cancer Institute in Mexico (018/001/OMI) (CEI/1219/17). All participants signed the informed consent form. The research complied with the principles of the Declaration of Helsinki for human study.
The collection of sociodemographic data, information on clinical and pathological variables was obtained through the clinical record and an interview conducted by one of the researchers. To know the perception of patients regarding their quality of sleep or if they have any sleep disturbances, the following questionnaires were applied: 1) The Pittsburgh Sleep Quality Index (PSQI) was used to characterize sleep quality. It is a 4-point rating scale ranging from 0 (not during the past month) to 3 (three or more times per week) and measures seven areas including subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction over the past month. It provides a global sum score and a score of >5 indicates that the individual is a poor sleeper. The PSQI is validated for the Mexican population and has high internal consistency reliability21; 2) The Athens Insomnia Scale (AIS), validated for the Mexican population,22 allowed to confirm if the participants had insomnia. It is a self-application scale, which consists of eight items, each can be scored from 0 to 3; whose total score of the test ranges from 0 (denoting a total absence of insomnia) to 24 (which would represent the more severe degree of insomnia), 3) The Epworth Sleepiness Scale (ESS) was used to assess daytime sleepiness. The ESS is an 8-item measure of daytime sleepiness, which asks respondents to indicate the probability of their dozing or sleeping during typical daytime activities on a 4-point rating scale ranging from 0 (never dozing) to 3 (high chance of dozing). A total score of less than 10 is considered normal, 10–12 as indicative of marginal sleepiness and above 12 is suggestive of excessive sleepiness. Validated for the Mexican population, the scale has been found to have an acceptable internal consistency23 and 4) STOP-Bang (snoring, tiredness, observed apnea, blood pressure, body mass index, age, neck size, gender) questionnaire, which was applied to assess the risk of obstructive sleep apnea (OSA). Since there exists a high prevalence of respiratory disorders during sleep such as OSA in the general population, a great proportion of which remains undiagnosed. It consists of eight dichotomous (yes/no) items related to the clinical features of sleep apnea. The total score ranges from 0 to 8. Patients can be classified for OSA risk based on their respective scores. For example; the results of the Stop-Bang questionnaire are classified as: low risk if there are affirmative answers of 0 to 2 questions, intermediate risk of 3 to 4 questions and high risk of 5 to 8 questions, or if they answered “yes” to 2 or more of the first 4 questions and is man, or if there are affirmative answers to 2 or more of the first 4 questions and your body mass index is more than 35 kg/m2, or if you answered “yes” to 2 or more of the first 4 questions and your neck circumference is >43 cm for men or > 41 cm in women. STOP-Bang questionnaire has demonstrated a high sensitivity and has been widely adopted and validated in various populations and among patients with assorted medical conditions.24
Also, two inventories were applied to know the possible emotional effects in the patients. The first was the Beck Depression Inventory-II (BDI-II) was used to self-report of depressive symptomatology. The BDI contains 21-items, which are rated from 0 to 3 in terms of intensity. The ratings are summed to calculate total depression scores, which can range from 0 to 63 with the following cut-offs: 0-13, minimally depressed; 14–19, mildly depressed; 20–28, moderately depressed; and 29–63, severely depressed. The adaptation to the Mexican population was used and has a high internal consistency.25 The second inventory applied was the State–Trait Anxiety Inventory (STAI), for the evaluation of anxiety. The STAI is a psychological inventory based on a 4-point Likert scale and consists of 40 questions on a self-report basis. The STAI measures two types of anxiety; state anxiety (STAI-S), or anxiety about an event, and trait anxiety (STAI-T), or anxiety level as a personal characteristic. The validated version for the Mexican population was used.26
Regarding the sleep study, two nights of PSG registration (first night of habituation) were recorded at the Sleep Laboratory of the Faculty of Psychology at the UNAM, in a faradized room, soundproofed and with adequate acclimatization. The PSG recording consisted of electroencephalogram leads, 19 electrodes were placed according to the 10/20 international system, with monopolar montage from Fp1, Fp2, F3, F4, C3, C4, T3, T4, P3, P4, O1, and O2 referred to contralateral earlobes (A1 and A2), sampled at 400 Hz and filters set at 0.5 and 70 Hz; right and left electrooculograms and submental electromyograms. For the recording and amplification of the signal, a Cadwell polygraph was used. The sleep recording started from the participant's usual sleep time and they were also allowed to sleep until spontaneous awakening the next day or until they had completed their usual hours of sleep determined by a sleep diary.
Physiologic signals were digitized for offline analysis of sleep patterns. The initial night of recording was not used in the analysis (in order to control for first night effects). Sleep-stage scoring was performed on 30-s epochs according to the criteria of the American Academy of Sleep Medicine (AASM) Version 2.527 by two sleep experts trained in sleep staging. The following sleep variables were determined: sleep period time (SPT), time from sleep onset until final awakening (TST), sleep efficiency (SE) (ratio of TST to time in bed/100%) sleep onset latency (SOL); wake after sleep onset (WASO), REM sleep latency (time from sleep onset until first epoch of stage R sleep), amount of wake and stage 1, 2, 3, and REM sleep as a percentage of SPT.
Data analysesThe data analysis was conducted with the software IBM SPSS Statistics 21 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were calculated for all measures. The PSG variables were compared between patients with brain tumors and normative values.28 The Mann–Whitney U test was performed to evaluate differences between PSG variables of patients with brain tumors and normative values. As well as to know if there are differences by sex, laterality of the tumor and antiepileptic treatment in the PSG variables and the self-report questionnaires. Additionally, an analysis was carried out using the Kruskal–Wallis Rank test, to know if there were differences between the types of antiepileptic drugs and the size of the lesion (volume and edema), which was classified as small (<10 cm3), medium (between 10.001 and 50 cm3) and large (>50 cm3). Finally, the Spearman correlation was used to determine the association among PSG variables, PSQI, AIS, ESS, BDI, STAI-T, STAI-S and STOP-Bang. The level of statistical significance was set at p ≤ 0.05 for all tests.
ResultsThe sample consisted of eight patients with frontal brain tumor, with mean age of 46.9 years (SD = 9.2, range = 30-57, 3 women, 3 left hemisphere). Regarding seizures, five patients had a diurnal predominance of seizures. Six patients were under treatment with antiepileptics; two with Carbamazepine, three with Levetiracetam and one patient with Phenytoin and Valproate. In four patients, seizure control was obtained. Only one patient had a seizure during sleep recording, which had a focal onset in the central regions of the left hemisphere, with a duration of 90 s during the second sleep cycle. Following seizure, the patient decided to continue with the recording, because he did not present an affectation that required medical assistance.
Through the Mann–Whitney U test no significant differences were found between patients with and without antiepileptic treatment in PSG variables and self-report questionnaires (p ≥ 0.05) and by subsequent analysis with the Kruskal–Wallis Rank test, no differences were observed between the types of antiepileptic treatment.
The patients did not report moderate or severe depressive symptoms. With respect to trait anxiety, two patients reported high symptoms and five patients in the middle range. In state anxiety of the second night of PSG registration, none had high symptoms and six had in the middle range. On the PSQI, three patients were characterized as poor sleepers. None of participants met criteria for excessive daytime sleepiness and insomnia as measured by the ESS and the AIS, respectively. According to the STOP-Bang questionnaire, two patients are at high risk for OSA, three are at intermediate risk and three are at low risk.
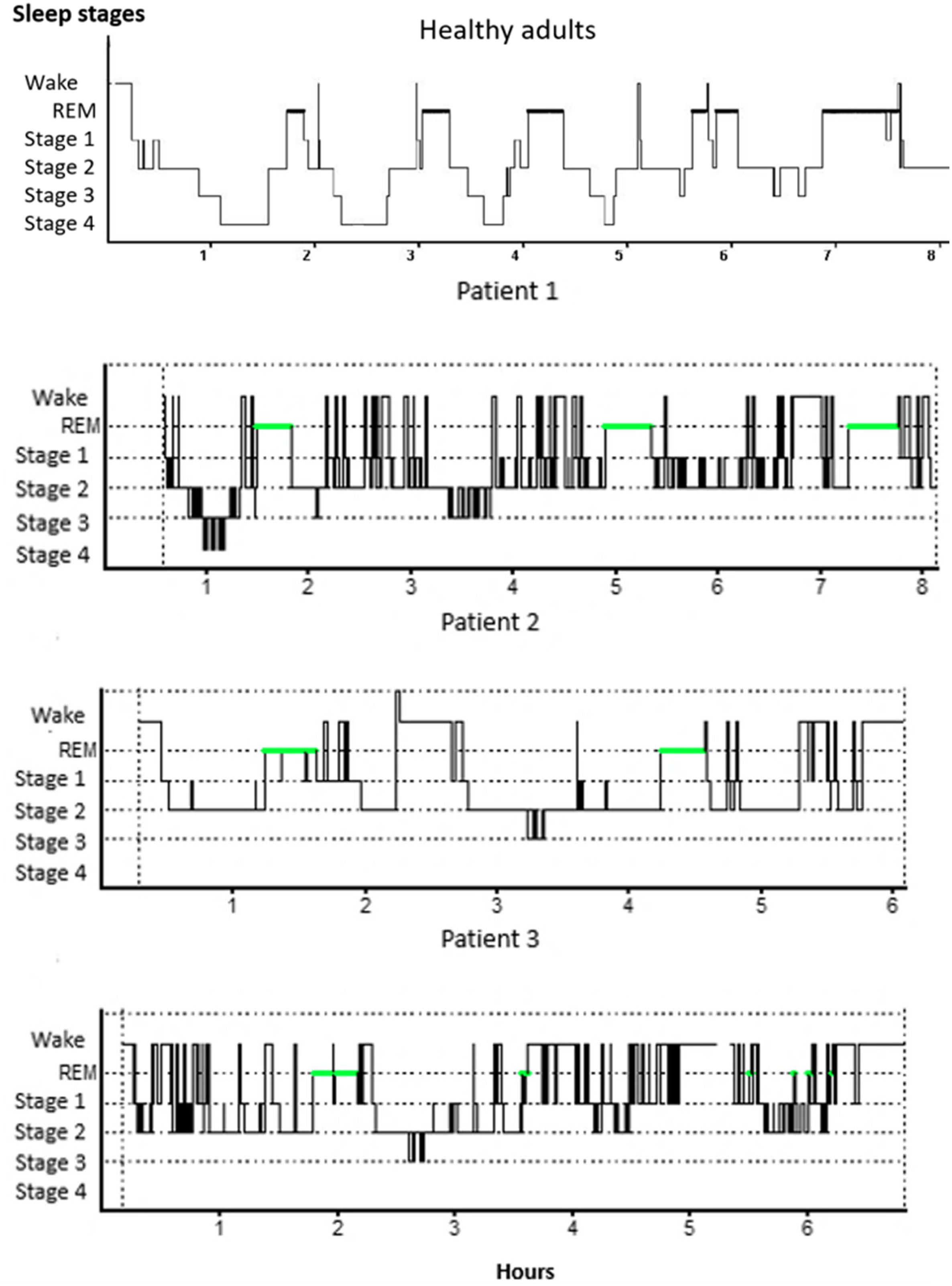
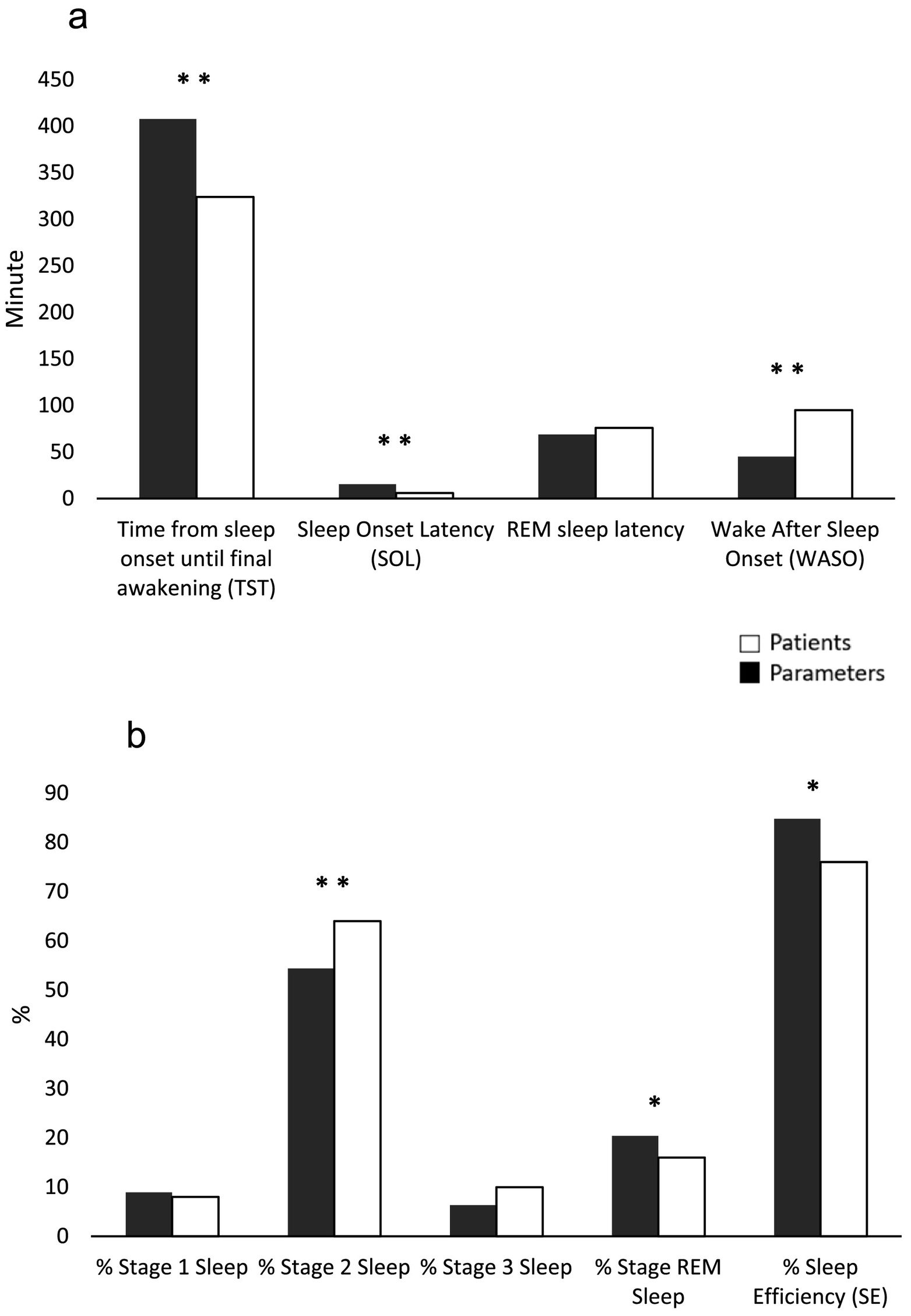
In relation to PSG variables, results reveal to changes in some of the parameters evaluated, between the obtained values in patients and the expected values according to sex and age28 (Fig. 1 - hypnogram examples of some of the patients of the sample). Descriptive statistics for self-report questionnaires and PSG variables are depicted for the total sample, as well as PSG criteria values and results of comparison between criteria and patient values, can be seen in Table 1. By means of a statistical analysis, it was found that patients with frontal tumor present an increase of wake time after sleep onset (p = 0.005) and percentage of stage 2 sleep (p = 0.01). Also, a shorter total sleep time is observed (p = 0.005), a decrease in the latency of sleep onset (p = 0.0), percentage of REM sleep (p = 0.038) and sleep efficiency (p = 0.021) (Fig. 2).
The first hypnogram shows the record of a night of sleep of a healthy adult (image courtesy of Dr. Haro, Sleep Clinic, UNAM). The following are hypnograms of three registered patients, note the presence of various awakenings and irregularities in the phase sequence, making it a bad night's sleep in all three patients.
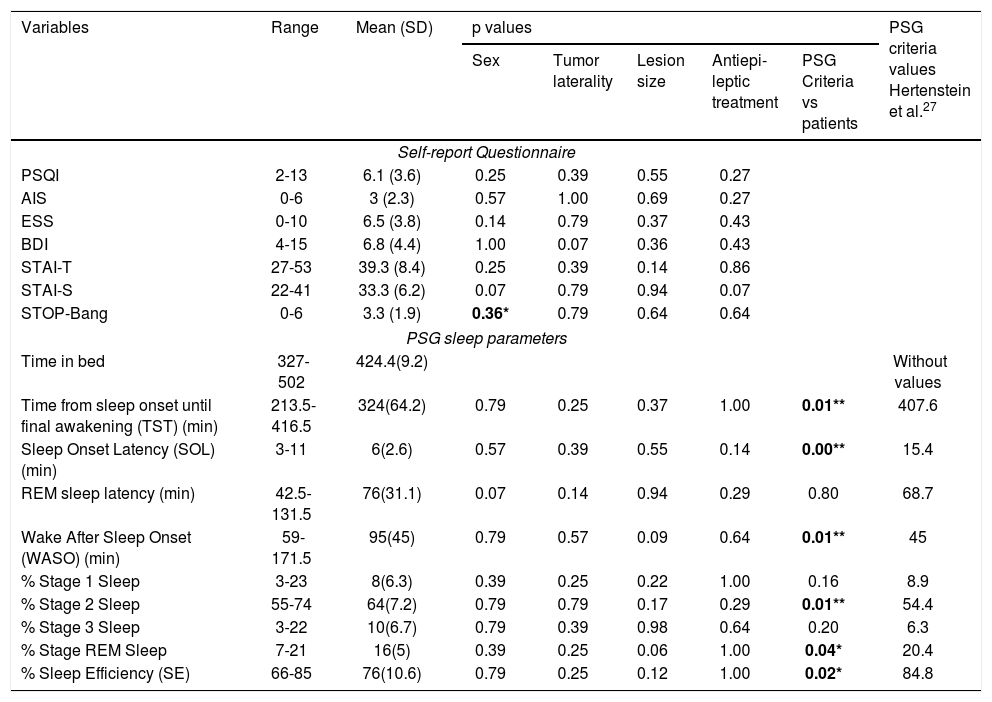
Summary of the results of statistical analyzes of self-report questionnaires and sleep polysomnography variables, according to the socio-demographic and tumor characteristics of the patients. As well the PSG criteria values and results of comparison with PSG values of patients.
| Variables | Range | Mean (SD) | p values | PSG criteria values Hertenstein et al.27 | ||||
|---|---|---|---|---|---|---|---|---|
| Sex | Tumor laterality | Lesion size | Antiepi-leptic treatment | PSG Criteria vs patients | ||||
| Self-report Questionnaire | ||||||||
| PSQI | 2-13 | 6.1 (3.6) | 0.25 | 0.39 | 0.55 | 0.27 | ||
| AIS | 0-6 | 3 (2.3) | 0.57 | 1.00 | 0.69 | 0.27 | ||
| ESS | 0-10 | 6.5 (3.8) | 0.14 | 0.79 | 0.37 | 0.43 | ||
| BDI | 4-15 | 6.8 (4.4) | 1.00 | 0.07 | 0.36 | 0.43 | ||
| STAI-T | 27-53 | 39.3 (8.4) | 0.25 | 0.39 | 0.14 | 0.86 | ||
| STAI-S | 22-41 | 33.3 (6.2) | 0.07 | 0.79 | 0.94 | 0.07 | ||
| STOP-Bang | 0-6 | 3.3 (1.9) | 0.36* | 0.79 | 0.64 | 0.64 | ||
| PSG sleep parameters | ||||||||
| Time in bed | 327-502 | 424.4(9.2) | Without values | |||||
| Time from sleep onset until final awakening (TST) (min) | 213.5-416.5 | 324(64.2) | 0.79 | 0.25 | 0.37 | 1.00 | 0.01** | 407.6 |
| Sleep Onset Latency (SOL) (min) | 3-11 | 6(2.6) | 0.57 | 0.39 | 0.55 | 0.14 | 0.00** | 15.4 |
| REM sleep latency (min) | 42.5-131.5 | 76(31.1) | 0.07 | 0.14 | 0.94 | 0.29 | 0.80 | 68.7 |
| Wake After Sleep Onset (WASO) (min) | 59-171.5 | 95(45) | 0.79 | 0.57 | 0.09 | 0.64 | 0.01** | 45 |
| % Stage 1 Sleep | 3-23 | 8(6.3) | 0.39 | 0.25 | 0.22 | 1.00 | 0.16 | 8.9 |
| % Stage 2 Sleep | 55-74 | 64(7.2) | 0.79 | 0.79 | 0.17 | 0.29 | 0.01** | 54.4 |
| % Stage 3 Sleep | 3-22 | 10(6.7) | 0.79 | 0.39 | 0.98 | 0.64 | 0.20 | 6.3 |
| % Stage REM Sleep | 7-21 | 16(5) | 0.39 | 0.25 | 0.06 | 1.00 | 0.04* | 20.4 |
| % Sleep Efficiency (SE) | 66-85 | 76(10.6) | 0.79 | 0.25 | 0.12 | 1.00 | 0.02* | 84.8 |
SD = standard deviation. PSQI = Pittsburgh Sleep Quality Index, AIS = Athens Insomnia Scale, ESS = Epworth Sleepiness Scale, BDI = Beck Depression Inventory, STAI-S = State–Trait Anxiety Inventory (state), STAI-T = State–Trait Anxiety Inventory (trait). *p < 0.05, **p < 0.01.
Comparison between patients and expected parameters27 of (a) time and latencies, and (b) percentage of sleep stages and efficiency. *p < 0.05, **p < 0.01.
No significant differences between the groups by laterality of the tumor and size of the lesion (volume and edema) in the sleep scales, the depression and anxiety inventories, and the polysomnographic variables (p ≥ 0.05). And by sex, only STOP-Bang questionnaire showed significant differences, with higher scores by men (See Table 1).
The results of the Spearman correlation test suggest no relation between PSG variables and self-reported sleep questionnaires. On the contrary, it was found a significant positive correlation between STOP-Bang scores with the assessment of the Epworth Sleepiness Scale (ESS) (Rho = 0.770; p = 0.025).
DiscussionThe sleep patterns can be modified due to a brain pathology such as tumors, that permit to know thoroughly that occur when these lesions affect one particular area of brain. With respect to sleep architecture, although the effects in the PSG parameters of some space-occupying pathologies indicate the impact of the lesion,11,12,29 it is difficult to delimit the participation of the frontal lobes, due to these studies do not counted with an adequate delimitation of specific study area by the characteristics of type of pathology, for example; cerebrovascular and neurodegenerative disease among others.
Precisely, in a similar study carried out with frontal brain tumor,10 divergent results were observed with our study, specifically with respect to the percentages of some stages, since in this study a decrease in stage 2 and an increase in SWS were observed; unlike our study, where stage 2 presented an increase and SWS did not show significant differences. This discrepancy could be due to differences in the use of the normative values used by both studies. In our case, it was decided to take the values of Hertenstein et al.,28 which provide reliable reference data for the characterization of sleep architecture, since it differs from other studies that have smaller samples, where there was only one night of recording (without habituation night), with narrower age ranges and whose staging of sleep stages was performed according to older criteria, such as those of Rechtschaffen and Kales.30
According to our results, in general were found changes in the sleep architecture in the patients with frontal brain tumor, principally, sleep deficiency given by increase in the number of awakenings and light sleep (stage 2), which in turn affects to the quantity of total sleep. This findings of the sleep disruption are consistent with what is reported in the literature-namely due to brain pathology.11,29 With respect to decline of REM sleep, let us remember that the frontal lobes have been implicated in the regulation of eye movements characteristic of this stage3–5 and in the presence of a pathology, the frontal cortex plays an inhibitory role in these movements.31 Therefore, this tumor growth can affect these REM sleep generation mechanisms. Likewise, REM sleep can be a vulnerable stage related to increased sleep disruption, as observed in other studies of brain pathology11,12,32,33 and along with the significant increase in stage 2, a cycling back to light sleep stage (stage 1 or 2) after disruption of sleep is suggested.34
Despite the fact that previous studies have reported the presence of depression in patients with brain tumors,14,18 in this study this aspect was not found to be present. Another of the most frequent complaints is anxiety,18 however the presence of high symptoms of trait anxiety is observed in only two patients and none in state anxiety for registration nights. Such findings could explain why patients also do not report insomnia and drowsiness, and only three patients report poor sleep quality (PSQI), since these sleep complaints may also be clustered or related to symptoms of depression and anxiety in the patients with brain tumors.15,18
Likewise, patients do not report insomnia and drowsiness and few refer poor quality of sleep in self-report questionnaires, but they do show changes in sleep architecture through the PSG recording, so there is a discrepancy between what patients perceive regarding your sleep with objective values. Although previously a high correlation between objective and subjective measures has been reported both in healthy people and in patients with different mental, neurological and sleep disorders,35 other factors also influence this relationship, such as emotional aspects.36 In accordance with the above, the absence of depressive and anxious symptoms could an explanation for this lack of correlation.
Variables such as sex, laterality of the tumor and size of the lesion were not found to influence sleep architecture and subjective aspects of sleep and mood. Although it has been reported that a lesion in a cerebral hemisphere can lead to changes in PSG patterns, suggesting a hemispheric inductive and regulatory function; mainly a greater specialization of the right hemisphere as regards REM sleep.11 Previous studies are also inconclusive regarding hemispheric lateralization during sleep.31,37 Equally, the fact of not finding effects due to laterality of the tumor, a plasticity effect (compensatory reaction) could be considered in the sample recorded in this study.
Regarding the risk assessment of OSA (one of the most prevalent respiratory disorders during sleep), despite the fact that some patients presented an intermediate and high risk, this did not impact on the architecture of sleep. On the other hand, it was observed that the men evaluated have a higher risk of OSA, in addition to a significant relationship with the evaluation of sleepiness, which coincides with the parameters evaluated in the STOP-Bang questionnaire.24
Finally, in this study it was possible to carry out the association between structural changes and sleep characteristics in patients with frontal brain tumor. These changes are of interest, not only from the perspective of the mechanisms responsible for the neuroanatomical changes in sleep architecture, but also from a clinical perspective, since the cognitive and behavioral effects of sleep disturbances are known.38
The limitations of our study, can be low number of patients studied, since we tried to control as much as possible the criteria of interest such as the type and location of the lesion, among others. Also, this study lacked a control group of individuals without brain tumor, however, despite this limitation, valid normality criteria were used for the population evaluated. Another important aspect to consider is that some of the patients were on anticonvulsant medication and even one patient had a seizure during sleep recording. Despite the fact that a statistical analysis carried out did not find significant differences in the variables evaluated in this study, it is a factor that we believe should be taken with caution, since previous studies report effects on sleep architecture due to antiepileptic treatment.39,40 Further investigation is needed to better characterize these effects of medication on sleep in these patients. Finally, the evaluation of other PSG parameters such as respiratory and movement aspects in future research, could help to clarify the changes observed in the architecture of sleep.
ConclusionsA disruption of the sleep architecture of patients with frontal tumors was observed, characterized by changes in the pattern and in the sleeping time. Such changes could you tell us that the presence of this type of pathology, which causes changes structural and functional, allows us to understand the organization of the brain during this state.
This study provides novel information about sleep disturbances in these patients and although the disruption of sleep through objective methods does not necessarily correspond to its perception of alteration through subjective methods, our results provide important clinical implications, since the neurocognitive and functional repercussions of sleep disruptions are known. Therefore, the findings will allow to guide more timely interventions and directed to their needs.
FundingThis work was financed by a graduate fellowship from Mexico's Consejo Nacional de Ciencia y Tecnología (CONACYT) (CVU 363836) and partially by the Universidad Nacional Autónoma de México DGAPA (UNAM) [Project IN231720].
Declarations of Competing InterestNone.
We thank to the Neuroscience Unit of the Instituto Nacional de Cancerología and the Neurosurgery, Magnetic Resonance and Cognition and Behavior Departments of the Instituto Nacional de Neurología y Neurocirugía for the use of their installations. Also to Carlos Raúl Castillo Montoya from the company “Sueño México, Medicina y Neurofisiología del Sueño S.C.”, for the loan of his equipment.