Migraine is the most common reason for neurology outpatient consultation; more than 80% of patients with migraine suffer some degree of associated disability. Migraine is an underdiagnosed disorder and patients frequently receive inappropriate treatment, which is why it is necessary to establish recommendations to improve patient management. The objective of this document is to establish a consensus position on the pharmacological treatment of migraine, including general measures and detailed analysis of the different options for the treatment of attacks and their prevention, from the perspective of Ibero-American countries.
Methods and resultsBased on the recommendation documents published by the scientific societies of different Ibero-American countries, 2 surveys were prepared; through the application of the Delphi methodology and discussions in subsequent meetings, a document was developed in consensus format, supported by evidence from the literature and expert recommendations.
ConclusionsA series of recommendations were agreed upon for the treatment of episodic migraine and chronic migraine, both for the acute management of attacks and for their prevention. These recommendations are based on the consensus process and a literature review, and are adapted to the regional conditions of the Ibero-American countries.
La migraña es el motivo ambulatorio neurológico de consulta más frecuente, más del 80% de los pacientes con migraña sufre algún grado de discapacidad asociada. La migraña es un trastorno subdiagnosticado y frecuentemente los pacientes reciben un tratamiento inadecuado por lo que es necesario establecer recomendaciones que ayuden a un mejor manejo de los pacientes. El objetivo de este trabajo es realizar un consenso sobre el tratamiento farmacológico de la migraña, incluyendo medidas generales y el análisis pormenorizado de las diferentes opciones para el tratamiento de las crisis y de la prevención de las mismas con la óptica de los países iberoamericanos.
Métodos y resultadosPartiendo de los documentos de recomendaciones publicados por las sociedades científicas de diferentes países latinoamericanos se elaboraron dos encuestas y mediante la aplicación de la metodología Delphi y discusiones en reuniones posteriores, se desarrolló un documento en formato de consenso soportado en literatura y recomendaciones de expertos.
ConclusionesSe han consensuado una serie de recomendaciones para el tratamiento de la migraña episódica (ME)y de la migraña crónica (MC), tanto para el manejo de los ataques como para su prevención. Dichas recomendaciones se han basado en el consenso y en la revisión bibliográfica y se han adaptado a las condiciones regionales de los países iberoamericanos.
Migraine is the most frequent reason for consultation at outpatient neurology clinics.1 Numerous population-based studies have confirmed its high prevalence rate, estimated at 5%–8% among men and 15%–20% among women. Over 80% of patients with migraine present some degree of associated disability. The World Health Organisation includes migraine among the most disabling disorders. It is the third most prevalent disorder and the seventh leading cause of disability worldwide (the second greatest cause of disability among neurological disorders, after stroke).2
Despite its high prevalence and severe impact on daily life, migraine is underdiagnosed and patients often receive inappropriate treatments, due to a number of factors, ranging from insufficient academic training at medical schools and faculties to low societal awareness about the problem and the stigma attached to the disorder. We also lack biomarkers to confirm diagnosis; nonetheless, the diagnostic criteria of the International Headache Society3 enable precise clinical diagnosis and differentiation between episodic and chronic migraine (EM and CM, respectively), according to the frequency of attacks.
This study aims to establish consensus on the pharmacological treatment of migraine in Ibero-American countries. To that end, we analysed the different consensus statements, guidelines, and recommendations on migraine published in Ibero-America (Table 1); studies were classified according to level of evidence (Table 2). Based on the proposals included in documents published by different scientific societies, we prepared 2 surveys, which were electronically distributed to neurologists belonging to the Latin American Headache Association (ASOLACE, for its Spanish initials). This study aims to develop a series of consensus recommendations on the acute and preventive management of migraine.
Ibero-American consensus statements on migraine included in the review.
| Country | Consensus |
|---|---|
| Argentina | Argentinian consensus guidelines on the use of monoclonal antibodies in patients with migraine4 |
| Brazil | Consensus of the Brazilian Headache Society (SBCe) for prophylactic treatment of episodic migraine: parts I and II5,6 |
| Chile | Migraña crónica. Una visión global de este gran desafío para manejar nuestros pacientes7 |
| Colombia | Expert consensus on the preventive and acute treatment of migraine on behalf of the Colombian Association of Neurology8Guideline on the preventive treatment of chronic migraine, chronic tension type headache, hemicrania continua, and new daily persistent headache on behalf of the Colombian Association of Neurology9Recommendations on the use of the anti-CGRP monoclonal antibodies for migraine prophylaxis on behalf of the Colombian Association of Neurology10 |
| Spain | Recommendations guide for the treatment of migraine in the clinical practice11Manual de Práctica Clínica en Cefaleas. Recomendaciones diagnóstico-terapéuticas12 |
| Latin America | Latin American consensus on guidelines for chronic migraine treatment13 |
| Mexico | Comprehensive management of adults with chronic migraine: clinical practice guidelines in Mexico14 |
CGRP: calcitonin gene-related peptide.
This consensus statement is based on 2 online surveys that were completed by ASOLACE members from multiple countries: Argentina, Bolivia, Brazil, Chile, Colombia, the Dominican Republic, Ecuador, Mexico, Paraguay, Peru, Spain, Uruguay, and the USA. We received 98 responses in the first phase and 84 in the second. They included questions on all aspects of migraine management, from general definitions and general measures for migraine prevention to therapeutic management of different clinical situations. We considered consensus to have been established if 70% or more of respondents approved a statement. Statements for which consensus was not reached were included in the second survey, with the same cut-off point for consensus. When consensus was not reached, the statements in question were reviewed by a smaller expert panel to assess which issues could be resolved based on reports in the literature and which required discussion to reach a consensus recommendation.
We also used artificial intelligence tools including Sourcely, Scite, Connected Maps, GPT4, and Readcube AI to improve access to and analysis of the information available in the literature.
Consensus statementGeneral measures in migraine treatmentIn the great majority of patients with migraine, treatment focused exclusively on avoiding trigger factors achieves only a marginal effect, and pharmacological treatment is required.15,16 Despite this, it is important to advise patients to exercise, practise good sleep hygiene, control stress, avoid fasting, avoid excessive caffeine consumption, stay hydrated, and identify and avoid migraine triggers; these measures contribute to reducing the frequency of migraine attacks. Well-designed studies assessing certain “non-conventional” therapies, such as cryotherapy, chiropractic cervical adjustment, acupuncture, and homoeopathy, have found them not to be efficacious for treating migraine. Other non-pharmacological treatments, such as biofeedback, relaxation techniques, and cognitive behavioural therapy, may be considered an option in migraine prevention, may help reduce attacks, and may be combined with preventive medications to achieve an additional improvement.17
Treatment of migraine attacks or crisesThe treatment of migraine attacks, known as acute, symptomatic, or abortive treatment, is indicated for all patients with migraine. This treatment should be tailored to the individual patient using a stratified analgesia approach.12 The drugs indicated for managing migraine attacks are classified as non-specific, specific, and adjuvant medications. Non-specific drugs include simple analgesics and non-steroidal anti-inflammatory drugs (NSAID); specific drugs include ergot derivatives, triptans (5-HT1B/1D/1F receptor agonists), ditans (selective 5-HT1D/1F receptor agonists), and calcitonin gene-related peptide (CGRP) receptor antagonists (oral gepants). Adjuvant drugs fundamentally include antiemetic/prokinetic agents (domperidone, metoclopramide), used to treat nausea and vomiting.12,18
Like other guidelines, we recommend avoiding opioids, opiates, and combinations of these with other analgesics, given their poor effectiveness in treating migraine attacks compared to the other available options; furthermore, they increase the risk of medication overuse headache (MOH).15,16 However, we must accept that there are situations in which such opioids as tramadol, with similar potency to a dose of intravenous diclofenac, may be considered when other drugs cannot be used or in patients with contraindications for the occasional use of options other than opioids.
Reports on the daily use of metamizole, a drug commonly used in Spain, suggest a degree of efficacy, although no studies have been performed that enable stronger recommendation; therefore, its indication should be limited.12
In these patients, analgesics must be selected on an individual basis, taking into account such factors as time of attack onset, presence of early vomiting or nausea, pain severity and duration, response and tolerance to previous analgesic drugs, and comorbidities. Similarly, subsequent evaluation must consider analgesic response and achievement of treatment goals: pain control in the following 2–4&#¿;h, recurrence or need for rescue doses in the following 24&#¿;h, adverse reactions, tolerability, and reduction in pain-related disability.
Treatment plans must be based on a stratified model, with non-specific drugs indicated for mild–moderate attacks and specific drugs for severe attacks. While this approach may be effective in the great majority of patients, it is not applicable in all cases due to idiosyncratic responses. Some patients will require specific treatments for migraine attacks, regardless of their severity; adjustment of this analgesic treatment regimen will depend on the response to analgesics previously used by the patient. Another important concept is determining what drugs to use for abortive (first 4&#¿;h of pain) and rescue therapy (after failure of the first drug). Analgesics lose their effectiveness due to biomolecular and neuromodulatory changes that are directly related to the time elapsed between migraine attack onset/amplification/persistence and administration of the abortive drug. This requires educating patients about taking the acute treatment in the initial phases of pain.
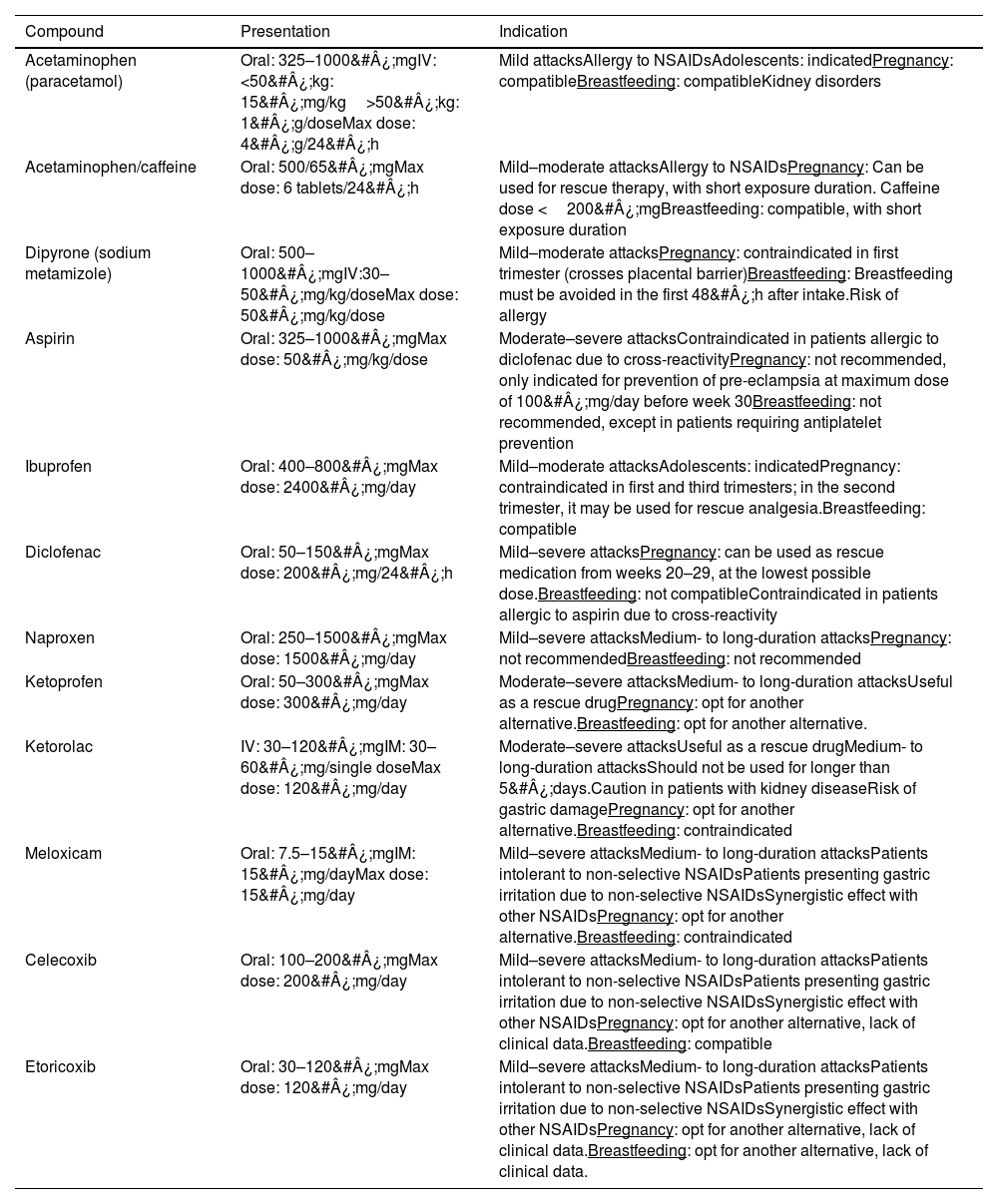
Non-specific drugsPatients with mild–moderate migraine attacks can initially be treated with non-specific drugs, such as oral NSAIDs. In patients with early nausea/vomiting, or whose symptoms cause moderate–severe disability, these may be combined with metoclopramide or domperidone. Not all NSAIDs are useful for the symptomatic treatment of migraine; those whose efficacy has been demonstrated are listed in Table 3.12 Another analgesic that should be considered in this type of attack is metamizole (dipyrone). In a meta-analysis of 209 studies, published in 2022, Peres et al.19 indirectly compared metamizole (5 studies) against triptans; analysing a primary outcome variable of analgesic response (pain relief or freedom from pain at 2&#¿;h), they concluded that metamizole may be equally as effective as triptans, with a smaller economic burden for the healthcare provider.12
Non-specific analgesics in migraine.
| Compound | Presentation | Indication |
|---|---|---|
| Acetaminophen (paracetamol) | Oral: 325–1000&#¿;mgIV:<50&#¿;kg: 15&#¿;mg/kg>50&#¿;kg: 1&#¿;g/doseMax dose: 4&#¿;g/24&#¿;h | Mild attacksAllergy to NSAIDsAdolescents: indicatedPregnancy: compatibleBreastfeeding: compatibleKidney disorders |
| Acetaminophen/caffeine | Oral: 500/65&#¿;mgMax dose: 6 tablets/24&#¿;h | Mild–moderate attacksAllergy to NSAIDsPregnancy: Can be used for rescue therapy, with short exposure duration. Caffeine dose <200&#¿;mgBreastfeeding: compatible, with short exposure duration |
| Dipyrone (sodium metamizole) | Oral: 500–1000&#¿;mgIV:30–50&#¿;mg/kg/doseMax dose: 50&#¿;mg/kg/dose | Mild–moderate attacksPregnancy: contraindicated in first trimester (crosses placental barrier)Breastfeeding: Breastfeeding must be avoided in the first 48&#¿;h after intake.Risk of allergy |
| Aspirin | Oral: 325–1000&#¿;mgMax dose: 50&#¿;mg/kg/dose | Moderate–severe attacksContraindicated in patients allergic to diclofenac due to cross-reactivityPregnancy: not recommended, only indicated for prevention of pre-eclampsia at maximum dose of 100&#¿;mg/day before week 30Breastfeeding: not recommended, except in patients requiring antiplatelet prevention |
| Ibuprofen | Oral: 400–800&#¿;mgMax dose: 2400&#¿;mg/day | Mild–moderate attacksAdolescents: indicatedPregnancy: contraindicated in first and third trimesters; in the second trimester, it may be used for rescue analgesia.Breastfeeding: compatible |
| Diclofenac | Oral: 50–150&#¿;mgMax dose: 200&#¿;mg/24&#¿;h | Mild–severe attacksPregnancy: can be used as rescue medication from weeks 20–29, at the lowest possible dose.Breastfeeding: not compatibleContraindicated in patients allergic to aspirin due to cross-reactivity |
| Naproxen | Oral: 250–1500&#¿;mgMax dose: 1500&#¿;mg/day | Mild–severe attacksMedium- to long-duration attacksPregnancy: not recommendedBreastfeeding: not recommended |
| Ketoprofen | Oral: 50–300&#¿;mgMax dose: 300&#¿;mg/day | Moderate–severe attacksMedium- to long-duration attacksUseful as a rescue drugPregnancy: opt for another alternative.Breastfeeding: opt for another alternative. |
| Ketorolac | IV: 30–120&#¿;mgIM: 30–60&#¿;mg/single doseMax dose: 120&#¿;mg/day | Moderate–severe attacksUseful as a rescue drugMedium- to long-duration attacksShould not be used for longer than 5&#¿;days.Caution in patients with kidney diseaseRisk of gastric damagePregnancy: opt for another alternative.Breastfeeding: contraindicated |
| Meloxicam | Oral: 7.5–15&#¿;mgIM: 15&#¿;mg/dayMax dose: 15&#¿;mg/day | Mild–severe attacksMedium- to long-duration attacksPatients intolerant to non-selective NSAIDsPatients presenting gastric irritation due to non-selective NSAIDsSynergistic effect with other NSAIDsPregnancy: opt for another alternative.Breastfeeding: contraindicated |
| Celecoxib | Oral: 100–200&#¿;mgMax dose: 200&#¿;mg/day | Mild–severe attacksMedium- to long-duration attacksPatients intolerant to non-selective NSAIDsPatients presenting gastric irritation due to non-selective NSAIDsSynergistic effect with other NSAIDsPregnancy: opt for another alternative, lack of clinical data.Breastfeeding: compatible |
| Etoricoxib | Oral: 30–120&#¿;mgMax dose: 120&#¿;mg/day | Mild–severe attacksMedium- to long-duration attacksPatients intolerant to non-selective NSAIDsPatients presenting gastric irritation due to non-selective NSAIDsSynergistic effect with other NSAIDsPregnancy: opt for another alternative, lack of clinical data.Breastfeeding: opt for another alternative, lack of clinical data. |
IM: intramuscular; IV: intravenous; NSAID: non-steroidal anti-inflammatory drug.
According to the first Chilean consensus statement on CM,7 the non-specific analgesics to be considered in patients with migraine are acetylsalicylic acid (ASA), acetaminophen (paracetamol), or combinations of analgesics plus caffeine (e.g., ASA&#¿;+&#¿;acetaminophen&#¿;+&#¿;caffeine) for mild–moderate pain. The American Headache Society (AHS) and Canadian Headache Society attribute the highest level of evidence to the use of these treatments as first-line therapy. This class of drugs is considered to be less efficacious than triptans. They are generally well tolerated and may be useful for treating mild–moderate pain.
In the opinion of our study participants, non–COX-2–selective NSAIDs are preferable for treating mild–moderate attacks; by order of preference, they recommended naproxen, ibuprofen, immediate-release diclofenac, and dexketoprofen. Simple analgesics such as acetaminophen may be useful in some cases. The use of such COX-2–selective NSAIDs as celecoxib and etoricoxib, COX-2–preferential NSAIDs such as meloxicam, and non-selective NSAIDs (particularly delayed-release diclofenac) are second-line treatment options to be considered in patients presenting gastrointestinal intolerance or treatment failure of the first-line options.
Specific drugsConsensus was reached on the position that, in moderate–severe attacks, specific analgesics should be the treatment of first choice, with triptans as the first-line treatment and ditans or gepants as the second-line treatment, as suggested in the literature, in patients presenting treatment failure or intolerance to triptans. Although ergot derivatives do control pain, their use should be restricted to certain situations (e.g., in long attack duration or intolerance to triptans) due to the risk of misuse and MOH. Economic cost continues to be an important consideration in the selection of ergot derivatives over triptans, ditans, or oral gepants, and explains the widespread use of these drugs in many countries.
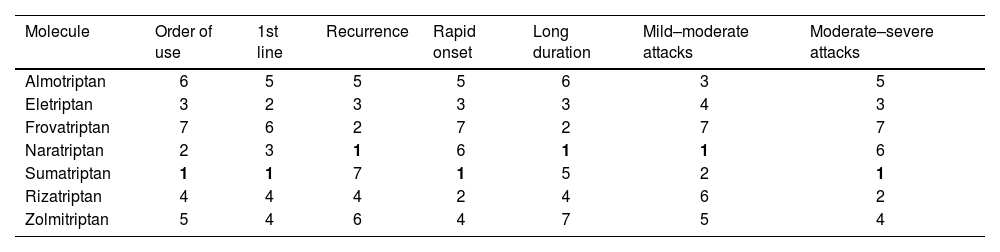
The availability of triptans varies between countries: they are most widely available in Spain, the USA, and Argentina, and least available in Ecuador. The most commonly available triptans, in ascending order, are naratriptan, eletriptan, and sumatriptan; these offer good coverage for the treatment of migraine attacks, with specific molecules available for poor tolerance, long attack duration, and moderate–severe pain intensity. However, we must account for idiosyncratic responses to analgesic treatment. Table 4 lists the most frequently selected molecules in different clinical situations. In most cases, these drugs scored the highest among participants in the consensus process, no doubt partly due to their greater availability. This demonstrates one of the limitations of triptans: their underuse and limited commercialisation and availability, despite their being the drugs with the highest described clinical effectiveness. Caution is frequently recommended for their use in hypertensive patients, although the great majority of neurologists consider them safe in patients with controlled hypertension.
Selection of triptans used to treat migraine attacks in Latin America. Consensus findings.
| Molecule | Order of use | 1st line | Recurrence | Rapid onset | Long duration | Mild–moderate attacks | Moderate–severe attacks |
|---|---|---|---|---|---|---|---|
| Almotriptan | 6 | 5 | 5 | 5 | 6 | 3 | 5 |
| Eletriptan | 3 | 2 | 3 | 3 | 3 | 4 | 3 |
| Frovatriptan | 7 | 6 | 2 | 7 | 2 | 7 | 7 |
| Naratriptan | 2 | 3 | 1 | 6 | 1 | 1 | 6 |
| Sumatriptan | 1 | 1 | 7 | 1 | 5 | 2 | 1 |
| Rizatriptan | 4 | 4 | 4 | 2 | 4 | 6 | 2 |
| Zolmitriptan | 5 | 4 | 6 | 4 | 7 | 5 | 4 |
Results are presented according to the number of responses, with 1 being the option receiving the most votes and 7 receiving the least.
In this consensus process, similarly to reports in the literature, triptans were the recommended first line of treatment for migraine attacks in patients showing no response to non-specific drugs. As the second line of treatment, novel molecules such as ditans and oral gepants should be considered, depending on analgesic response and tolerance to triptans. Finally, ergot derivatives should be considered as a third line of treatment in individual cases.
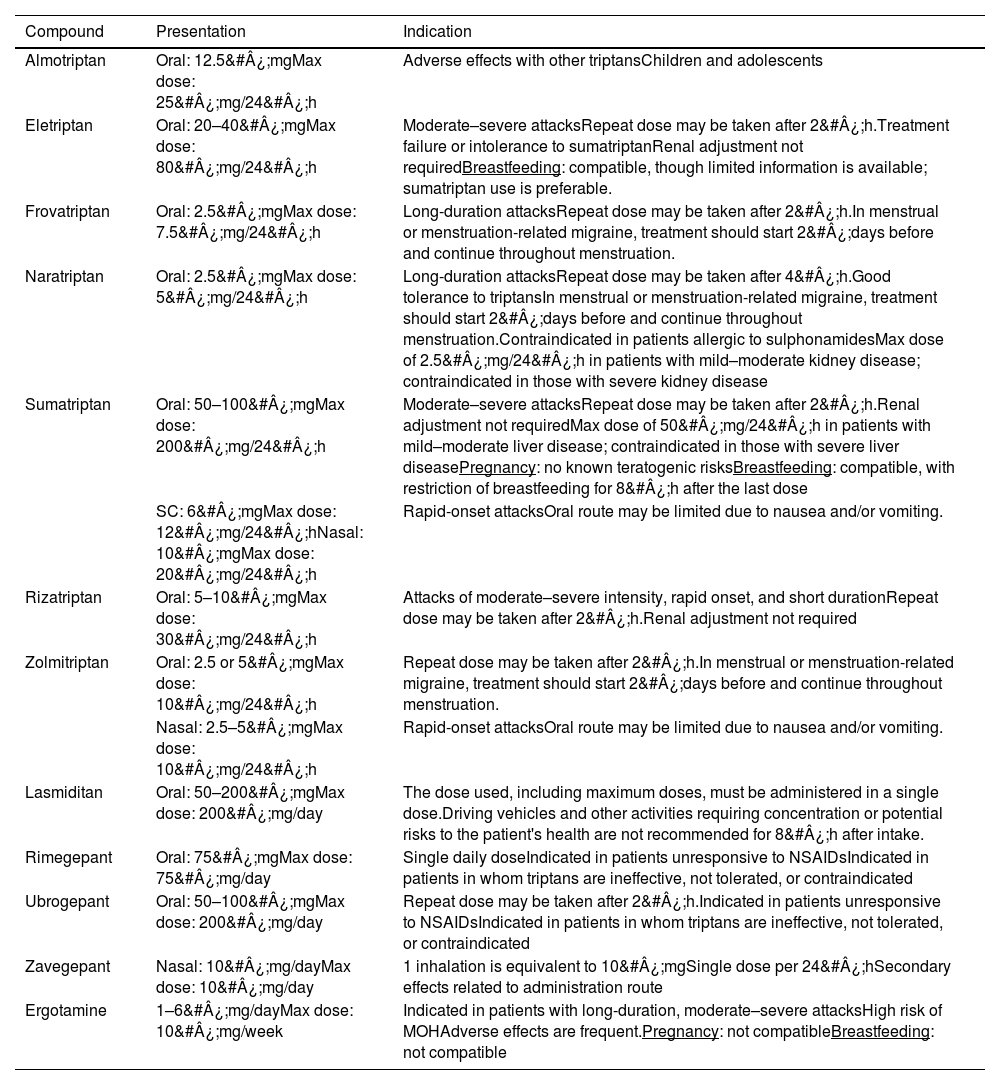
Table 5 describes specific drugs, such as triptans, ditans, and oral gepants, and their indications and recommended doses. These molecules are efficacious in treating migraine attacks; ergotamine and other non-inhaled forms of dihydroergotamine present an intermediate level of efficacy between those of NSAIDs and triptans, with the latter being the first-line treatment in most cases. Treatment with ergot derivatives may be maintained in patients with long-term, appropriate use of these drugs and satisfactory response, taking into consideration adverse reactions, frequency of use, and risk of MOH. Due to their prolonged effects, another possible indication of ergot derivatives is in patients with long-duration attacks and high rates of recurrence. Although triptans are the drugs of choice in this type of attack, on account of their greater efficacy (frovatriptan and naratriptan are also indicated for these patients), ergot derivatives may be a treatment option in patients presenting poor tolerance or response to these drugs.20–22
Specific analgesics in migraine. Indications and recommended doses.
| Compound | Presentation | Indication |
|---|---|---|
| Almotriptan | Oral: 12.5&#¿;mgMax dose: 25&#¿;mg/24&#¿;h | Adverse effects with other triptansChildren and adolescents |
| Eletriptan | Oral: 20–40&#¿;mgMax dose: 80&#¿;mg/24&#¿;h | Moderate–severe attacksRepeat dose may be taken after 2&#¿;h.Treatment failure or intolerance to sumatriptanRenal adjustment not requiredBreastfeeding: compatible, though limited information is available; sumatriptan use is preferable. |
| Frovatriptan | Oral: 2.5&#¿;mgMax dose: 7.5&#¿;mg/24&#¿;h | Long-duration attacksRepeat dose may be taken after 2&#¿;h.In menstrual or menstruation-related migraine, treatment should start 2&#¿;days before and continue throughout menstruation. |
| Naratriptan | Oral: 2.5&#¿;mgMax dose: 5&#¿;mg/24&#¿;h | Long-duration attacksRepeat dose may be taken after 4&#¿;h.Good tolerance to triptansIn menstrual or menstruation-related migraine, treatment should start 2&#¿;days before and continue throughout menstruation.Contraindicated in patients allergic to sulphonamidesMax dose of 2.5&#¿;mg/24&#¿;h in patients with mild–moderate kidney disease; contraindicated in those with severe kidney disease |
| Sumatriptan | Oral: 50–100&#¿;mgMax dose: 200&#¿;mg/24&#¿;h | Moderate–severe attacksRepeat dose may be taken after 2&#¿;h.Renal adjustment not requiredMax dose of 50&#¿;mg/24&#¿;h in patients with mild–moderate liver disease; contraindicated in those with severe liver diseasePregnancy: no known teratogenic risksBreastfeeding: compatible, with restriction of breastfeeding for 8&#¿;h after the last dose |
| SC: 6&#¿;mgMax dose: 12&#¿;mg/24&#¿;hNasal: 10&#¿;mgMax dose: 20&#¿;mg/24&#¿;h | Rapid-onset attacksOral route may be limited due to nausea and/or vomiting. | |
| Rizatriptan | Oral: 5–10&#¿;mgMax dose: 30&#¿;mg/24&#¿;h | Attacks of moderate–severe intensity, rapid onset, and short durationRepeat dose may be taken after 2&#¿;h.Renal adjustment not required |
| Zolmitriptan | Oral: 2.5 or 5&#¿;mgMax dose: 10&#¿;mg/24&#¿;h | Repeat dose may be taken after 2&#¿;h.In menstrual or menstruation-related migraine, treatment should start 2&#¿;days before and continue throughout menstruation. |
| Nasal: 2.5–5&#¿;mgMax dose: 10&#¿;mg/24&#¿;h | Rapid-onset attacksOral route may be limited due to nausea and/or vomiting. | |
| Lasmiditan | Oral: 50–200&#¿;mgMax dose: 200&#¿;mg/day | The dose used, including maximum doses, must be administered in a single dose.Driving vehicles and other activities requiring concentration or potential risks to the patient's health are not recommended for 8&#¿;h after intake. |
| Rimegepant | Oral: 75&#¿;mgMax dose: 75&#¿;mg/day | Single daily doseIndicated in patients unresponsive to NSAIDsIndicated in patients in whom triptans are ineffective, not tolerated, or contraindicated |
| Ubrogepant | Oral: 50–100&#¿;mgMax dose: 200&#¿;mg/day | Repeat dose may be taken after 2&#¿;h.Indicated in patients unresponsive to NSAIDsIndicated in patients in whom triptans are ineffective, not tolerated, or contraindicated |
| Zavegepant | Nasal: 10&#¿;mg/dayMax dose: 10&#¿;mg/day | 1 inhalation is equivalent to 10&#¿;mgSingle dose per 24&#¿;hSecondary effects related to administration route |
| Ergotamine | 1–6&#¿;mg/dayMax dose: 10&#¿;mg/week | Indicated in patients with long-duration, moderate–severe attacksHigh risk of MOHAdverse effects are frequent.Pregnancy: not compatibleBreastfeeding: not compatible |
NSAID: non-steroidal anti-inflammatory drug; SC: subcutaneous.
Additional sources of information: Epocrates®, MedLink®, UpToDate®, LactMed®, Drugs®, Vademecum®.
Triptans are selective agonists of serotonin (5-HT1B/1D/1F) receptors, and are generally safe, with demonstrated efficacy and good tolerability. They do not differ in terms of action mechanism or pharmacodynamics, but do present relevant pharmacokinetic differences, which make certain triptans more appropriate for some patients. In patients with nausea, lyophilised forms may be administered orally or nasally, although all forms are essentially absorbed by digestion. In non-responders, subcutaneous sumatriptan may be used; this is currently the treatment of choice for moderate–severe refractory migraine and the most efficacious drug (in this presentation) for treating migraine attacks. Triptans may cause vasoconstriction, and therefore are contraindicated in patients with uncontrolled hypertension, coronary artery disease, cerebrovascular disease, or uncontrolled peripheral vascular disease, but have been shown to be very safe in patients without vascular disease.23–25
According to the first Chilean consensus statement on CM,7 specific analgesics, such as triptans and dihydroergotamine, should be considered for first-line treatment of moderate–severe attacks, or in mild–moderate attacks that do not respond to non-specific treatments. Although orodispersible forms (rizatriptan and zolmitriptan) are pharmacokinetically similar to standard tablets, it has been suggested that they may confer greater benefits than oral triptans.
In the Mexican consensus statement on CM,14 such drugs as oral sumatriptan (in monotherapy or combined with naproxen), oral zolmitriptan, eletriptan, and rizatriptan are recommended as the first-line symptomatic treatment for moderate–severe attacks without intense nausea or vomiting. Lasmiditan, ubrogepant, and rimegepant are recommended in patients who are unresponsive or intolerant to triptans, with mild cardiovascular disease, or who are not receiving moderate or potent CYP3A4 inductors or inhibitors.
The clinical practice guidelines of the Spanish Society of Neurology12 define triptans as specific drugs that are generally very safe and well tolerated, with demonstrated efficacy for the symptomatic treatment of migraine attacks; they are recommended as the treatment of choice for moderate–severe attacks.
Migraine attacks at the emergency department and status migrainosusWhen selecting a drug to control migraine attacks in the emergency department, we must assume that the attack presents severe intensity or long duration, and that the patient's usual abortive and rescue treatments have failed (the most frequent reasons for a patient with migraine to visit the emergency department). Two questions should be put to the patient: what treatment options they have already tried, to avoid repeat administration of a drug; and how long they have been in pain, as certain drugs, such as triptans, become less effective once the attack is established and the patient presents such symptoms as hyperalgesia and allodynia. Finally, we must consider what drugs are available at the emergency department.
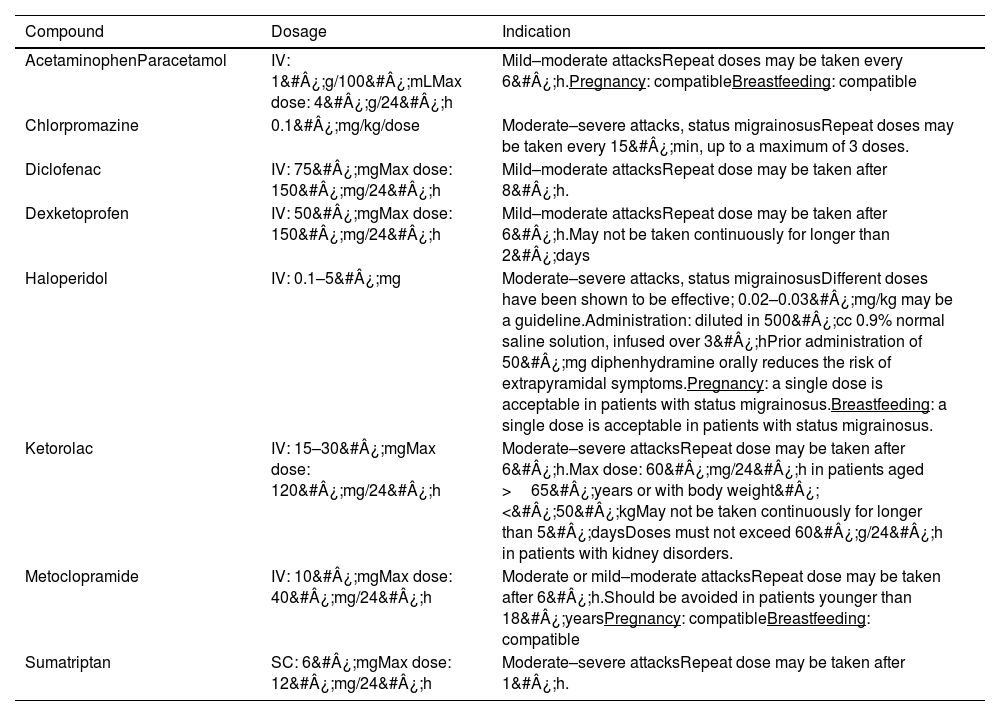
The main drugs recommended in scientific publications are such NSAIDs as diclofenac, dexketoprofen, and ketorolac, and antipsychotics targeting dopamine receptors, with metoclopramide, chlorpromazine, and haloperidol being the most frequently used intravenous options (Table 6).
Drugs used to treat migraine attacks and status migrainosus in the emergency department.
| Compound | Dosage | Indication |
|---|---|---|
| AcetaminophenParacetamol | IV: 1&#¿;g/100&#¿;mLMax dose: 4&#¿;g/24&#¿;h | Mild–moderate attacksRepeat doses may be taken every 6&#¿;h.Pregnancy: compatibleBreastfeeding: compatible |
| Chlorpromazine | 0.1&#¿;mg/kg/dose | Moderate–severe attacks, status migrainosusRepeat doses may be taken every 15&#¿;min, up to a maximum of 3 doses. |
| Diclofenac | IV: 75&#¿;mgMax dose: 150&#¿;mg/24&#¿;h | Mild–moderate attacksRepeat dose may be taken after 8&#¿;h. |
| Dexketoprofen | IV: 50&#¿;mgMax dose: 150&#¿;mg/24&#¿;h | Mild–moderate attacksRepeat dose may be taken after 6&#¿;h.May not be taken continuously for longer than 2&#¿;days |
| Haloperidol | IV: 0.1–5&#¿;mg | Moderate–severe attacks, status migrainosusDifferent doses have been shown to be effective; 0.02–0.03&#¿;mg/kg may be a guideline.Administration: diluted in 500&#¿;cc 0.9% normal saline solution, infused over 3&#¿;hPrior administration of 50&#¿;mg diphenhydramine orally reduces the risk of extrapyramidal symptoms.Pregnancy: a single dose is acceptable in patients with status migrainosus.Breastfeeding: a single dose is acceptable in patients with status migrainosus. |
| Ketorolac | IV: 15–30&#¿;mgMax dose: 120&#¿;mg/24&#¿;h | Moderate–severe attacksRepeat dose may be taken after 6&#¿;h.Max dose: 60&#¿;mg/24&#¿;h in patients aged >65&#¿;years or with body weight&#¿;<&#¿;50&#¿;kgMay not be taken continuously for longer than 5&#¿;daysDoses must not exceed 60&#¿;g/24&#¿;h in patients with kidney disorders. |
| Metoclopramide | IV: 10&#¿;mgMax dose: 40&#¿;mg/24&#¿;h | Moderate or mild–moderate attacksRepeat dose may be taken after 6&#¿;h.Should be avoided in patients younger than 18&#¿;yearsPregnancy: compatibleBreastfeeding: compatible |
| Sumatriptan | SC: 6&#¿;mgMax dose: 12&#¿;mg/24&#¿;h | Moderate–severe attacksRepeat dose may be taken after 1&#¿;h. |
IV: intravenous; SC: subcutaneous.
Additional sources of information: Epocrates®, MedLink®, UpToDate®, LactMed®, Drugs®, Vademecum®.
The first line of treatment will depend on the conditions at the time of assessment at the emergency department. Several guidelines recommend subcutaneous sumatriptan in patients without contraindications and who have not used triptans or ergot derivatives in the last 24&#¿;h,12 although this recommendation is not widely applicable due to the limited availability of this drug at many hospital emergency departments. Therefore, the first line of treatment is typically intravenous NSAIDs, with such drugs as diclofenac,25,26 ketorolac,27,28 and dexketoprofen typically being recommended.29
Intravenous metamizole (dipyrone) is an effective treatment option in mild–moderate attacks, with superior results to placebo; this treatment is associated with a reduction of approximately 50% in pain recurrence or administration of rescue therapies.30 Some cases have been described of allergic reactions; therefore, prior skin allergy testing of the medication is recommended. Rare cases have been described of severe hypotension, anaphylactic shock, and agranulocytosis (more common with long-term use); therefore, it seems reasonable to restrict the use of the drug to patients in whom other options are contraindicated, such as those with allergies to NSAIDs or with kidney disorders, who have previously shown good response to this treatment option.31,32
Among the dopamine receptor agonists, particular attention should be paid to metoclopramide,33,34 chlorpromazine,30 and haloperidol.35,36
In the event of treatment failure with the first- and second-line treatments at the emergency department, other options to be considered include such intravenous drugs as valproic acid37 and occipital nerve block with such local anaesthetics as lidocaine without epinephrine and/or bupivacaine without epinephrine; these treatments should not be mixed with steroids.12
The first Chilean consensus statement on CM7 recommended the following treatments for migraine attacks at the emergency department: ketorolac, metoclopramide, chlorpromazine, and anaesthetic block. Combined therapy with dexamethasone should only be administered to prevent pain recurrence in the following 24&#¿;h, and must not be administered more than 3 times per year.
The Mexican consensus statement on CM14 recommends metamizole (dipyrone) as the first-line symptomatic treatment for patients with moderate–severe attacks. Likewise, intravenous metoclopramide is recommended as a first-line treatment for adults with moderate–severe attacks associated with intense nausea or vomiting.
Participants' responses regarding the management of migraine attacks at the emergency department were consistent with existing recommendations, with NSAIDs, dopamine agonists, sumatriptan, and dipyrone being the first-line treatments. NSAIDs (diclofenac, dexketoprofen, ketorolac) and the dopamine antagonist metoclopramide are often used as the first line of treatment by emergency department physicians, and when assessment by the neurology department is requested due to treatment resistance. In the event of treatment resistance, the first-line treatment options are antipsychotics (metoclopramide, chlorpromazine, haloperidol) and valproic acid. Ketorolac may be used in this phase of the attack, as it may be an appropriate rescue medication; in these cases, it is essential to take into account maximum doses and limitations in patients with kidney disorders. Opioids and benzodiazepines are not recommended for patients with migraine attacks at the emergency department.
Status migrainosus is a special case, as it is a complication of migraine. Participants in the consensus process recommended such antipsychotics as chlorpromazine and haloperidol as the first line of therapy, with metoclopramide being the last choice. Migraine showing no response to these options is considered to be refractory; in these cases, infusion of such coadjuvant treatments as valproic acid or myoneural anaesthetic blocks should be considered as rescue measures. If pain remains uncontrolled, continuous infusion of such drugs as ketamine or propofol at the intensive care unit should be considered.
Dexamethasone is not a good analgesic, but is associated with a reduction in pain recurrence in the next 24&#¿;h, and may be used to treat both migraine attacks and status migrainosus.
Opioids, opiates, and benzodiazepines are not recommended for the treatment of status migrainosus. They may be appropriate for use as coadjuvants in specific super-refractory cases, in continuous infusion for analgesia or to decrease hallucinations (concomitant use with ketamine); these situations are rare and should be assessed on an individual basis.
Preventive treatment of migraine. General considerationsAt least 25% of patients consulting due to migraine are thought to require preventive or prophylactic treatment. Selection of a preventive treatment involves analysis of various migraine-related parameters, such as attack frequency and severity, associated disability, and analgesic response. These parameters are then converted into treatment objectives to be evaluated: the reduction in the number of attacks per month, decrease in the use of analgesics, reduction in disease burden, analgesic response time (2–4&#¿;h after abortive drug administration), and percentage of response.38,39 Another important objective of preventive treatment is avoiding chronic transformation of EM. These recommendations for treatment onset, follow-up, optimisation, and discontinuation are applicable to both CM and EM.
Occurrence of 4 or more migraine attacks per month is a frequently considered requirement for onset of preventive treatment39,40; for several newer drugs, such as anti-CGRP monoclonal antibodies (CGRP mAb), this recommendation is included in the approval issued by the United States Food and Drug Administration and the European Medicines Agency. However, attack frequency alone is insufficient to establish preventive treatment, and other characteristics must be considered. A patient presenting 4–8 attacks per month may require only a specific analgesic, rather than daily preventive treatment, if attacks are well controlled with acute treatment.
Regardless of pain intensity and analgesic treatment response, preventive treatment is recommended for patients requiring analgesics ≥2&#¿;days per week due to the risk of analgesic misuse and the development of MOH or CM. Patients with prolonged aura or aura with hemiplegia, aphasia, or brainstem symptoms (impaired awareness, vertigo, gait instability, diplopia, etc) are also eligible for preventive treatment, as aura does not respond to abortive treatment. Finally, preventive treatment is also indicated in patients presenting epileptic seizures associated with migraine attacks (“migralepsy”).17,41–48
The duration of preventive treatment also varies. As an initial recommendation, once the patient has received a drug at an appropriate dose that reduces attack frequency and achieves therapeutic goals, this treatment should be continued, with a cumulative treatment effect of at least 3&#¿;months (typically 6&#¿;months in EM and 6–12&#¿;months in CM). However, many patients with CM will require periods of over 1 year before withdrawal of the drug. As a second recommendation, treatment duration should be guided by achievement of the therapeutic goals set.49
Other key aspects to consider when selecting a preventive treatment are the patient's comorbidities, which may support or contraindicate the use of a particular group of drugs. For instance, such psychiatric disorders as anxiety or depression are the most frequently observed in patients with migraine; therefore, we may opt for preventive treatment with such antidepressants as amitriptyline or venlafaxine, avoiding the need for combination therapy, where feasible. In more complex cases, we must combine the action mechanisms of different groups of drugs with coadjuvant effects.16,50,51
Studies on migraine prevention have evaluated the effectiveness, early safety, and treatment response within the exposure time, but have not established when treatment should be discontinued. Treatment discontinuation should be determined according to parameters evaluated during clinical follow-up: attack frequency, duration, and severity, and the treatment response achieved. Though we should ideally aim for patients to remain free of migraine attacks, this is not a realistic expectation. While some patients are so-called super-responders, this situation is not sustainable over time; as a result, our real goal must be to reduce attack frequency as much as possible, and to achieve a satisfactory response to symptomatic treatment.
Treatment duration after therapeutic objectives are achieved may differ between CM and EM (generally, 6&#¿;months for EM and 12&#¿;months for CM). However, the longer the duration of poorly controlled chronic migraine, the smaller the likelihood of achieving total discontinuation of preventive treatment. Furthermore, patients may present recurrence in high-frequency EM or CM, after a period of medication use or without treatment. Therefore, it is important to identify factors related to chronic transformation and comorbidities, and to educate patients about healthy lifestyle choices.46
Thus, the time of medication use is one of the parameters to be accounted for when evaluating preventive treatment duration; the question of when is the most appropriate time to withdraw treatment, especially in high-frequency EM and CM, continues to be debated. Therefore, where other preventive therapies have failed, the continued use of costly but efficacious treatments may be indicated for very long periods (years); as with treatment onset, treatment duration and discontinuation must be considered on an individual basis. Generally, discontinuation of preventive treatment is more straightforward in patients who can achieve low attack frequency in EM with a cumulative treatment effect of 6&#¿;months.52
The consensus process generated the following indications for starting preventive treatment: ≥4 attacks per month, attacks of ≥4&#¿;h' duration with medication use, unsatisfactory analgesic response, moderate–severe disability, attacks with brainstem symptoms, and hemiplegic migraine. The participants recommended the following treatment objectives: reduction in attack frequency (attacks/month) of 50%–75% for EM and 30%–50% for CM, and a reduction in attack duration and severity. The participants established a latency time of 2–3&#¿;months after treatment onset before positive response or treatment failure can be confirmed. Finally, they recommended that preventive treatment be tapered or suspended after 6–12&#¿;months of good migraine control for EM and after ≥12&#¿;months for CM. Nonetheless, assessment of therapeutic objectives during follow-up is the best strategy for establishing the duration of preventive treatment. There was consensus that if, following discontinuation of preventive treatment, the patient presents recurrence with similar or greater attack frequency, the discontinued treatment should be resumed and its duration increased, with a greater cumulative treatment effect than the first time it was used.
Patients with migraine present poor adherence to preventive treatment, and it is therefore important to minimise the number of concomitant medications. Monotherapy is the general recommendation par excellence; treatment should start with low doses to improve tolerance, with gradual increases until a therapeutic effect is achieved or treatment must be limited due to adverse reactions. A range of treatment schedules may be followed, with periodic increases until a therapeutic dose is reached.53,54
It is important to be familiar with several concepts in migraine prevention. Treatment failure is defined as failure to achieve treatment objectives after the patient has received a drug at the optimal dose for at least 2&#¿;months. Resistant migraine is defined as treatment failure of at least 3 molecules from different groups of drugs. Refractory migraine is defined as lack of response to any of the available pharmacological options; these patients must also meet the clinical criterion of presenting disabling headache with ≥8 attacks/month for the last 3&#¿;months. A frequent mistake at non-specialised consultations is the use of subtherapeutic doses and shorter treatment durations than those recommended for evaluating treatment response; this favours chronic transformation of headache and the development of MOH.38,10,55,56
Oral preventive treatments often present secondary or adverse effects, although these tend to be mild and do not necessitate withdrawal of the drug. Patients must be educated about the existence of these effects. Slow onset and gradual up-titration improve tolerability and treatment adherence. The risk of adverse reactions alone should not be considered a contraindication for treatment.57
The brains of patients with EM and CM are neurobiologically distinct, and each form must be understood on the basis of neurobiological changes, rather than the arbitrary distinction of the number of pain days. CM is more complex in terms of pain-related neural networks and analgesic and modulatory response; migraine attacks may occur as inappropriate responses to internal stimuli, in a process known as central sensitisation. Patients with CM report lower levels of treatment satisfaction than those with EM (4/10 vs 6/10). Not all of the available oral preventive treatments can be recommended for CM. On the other hand, EM and CM are similar in terms of scores on disability scales, disease burden, and limitations in accessing specialised care; patients with ≥8 attacks/month may be more similar to patients with CM in clinical and neurobiological terms. However, until this fact is accepted and reflected in the International Classification of Headache Disorders, third edition, migraine must be classified according to the current criteria.57–60
Clinical history is the most useful tool in the diagnostic and therapeutic assessment of patients with migraine. During history-taking, physicians should record the previous preventive treatments and the responses obtained, which will help establish whether any options considered to have failed were in fact not administered correctly, and facilitate decision-making about preventive treatment. A simple way of recording this is noting the drugs used and an abbreviation for the outcome (e.g., no response [NR], intolerance [IT], allergy [A*], good response [GR], partial response [PR], discontinuation [D&#¿;+&#¿;explanation]).
In patients requiring more structured management due to their particular situation or migraine characteristics, we may use follow-up tools such as headache diaries or migraine scales; scales are often needed to detect comorbidities in patients with CM. The evaluation and follow-up tools that were most recommended by participants in the consensus process were the clinical history interview, headache diaries, the Migraine Disability Scale, the Headache Impact Test (HIT-6), and pain evaluation with the Numeric Rating Scale or Visual Analogue Scale. Despite the availability of numerous tools for evaluating different aspects of migraine and patient characteristics, such as the Patient Global Impression scale, which is often used in studies of migraine, the objective of these tools is their ease of application in everyday practice, optimising time management and improving the selection of treatment options.61–64
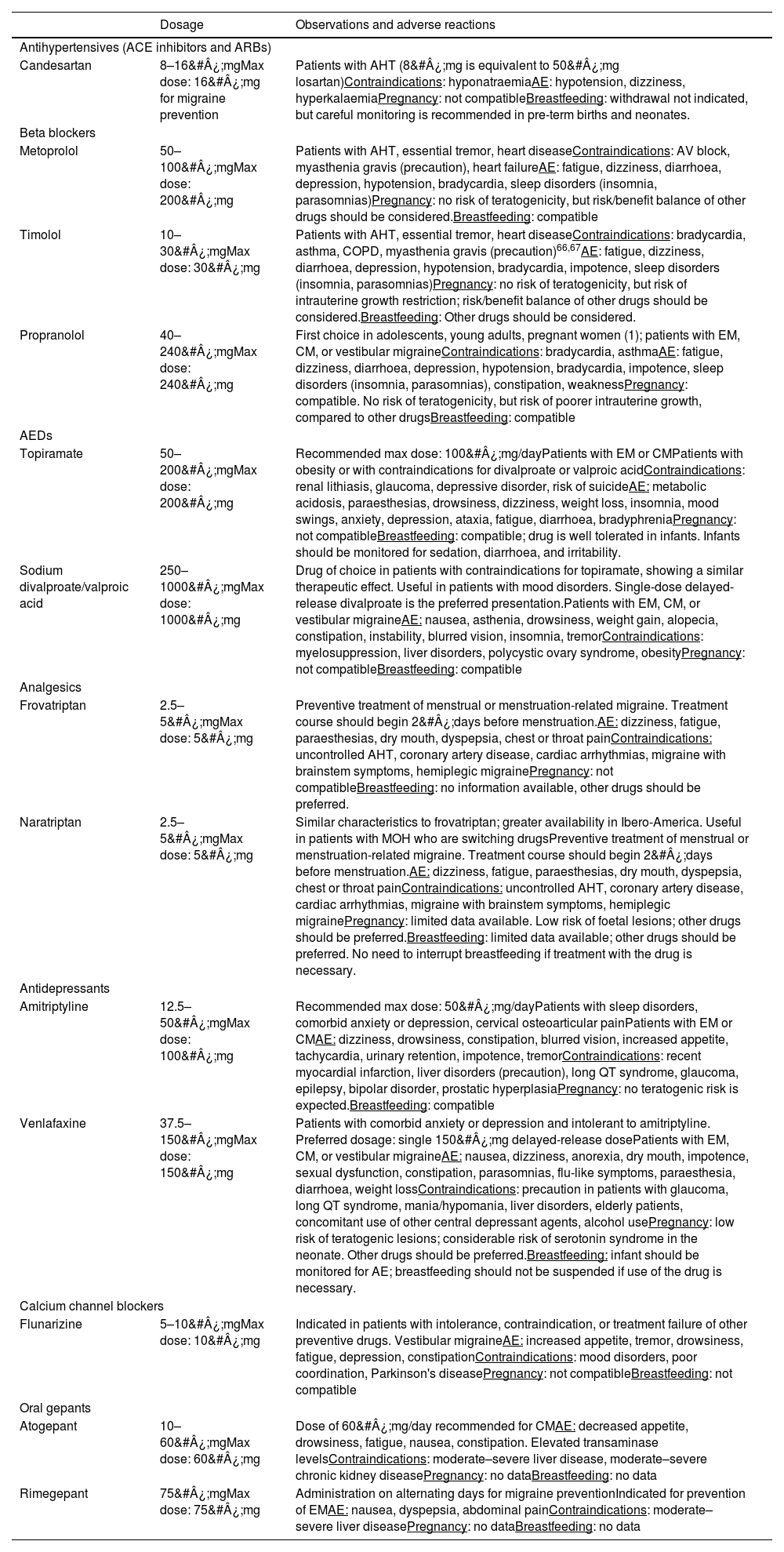
Oral preventive treatmentTwo studies, published in 2012 and 2021, respectively,42,65 reflect the evolution in the use of oral preventive drugs for migraine, which have enabled a simplification of the pharmaceutical options offered to patients. Drugs can be divided into 2 groups, those of probable and those of established effectiveness; in turn, each group includes antidepressants, antihypertensives, and antiepileptic drugs. Table 7 lists these drugs and their recommended doses; Table 8 lists the commonly used drugs evaluated in the consensus process. In addition to these recommendations, the heterogeneity of migraine means that certain special cases may require preventive treatments not described in this consensus statement, but which are supported by bibliographical evidence on migraine prophylaxis.
Frequently used oral preventive drugs.
| Dosage | Observations and adverse reactions | |
|---|---|---|
| Antihypertensives (ACE inhibitors and ARBs) | ||
| Candesartan | 8–16&#¿;mgMax dose: 16&#¿;mg for migraine prevention | Patients with AHT (8&#¿;mg is equivalent to 50&#¿;mg losartan)Contraindications: hyponatraemiaAE: hypotension, dizziness, hyperkalaemiaPregnancy: not compatibleBreastfeeding: withdrawal not indicated, but careful monitoring is recommended in pre-term births and neonates. |
| Beta blockers | ||
| Metoprolol | 50–100&#¿;mgMax dose: 200&#¿;mg | Patients with AHT, essential tremor, heart diseaseContraindications: AV block, myasthenia gravis (precaution), heart failureAE: fatigue, dizziness, diarrhoea, depression, hypotension, bradycardia, sleep disorders (insomnia, parasomnias)Pregnancy: no risk of teratogenicity, but risk/benefit balance of other drugs should be considered.Breastfeeding: compatible |
| Timolol | 10–30&#¿;mgMax dose: 30&#¿;mg | Patients with AHT, essential tremor, heart diseaseContraindications: bradycardia, asthma, COPD, myasthenia gravis (precaution)66,67AE: fatigue, dizziness, diarrhoea, depression, hypotension, bradycardia, impotence, sleep disorders (insomnia, parasomnias)Pregnancy: no risk of teratogenicity, but risk of intrauterine growth restriction; risk/benefit balance of other drugs should be considered.Breastfeeding: Other drugs should be considered. |
| Propranolol | 40–240&#¿;mgMax dose: 240&#¿;mg | First choice in adolescents, young adults, pregnant women (1); patients with EM, CM, or vestibular migraineContraindications: bradycardia, asthmaAE: fatigue, dizziness, diarrhoea, depression, hypotension, bradycardia, impotence, sleep disorders (insomnia, parasomnias), constipation, weaknessPregnancy: compatible. No risk of teratogenicity, but risk of poorer intrauterine growth, compared to other drugsBreastfeeding: compatible |
| AEDs | ||
| Topiramate | 50–200&#¿;mgMax dose: 200&#¿;mg | Recommended max dose: 100&#¿;mg/dayPatients with EM or CMPatients with obesity or with contraindications for divalproate or valproic acidContraindications: renal lithiasis, glaucoma, depressive disorder, risk of suicideAE: metabolic acidosis, paraesthesias, drowsiness, dizziness, weight loss, insomnia, mood swings, anxiety, depression, ataxia, fatigue, diarrhoea, bradyphreniaPregnancy: not compatibleBreastfeeding: compatible; drug is well tolerated in infants. Infants should be monitored for sedation, diarrhoea, and irritability. |
| Sodium divalproate/valproic acid | 250–1000&#¿;mgMax dose: 1000&#¿;mg | Drug of choice in patients with contraindications for topiramate, showing a similar therapeutic effect. Useful in patients with mood disorders. Single-dose delayed-release divalproate is the preferred presentation.Patients with EM, CM, or vestibular migraineAE: nausea, asthenia, drowsiness, weight gain, alopecia, constipation, instability, blurred vision, insomnia, tremorContraindications: myelosuppression, liver disorders, polycystic ovary syndrome, obesityPregnancy: not compatibleBreastfeeding: compatible |
| Analgesics | ||
| Frovatriptan | 2.5–5&#¿;mgMax dose: 5&#¿;mg | Preventive treatment of menstrual or menstruation-related migraine. Treatment course should begin 2&#¿;days before menstruation.AE: dizziness, fatigue, paraesthesias, dry mouth, dyspepsia, chest or throat painContraindications: uncontrolled AHT, coronary artery disease, cardiac arrhythmias, migraine with brainstem symptoms, hemiplegic migrainePregnancy: not compatibleBreastfeeding: no information available, other drugs should be preferred. |
| Naratriptan | 2.5–5&#¿;mgMax dose: 5&#¿;mg | Similar characteristics to frovatriptan; greater availability in Ibero-America. Useful in patients with MOH who are switching drugsPreventive treatment of menstrual or menstruation-related migraine. Treatment course should begin 2&#¿;days before menstruation.AE: dizziness, fatigue, paraesthesias, dry mouth, dyspepsia, chest or throat painContraindications: uncontrolled AHT, coronary artery disease, cardiac arrhythmias, migraine with brainstem symptoms, hemiplegic migrainePregnancy: limited data available. Low risk of foetal lesions; other drugs should be preferred.Breastfeeding: limited data available; other drugs should be preferred. No need to interrupt breastfeeding if treatment with the drug is necessary. |
| Antidepressants | ||
| Amitriptyline | 12.5–50&#¿;mgMax dose: 100&#¿;mg | Recommended max dose: 50&#¿;mg/dayPatients with sleep disorders, comorbid anxiety or depression, cervical osteoarticular painPatients with EM or CMAE: dizziness, drowsiness, constipation, blurred vision, increased appetite, tachycardia, urinary retention, impotence, tremorContraindications: recent myocardial infarction, liver disorders (precaution), long QT syndrome, glaucoma, epilepsy, bipolar disorder, prostatic hyperplasiaPregnancy: no teratogenic risk is expected.Breastfeeding: compatible |
| Venlafaxine | 37.5–150&#¿;mgMax dose: 150&#¿;mg | Patients with comorbid anxiety or depression and intolerant to amitriptyline. Preferred dosage: single 150&#¿;mg delayed-release dosePatients with EM, CM, or vestibular migraineAE: nausea, dizziness, anorexia, dry mouth, impotence, sexual dysfunction, constipation, parasomnias, flu-like symptoms, paraesthesia, diarrhoea, weight lossContraindications: precaution in patients with glaucoma, long QT syndrome, mania/hypomania, liver disorders, elderly patients, concomitant use of other central depressant agents, alcohol usePregnancy: low risk of teratogenic lesions; considerable risk of serotonin syndrome in the neonate. Other drugs should be preferred.Breastfeeding: infant should be monitored for AE; breastfeeding should not be suspended if use of the drug is necessary. |
| Calcium channel blockers | ||
| Flunarizine | 5–10&#¿;mgMax dose: 10&#¿;mg | Indicated in patients with intolerance, contraindication, or treatment failure of other preventive drugs. Vestibular migraineAE: increased appetite, tremor, drowsiness, fatigue, depression, constipationContraindications: mood disorders, poor coordination, Parkinson's diseasePregnancy: not compatibleBreastfeeding: not compatible |
| Oral gepants | ||
| Atogepant | 10–60&#¿;mgMax dose: 60&#¿;mg | Dose of 60&#¿;mg/day recommended for CMAE: decreased appetite, drowsiness, fatigue, nausea, constipation. Elevated transaminase levelsContraindications: moderate–severe liver disease, moderate–severe chronic kidney diseasePregnancy: no dataBreastfeeding: no data |
| Rimegepant | 75&#¿;mgMax dose: 75&#¿;mg | Administration on alternating days for migraine preventionIndicated for prevention of EMAE: nausea, dyspepsia, abdominal painContraindications: moderate–severe liver diseasePregnancy: no dataBreastfeeding: no data |
ACE: angiotensin-converting enzyme; AE: adverse events; AED: antiepileptic drug; AHT: arterial hypertension; ARB: angiotensin II receptor blocker; AV: atrioventricular; CM: chronic migraine; COPD: chronic obstructive pulmonary disease; EM: episodic migraine; MOH: medication overuse headache.
Additional sources of information: Epocrates®, MedLink®, UpToDate®, LactMed®, Drugs®, Vademecum®.
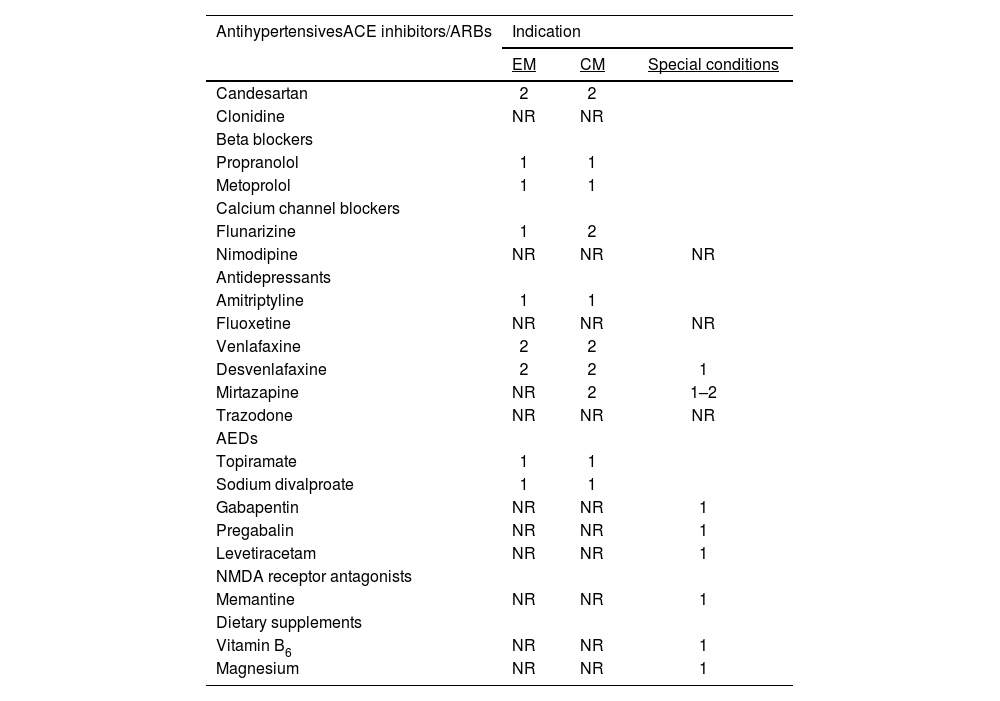
Consensus recommendations for the use of oral preventive drugs.
| AntihypertensivesACE inhibitors/ARBs | Indication | ||
|---|---|---|---|
| EM | CM | Special conditions | |
| Candesartan | 2 | 2 | |
| Clonidine | NR | NR | |
| Beta blockers | |||
| Propranolol | 1 | 1 | |
| Metoprolol | 1 | 1 | |
| Calcium channel blockers | |||
| Flunarizine | 1 | 2 | |
| Nimodipine | NR | NR | NR |
| Antidepressants | |||
| Amitriptyline | 1 | 1 | |
| Fluoxetine | NR | NR | NR |
| Venlafaxine | 2 | 2 | |
| Desvenlafaxine | 2 | 2 | 1 |
| Mirtazapine | NR | 2 | 1–2 |
| Trazodone | NR | NR | NR |
| AEDs | |||
| Topiramate | 1 | 1 | |
| Sodium divalproate | 1 | 1 | |
| Gabapentin | NR | NR | 1 |
| Pregabalin | NR | NR | 1 |
| Levetiracetam | NR | NR | 1 |
| NMDA receptor antagonists | |||
| Memantine | NR | NR | 1 |
| Dietary supplements | |||
| Vitamin B6 | NR | NR | 1 |
| Magnesium | NR | NR | 1 |
ACE: angiotensin-converting enzyme; AED: antiepileptic drug; ARB: angiotensin II receptor blocker; CM: chronic migraine; EM: episodic migraine; NMDA: N-methyl-D-aspartate.
Recommendations are classed as: 1: first line; 2: second line; NR: not recommended.
Special conditions: individual cases, intolerance to another drug in the same group. Data may be insufficient, and another drug should be preferred where possible.
The preventive treatments with greater quality of evidence and which are considered effective by consensus participants are listed in Table 8. Oral therapy continues to be valid and useful in migraine prevention, but presents poorer rates of treatment adherence when compared to certain injectable treatments; however, these drugs remain a valid option, as the biological components leading to chronic transformation are not the same in all patients. As a result of this, it is necessary to use different drug groups in the event of treatment failure, which may even occur in patients who have recently received specific drugs. The most frequent causes for withdrawal of oral preventive treatment (a frequent occurrence) are lack of treatment response, adverse reactions, complex treatment schedules, lack of patient education about the treatment, and the placebo effect. Recent studies with botulinum toxin type A (BTX-A) and CGRP mAb (particularly the latter) have included patients with greater degrees of complexity due to previous treatment failures, and have demonstrated the high rate of failure in migraine prevention. Approximately, 40%–50% of patients have experienced treatment failure of at least 2 oral drugs, and treatment failure is a possibility with all therapeutic options.68–70
Though it is not ideal, combined oral therapy for migraine prevention is frequently used in clinical practice, sometimes due to lack of treatment response, and on other occasions due to the need to manage other comorbidities. The use of 2 preventive drugs is most widely accepted in the latter situation. According to our consensus participants, the drug groups most frequently used in combination therapy are antiepileptic drugs and antidepressants. New data on the use of oral gepants in migraine prevention will probably enable the recommendation of these drugs in combined therapy, improving tolerability and response rates.71–73
In the last 5&#¿;years, few drugs have been evaluated and indicated for migraine prevention. Of these, only second-generation oral gepants have been included in the list of options to consider. Probably due to the positive results observed with first-generation gepants (olcegepant and telcagepant) in the acute treatment of migraine attacks (despite their early withdrawal from the market due to hepatotoxicity), it was possible to develop new CGRP antagonists. This, in addition to the good response to parenteral CGRP mAb, has drawn greater attention to this group of second-generation drugs, with oral atogepant and rimegepant being indicated for migraine prevention.
The AHS74 recently recommended that these molecules (as well as CGRP mAb) be considered for first-line preventive treatment, avoiding the trial-and-error use of non-specific preventive treatments. The main barrier to the use of these novel drugs is their cost, and greater scientific evidence is needed to establish their position as the first choice over other drugs. Other relevant preventive drugs addressed in recent studies are propranolol in CM, cinnarizine in migraine associated with vertigo, memantine, and levetiracetam.
Propranolol has been used for many years as a preventive treatment for EM, but is not considered superior to such other drugs as valproic acid, topiramate, or flunarizine.75–77 In the recent TOP-PRO study, which evaluated monotherapy with topiramate and with propranolol in the prevention of CM, the latter drug achieved a reduction of −7.3 monthly migraine days (MMD) (vs −5.3 for topiramate; P&#¿;=&#¿;.226). The difference between the 2 drugs was not statistically significant, and they may therefore be considered equally effective; patients in the study had a disease progression time of approximately 6&#¿;years, with 1–1.5&#¿;years of chronic migraine.78
Cinnarizine is a calcium channel blocker used for migraine prophylaxis in paediatric patients; some publications have suggested that it may be effective in the management of patients with migraine-associated vertigo. Given its similarities to flunarizine, both drugs have traditionally been considered for treating patients with vertigo.79 However, recent systematic reviews have not confirmed its therapeutic effect in patients with vestibular migraine, in whom valproic acid, propranolol, and venlafaxine do prevent migraine, whereas flunarizine and amitriptyline only reduce vertigo-related symptoms.80,81
One of the pain transmission pathways is regulated by glutamate, which can be inhibited by N-methyl-D-aspartate (NMDA) receptor activity. Memantine is a well-tolerated NMDA antagonist that has been evaluated in studies into the prevention of migraine by targeting this pathway, with results evaluated at 12 or 24&#¿;weeks. Observational studies of patients with EM have observed a treatment response, with a reduction of −5.3 to −9.1 MMD at doses of 10–20&#¿;mg/day. However, these results must be interpreted with caution due to the open-label designs of some of the studies, the small size of the active treatment groups (30–100 patients), with some patients presenting migraine frequencies lower than 6 MMD, and the concomitant use of other preventive therapies. Three systematic reviews evaluated the preventive effect of memantine in a total of 152 patients.82–84 It is not currently possible to recommend the regular use of memantine in migraine prevention, as there is a need for studies with greater statistical rigour and larger patient samples to robustly establish the drug's usefulness in these patients (currently, it may be an option in patients with intolerance to the rest of the available drugs).85
Levetiracetam is an antiepileptic drug with a different action mechanism to that of topiramate and valproic acid: it binds to the protein SV2A in the presynaptic membrane to regulate neuronal hyperexcitability. It is also reported to affect calcium channels, indirectly increasing GABAergic activity.86 The drug has been studied in open-label and controlled trials, with doses ranging from 500 to 2000&#¿;mg per day, with a preventive response observed in 40%–60% of patients, most of whom presented EM. The drug has been studied in patients with CM by Rapoport and Bigal87 and by Kashipazha et al.,88 with reductions of 6 and 6.7 MMD, respectively; levetiracetam was less effective in CM than in EM. As with studies into other drugs, small sample sizes and methodological quality constitute limitations.89,90 However, levetiracetam presents an interesting characteristic that must not be overlooked, its compatibility and safety during pregnancy and breastfeeding in women with epilepsy. This makes it a relevant option for consideration in patients requiring migraine preventive therapy during pregnancy and who cannot use such first-line treatments as propranolol and amitriptyline, whether due to intolerance or treatment failure.91–93
Finally, the action mechanism of second-generation gepants is reversible antagonism of the postsynaptic CGRP receptor. The first-generation oral gepants were developed before CGRP mAb, and were withdrawn from the market due to hepatotoxicity; therefore, studies of the second generation of drugs have focused both on effectiveness and on hepatotoxicity. Atogepant is currently approved for preventive treatment of EM and CM, while rimegepant is only approved for EM. In the ADVANCE study, in which patients reported 7 MMD and 9 monthly headache days at baseline, atogepant showed a preventive response with all doses evaluated (10&#¿;mg [−3.7], 30&#¿;mg [−3.9], and 60&#¿;mg [−4.2]), compared to placebo (−2.5), at 12&#¿;weeks of treatment.94 In the secondary data analysis, 50%–60% of patients exposed to the drug achieved a 50% reduction in migraine frequency at 12&#¿;weeks of treatment, compared to 29% in the placebo group; 70%–75% of patients were satisfied with the treatment at the doses evaluated, compared to 46% of patients in the placebo group.95 The PROGRESS study of CM treatment evaluated atogepant at doses of 30&#¿;mg every 12&#¿;h and 60&#¿;mg once daily in patients with 18 and 19 MMD, respectively. At 12&#¿;weeks, patients showed a reduction of 7.5 (30&#¿;mg) and 6.9 MMD (60&#¿;mg).96
Rimegepant is another oral CGRP antagonist; information on the drug is limited, compared to other gepants. It is approved for migraine prevention at a dose of 75&#¿;mg on alternating days. Its effectiveness and safety for preventive treatment have not been evaluated in patients with more than 18 attacks per month. The phase II/III trial by Croop et al.95 included patients with 4–18 MMD, 23% of whom were diagnosed with CM. At 12&#¿;weeks, the active treatment group presented a reduction of −3.6 MMD (vs 2.9 in the placebo group; P&#¿;=&#¿;.0017); a ≥50% reduction in moderate–severe migraine days was recorded in 49% of patients in the active treatment group and 41% of those in the placebo group.95 The open-label study of rimegepant included 1044 patients with ≥6 MMD in its analysis; the secondary endpoint was the decrease in MMD after 52&#¿;weeks of exposure; 40.6% of patients were enrolled, with a mean of 10.9 MMD (range, 2–14). The authors concluded that higher baseline migraine frequency was associated with longer delays in achieving reductions of ≥30% (median, 12&#¿;weeks) and ≥50% in MMD (median, 32&#¿;weeks). At the end of the 52&#¿;weeks, 78.6% of patients had achieved a ≥30% reduction, and 63.3% achieved a ≥50% reduction. The drug was dosed at 75&#¿;mg for acute treatment.97
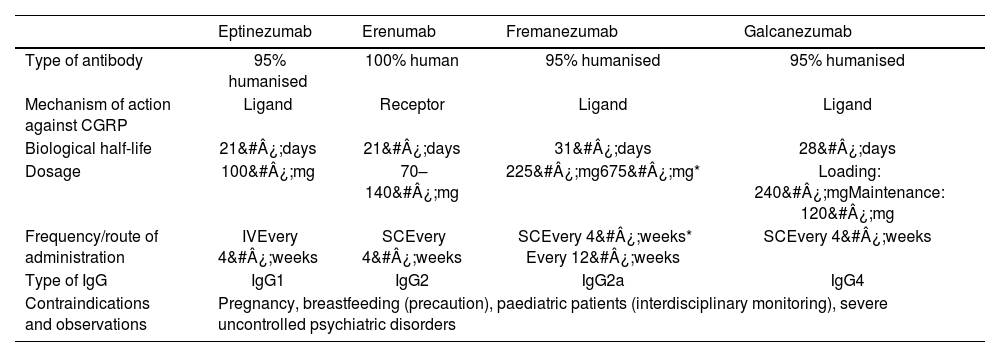
Monoclonal antibodiesNumerous publications, from phase II trials to studies of real-life data, have demonstrated the safety, tolerability, and efficacy of CGRP mAb (Table 9) in the preventive treatment of both EM and CM, even in patients with MOH.98 Currently, no particular molecule within this group of drugs is considered superior to any other, with comparative studies showing similar results in achieving a ≥50% reduction in MMD at 12&#¿;weeks. No study has compared these drugs against BTX-A, although data on the percentage of patients achieving a ≥30% reduction in MMD at 90&#¿;days are similar for both treatments.10,99 Relevant characteristics of these drugs include their ease of administration, low rate of adverse reactions, lack of drug interactions, shorter latency to treatment response, and greater likelihood of achieving a preventive effect in patients in whom multiple oral drugs (plus BTX-A) have failed.72 One disadvantage of CGRP mAb is their cost, in such a prevalent disease, with healthcare systems being responsible for the majority of expenditure; this is a limitation for early treatment onset. Recommendations have been issued to assess the appropriate time for withdrawal of the drugs.100 Another limitation is the fact that, although these drugs have been successful in patients with history of failure of other treatments, not all patients will achieve a clinical response, as chronic transformation of migraine is not always related to the CGRP pathway. Finally, although administration of this group of drugs should not be delayed in patients with indication for the treatment, it is essential to take care in selecting the patients most likely to benefit.101–103
Anti-CGRP monoclonal antibodies for migraine prevention.
| Eptinezumab | Erenumab | Fremanezumab | Galcanezumab | |
|---|---|---|---|---|
| Type of antibody | 95% humanised | 100% human | 95% humanised | 95% humanised |
| Mechanism of action against CGRP | Ligand | Receptor | Ligand | Ligand |
| Biological half-life | 21&#¿;days | 21&#¿;days | 31&#¿;days | 28&#¿;days |
| Dosage | 100&#¿;mg | 70–140&#¿;mg | 225&#¿;mg675&#¿;mg* | Loading: 240&#¿;mgMaintenance: 120&#¿;mg |
| Frequency/route of administration | IVEvery 4&#¿;weeks | SCEvery 4&#¿;weeks | SCEvery 4&#¿;weeks* Every 12&#¿;weeks | SCEvery 4&#¿;weeks |
| Type of IgG | IgG1 | IgG2 | IgG2a | IgG4 |
| Contraindications and observations | Pregnancy, breastfeeding (precaution), paediatric patients (interdisciplinary monitoring), severe uncontrolled psychiatric disorders | |||
CGRP: calcitonin gene-related peptide; IgG: immunoglobulin; IV: intravenous; SC: subcutaneous.
Consensus statements including recommendations about these molecules have required rapid updates due to the good outcomes observed. For reasons related to cost (not based on scientific evidence), the first consensus statements of the European Headache Federation104 and the AHS65 recommended that these drugs be reserved for cases of treatment failure of multiple oral treatments and/or BTX-A. Evidence from real-life studies led to the recommendation that these treatments be started earlier, and even considered as a first-line therapy.38
Numerous meta-analyses have confirmed the preventive effect of CGRP mAb in patients with EM and CM, and this recommendation is supported by the available data on their safety, tolerability, better results on assessment scales, and reduced analgesic use.105–108
Data remain scarce regarding the appropriate treatment duration and the timing of its withdrawal, and even the question of whether a decrease of only 50% in MMD should be considered a good clinical response: the other 50% of days on which headache is not controlled and the burden of disease remains high justify the use of combination therapy.109 It is rare for a patient with migraine who has required preventive treatment to achieve low headache frequency without medication, without first controlling factors related to chronic transformation or facilitation of migraine.110 The preventive effect is sustained over time with prolonged use of CGRP mAb,1,111 although approximately 75% of patients present relapses to higher migraine frequencies after withdrawal of the drugs.112
Similar treatment duration and objectives are applied in mAb therapy as in other preventive treatments, including a decrease in MMD. In EM, a ≥50% decrease in MMD is considered a good treatment response, whereas in CM, we seek to achieve a ≥30% reduction. Some studies, such as that published by Schoenen et al.,113 have described predictors of response to CGRP mAb therapy. Positive factors include low frequency of attacks, young age, positive analgesic response to triptans, attacks associated with autonomic symptoms, unilateral headache, and elevated salivary CGRP levels prior to treatment onset. Factors associated with poor response include CM, MOH, depression, failure of multiple previous treatments, and allodynia between attacks. Signs suggestive of central sensitisation are associated with a favourable treatment response.114
In some patients, longer periods of treatment and evaluation may be needed before a preventive response is observed. This is particularly true in CM, in which a clinically significant response may not be apparent for up to 6&#¿;months. This has been attributed to the need to modulate the central sensitisation process, a phenomenon that is also observed in EM (a late clinical response is observed in up to 36% of these patients).115,116 These drugs have a greater effect on patients with higher disease burden and higher numbers of previous treatment failures.117
In patients with continuous CM with no pain-free days, any reduction in the frequency or severity of migraine attacks will have a direct impact on their quality of life, and this is the first treatment to achieve a positive effect. Therefore, CGRP mAb may be considered as a first-line treatment in this patient group.10
Experience with the use of this medication has resolved academic questions, such as its combined use with other oral or parenteral drugs, with the concomitant use of BTX-A and CGRP mAb initially having been ruled out. This combination is currently considered to be potentially useful in patients with CM65; switching to a second mAb is a possibility in patients in whom a first mAb failed.118 This practice is mainly based on expert recommendations, which reflects once more the need to consider patients with migraine on an individual basis.119
Some peculiarities have been identified in the response to the different mAb available, with small differences in such secondary effects as constipation, which is more frequent with erenumab.72 Eptinezumab achieves the smallest decrease in MMD in EM99; within the group of ligand blockers, fremanezumab is the drug with the greatest preventive effectiveness in the first 6&#¿;weeks; of all mAb, erenumab dosed at 140&#¿;mg achieves the best preventive control between weeks 8 and 12.120 Despite these differences, however, these drugs present similar clinical behaviour, and their use in some countries will depend on their availability. The reductions in MMD are not much greater than those observed with BTX-A,99,121 although no comparative studies have been conducted, and they are efficacious in patients unresponsive to BTX-A.
Our consensus participants recommended all 4 CGRP mAb for the preventive treatment of EM and CM, with no preference for any particular molecule. In the light of the response to oral and parenteral preventive treatments in EM and CM, participants considered that mAb may be used in combination with other oral drugs or BTX-A, or that an oral drug may be added to mAb therapy, according to the preventive treatment plan established. A first follow-up assessment is recommended at 3&#¿;months after treatment onset to establish the response to mAb therapy and to perform any adjustments. The recommended minimum treatment duration for mAb is similar to that of other preventive drugs: 3–6&#¿;months for EM and 6–12&#¿;months for CM. Many patients, especially those with CM, may require treatment for much longer periods.
Botulinum toxin type AAn extensive body of research has analysed the effectiveness of BTX-A in migraine prevention over the last 30&#¿;years, although its action mechanism in migraine is not fully understood. Early studies described the blockade of acetylcholine release and a subsequent decrease in pericranial muscle contraction122; however, this mechanism would not explain its effectiveness in migraine. Peripheral molecular activity involving presynaptic C and Aδ fibres is currently the most widely accepted theory; following subcutaneous injection of the toxin, the light chain binds irreversibly to the SNAP-25 protein (a component of the SNARE complex, involved in the formation of the vesicular transport complex) in sensory neurons, preventing the transport and exocytosis into the synaptic cleft of such neuropeptides as CGRP, PACAP, substance P, serotonin, glutamate, GABA, norepinephrine, and dopamine, among others.123 This leads to blockade of pain transmission.124,125 Sites of action have been described in other transmembrane receptors in the trigeminal system, such as TRPV1, associated with thermal and inflammatory nociceptive information, which is involved in hyperalgesia processes and is most abundantly expressed in C fibres126,127; TRPA1, located in Aδ and C fibres,128 which is involved in nociceptive and neuropathic pain processes129; and the P2X3 receptor, which participates in hyperalgesia in the acute and chronic phases of pain through interaction with ATP and glutamate.130,131 Therefore, BTX-A reduces the frequency and severity of migraine attacks through complex interactions in the peripheral nervous system, involving blockade of neuropeptide and neurotransmitter release in the trigeminal system. The resulting modulation of pain transmission decreases the hyperactivation of the processes causing central sensitisation.
The PREEMPT-1132 and PREEMPT-2133 trials are considered the most influential studies in the consolidation of this preventive treatment. These studies included 1384 patients with CM, who were randomly assigned to receive active treatment with subcutaneous BTX-A dosed at 155–195&#¿;units every 4&#¿;weeks, or placebo treatment. At 24&#¿;weeks of follow-up, the active treatment group presented a decrease in attack frequency of −8.4 (PREEMPT-1) and −9 headache days (PREEMPT-2), compared to −6.6 and −6.7 headache days in the placebo group. The studies demonstrated an improvement in disability and disease burden according to the different scales used, and treatment was safe and well tolerated, even after two 12-week&#¿;cycles of exposure.134 The COMPEL study,135 an open-label extension study following up patients over 108&#¿;weeks of BTX-A treatment, analysed 715 patients with CM (intention to treat analysis), observing a reduction of −6.5&#¿;days in the frequency of moderate–severe headache at week 24, −8.1&#¿;days at week 60, and −9.5&#¿;days at week 108. Furthermore, at week 108, BTX-A achieved a reduction of −10.7 headache days, with a mean baseline frequency of 22 headache days and 18 moderate–severe headache days, and presented better results in the secondary outcomes analysed, demonstrating its usefulness in the preventive treatment of CM. Another noteworthy study is the REPOSE study,136 with 2&#¿;years' follow-up of patients with CM under treatment by their usual physician. The authors confirmed a reduction in the number of attacks per month, as well as improved scores on quality of life scales. Some deviations were observed with respect to the PREEMPT treatment protocol, with dosing intervals >13&#¿;weeks being observed at least once in 79.1% of patients and intervals >16 in 46%; the most frequently used dose was 155&#¿;units distributed across 31 injection sites.
Patients with chronic CM may present long latency in achieving treatment objectives; some studies in the literature recommend that BTX-A treatment should only be considered to have failed if objectives are not met after 2&#¿;cycles of treatment.137 By definition, a cycle of BTX-A in the PREEMPT protocol corresponds to 12&#¿;weeks' exposure to the drug; thus, 2&#¿;cycles amount to 24&#¿;weeks of exposure, before treatment failure can be established. However, allowing a patient to remain in pain for a period of 6&#¿;months pending evidence of treatment response, at a time when other parenteral options with shorter latencies are available, may not be the best decision. We must evaluate 2 situations. On the one hand, in patients presenting no response, we might opt for an early switch to another treatment; on the other, BTX-A administration should be continued in patients showing a partial response, progressively increasing the cumulative treatment effect.
Participants in our consensus survey recommended BTX-A therapy for all eligible patients, with a minimum of 3 administrations (1 cycle) before the treatment can be considered to have failed, at doses of 195&#¿;units per administration; they recommended following the PREEMPT protocol. Regarding this point, 43% of respondents stated that they sometimes departed from the PREEMPT protocol, a frequent approach within the process of tailoring treatment in order to improve therapeutic response; clinicians reported appropriate follow-up of the effect of the modifications applied.138,139 Furthermore, 86% of respondents considered that patients with high-frequency EM and failure of oral treatments or CGRP mAb may be eligible to receive a cycle of BTX-A as a therapeutic test. Finally, 74% of participants did not believe that there was a clinically significant difference in preventive effect between CGRP mAb and BTX-A when evaluated at 90&#¿;days.
It is difficult to establish whether one therapy is superior to the other when comparing BTX-A and CGRP mAb. The 2 treatments present significant pharmacological and pharmacokinetic differences, such as the greater ease of application, lower rate of treatment withdrawal, and faster onset of treatment response with CGRP mAb,140 which may be important and necessary characteristics for some patients. On the other hand, BTX-A is well tolerated, and the most frequent secondary effect (with the exclusion of dispersion effects or inappropriate administration technique) is neck pain, which lasts several days but is nearly always self-limited.141 Given the heterogeneity of migraine pathophysiology and the idiosyncrasies of treatment response, evaluation of therapeutic goals at 90&#¿;days reveals no significant differences in indirect comparisons between CGRP mAb and BTX-A in terms of reduction in MMD, ≥50% reduction in MMD, or HIT-6 scores. CGRP mAb are superior in terms of reducing the number of analgesics used and the associated secondary effects.102,142,143
Lanteri-Minet et al.144 published a meta-analysis of studies of real-world data published in the last 10&#¿;years, concluding that the treatment effect of BTX-A in real-life patients replicated the results of the PREEMPT and COMPEL studies, supporting the drug's effectiveness, importance, and validity in the treatment of CM.
No biochemical marker exists that has shown BTX-A to be superior to CGRP mAb, although associations have been identified between certain clinical conditions and a better preventive response, such as unilateral pain, ocular pain during the attack, presentation with implosive pain, severe allodynia, pericranial pain points, and response to triptans.143 Some of these predictors are also associated with good response to CGRP mAb.
Dual therapy with CGRP monoclonal antibodies and botulinum toxin type AAs patients refractory to treatment with BTX-A showed a preventive response to CGRP mAb, the 2 options were considered to be consecutive treatments. Although these treatments were considered mutually exclusive in early consensus statements, a group of patients with complex management, who did not achieve the desired response, showed only a partial response on an individual basis. As BTX-A acts on the presynaptic membrane, whereas CGRP mAb act postsynaptically, and because the preventive effect in these patients was achieved by targeting the CGRP pathway, some patients presenting a partial response to BTX-A and CGRP mAb, after a lack of response to previous treatments, would benefit from combination therapy due to complementary or synergistic pharmacological activity. Due to the pharmacological and pharmacokinetic characteristics of these 2 treatments, this combination is considered safe.142,145–147
While this recommendation has been issued in several consensus statements, insufficient evidence has been published on this matter. A meta-analysis by Scuteri et al.148 analysed the safety and effectiveness of this combination in 393 patients from 5 studies; 58% achieved the treatment goal of a ≥50% reduction in MMD. In a study of real-life data by Mechtler et al.,149 evaluating 148 patients exposed to CGRP mAb (erenumab in 56.7%, galcanezumab in 0.7%, and fremanezumab in 42.6%) and BTX-A (120–200&#¿;units), a mean reduction of −3.8 to −4.6 MMD was observed in the first 6–12&#¿;months of treatment; after 12&#¿;months, 30% of patients achieved a ≥50% reduction in MMD. Similar results were reported in the study by Blumenfeld et al.,150 in which 257 patients received CGRP mAb (erenumab in 77.8%, galcanezumab in 16.3%, and fremanezumab in 5.8%) and BTX-A (115–200&#¿;units), showing a reduction of 3–4 MMD at 6–12&#¿;months of treatment.
Erenumab is the most frequently prescribed mAb in these studies.151,152 We may consider the hypothesis that blockade of CGRP release by BTX-A and blockade of the CGRP receptor is the therapeutic pathway of choice in these difficult-to-manage cases, although the most likely reason is that it was the first CGRP mAb to be commercialised. However, insufficient data are available to confirm this, and the selection of a specific CGRP mAb is often determined by availability and coverage by the healthcare system.153
In conclusion, in patients with CM who present a partial response to BTX-A and CGRP mAb but do not achieve MMD targets, and in whom these are the preventive options that have shown the best treatment response, it is acceptable to administer combination therapy for a period not shorter than 12&#¿;months.
Refractory chronic migraineRefractory CM is defined as CM showing no preventive response to the recommended pharmacological treatment, administered with adequate doses and durations, including dual therapy with BTX-A plus CGRP mAb. Clinically, these patients present significant disease burden and disability.154–157
In this patient group, non-pharmacological alternatives should be considered. The relationship between diet and migraine has not been well studied, and the preventive response observed may be associated with weight loss, a reduction in allergens, increased intake of (omega) fatty acids, reduced intake of oestrogen-rich foods, or better glycaemic control, among other possible mechanisms.158 Similarly, increased intake or introduction of certain foods (cacao, cinnamon, turmeric, or magnesium, among other foods and supplements with a possible preventive effect) may have anti-inflammatory or immune regulatory effects.159,160 Comorbidity between migraine and such gastrointestinal disorders as irritable bowel syndrome has also been described, as well as gastroparesis during migraine attacks, in a relationship described as the gut–brain axis161; these phenomena involve neurotransmitters described in migraine pathophysiology, such as β-CGRP. Some authors have described non-pharmacological diet-based treatments159; among these, Bongiovanni et al.162 reported positive results with the ketogenic diet in patients with refractory CM, with a reduction in migraine attack duration from a median of 24&#¿;h/day at baseline to 5.5&#¿;h/day at the end of the study period. Attack frequency decreased from 30&#¿;days/month to 7.5&#¿;days/month. According to recent recommendations on the indication of ketogenic diet in patients with migraine, specific supplements should be prescribed if this option is selected (for instance in patients with refractory migraine).163
Another treatment option studied in patients with refractory CM is lidocaine infusion. Though its action mechanism is unknown, in theory it blocks voltage-dependent sodium channels and GABA and NMDA receptors; interaction with second messengers is the pathway proposed to explain the sustained effect of the treatment.164 A retrospective study published in 2022 by Schwenk et al.165 analysed the response to in-hospital administration of this treatment, with 43% of patients scoring 1 on the Numeric Rating Scale at 1 month after infusion. Interestingly, response to lidocaine seems not to be associated with levels of the drug in the blood.166 The limitations of lidocaine treatment include the need for hospitalisation, which may last up to 15&#¿;days before a clinical response is achieved.164
Another drug used in patients with chronic refractory pain is ketamine, which has been studied in patients with refractory CM. In a series of 6 cases published in 2016 by Lauritsen et al.,167 the mean time before a sustained reduction in pain (lasting 8&#¿;h) to 3/10 on the NRS was 3–5&#¿;days. In a study published by Schwenk et al.168 in 2021, 6 patients receiving ketamine infusion for 5&#¿;days presented a decrease from 7.5&#¿;±&#¿;1.4 to 3.7&#¿;±&#¿;2.3 on the NRS at the end of treatment. All patients presented treatment-related adverse effects, and the duration of the treatment effect was short; at an assessment 6&#¿;weeks after treatment, pain had returned to baseline values. Other studies suggest that the duration of the effect may be related to the duration of the infusion; in patients with refractory chronic pain and prolonged infusions of up to 14&#¿;days, a treatment effect lasting up to 3&#¿;months was observed.169 A descriptive study published in 2023 by Yuan et al.170 included 169 patients with refractory CM (67.5% presenting chronic daily headache) who were administered intranasal ketamine for analgesic treatment; 49.1% of patients considered the therapy to have been very effective, with 39.6% considering it partially effective.
Invasive or non-invasive nerve stimulation is restricted to CM refractory to pharmacological treatments, and is indicated more as a rescue measure in patients with elevated disease burden, as it is not feasible to recommend this treatment in other patient groups based on the available evidence. Of the devices studied, the gammaCore vagus nerve stimulator, which was evaluated in the study with the best methodological design, failed to achieve its primary objective; the remaining studies present biases due to their open-label designs, or use target populations other than patients with CM. No evidence is currently available to support the use of invasive stimulation techniques in patients with refractory migraine.2,171,172
This particular patient group will require interdisciplinary management, seeking to reduce their significant disease burden.
Closing remarksDespite the published information on the new preventive drugs for migraine, we cannot rule out the demonstrated therapeutic effect of the traditional oral and parenteral treatment options, which have been used for years. The heterogeneity of migraine transformation is responsible for the incomplete response to or the failure of both the novel and the traditional treatments. Without a doubt, treatment must be tailored and must always take into account patient characteristics, migraine phenotype, pharmacological contraindications, the characteristics of specific populations, and secondary effects. In future, treatment selection will probably be based on artificial intelligence models and/or biomarkers enabling prediction of the response to treatment with current or novel molecules. Currently, neurologists are and will remain the party responsible for selecting the treatment considered most likely to achieve a preventive response in each case, optimising the dose and exposure time, avoiding delays in treatment selection and switching, and seeking to achieve the treatment objectives established.
FundingThis project received financial support from Teva for its logistical development. Teva had no involvement in the design, data collection, analysis, or conclusions of the study, nor in decisions related to its publication.
Informed consentNot applicable for this type of publication.
Ethical responsibilities and protection of humans and animalsThe authors declare that no human or animal experiments were performed for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
The authors thank the following neurologists for responding to the consensus surveys: Abouch V Krymchantowski, Alcantara Ramos De Assis César, Alejandro Marfil Rivera, Alex Espinoza Giacomozzi, Álvaro Vidal Santoro, Ana M. Robles Goris, Analuiza S, Tenório Luna Sarmento, Anibal Elías Molinas Ayala, Bernardo Dror Felsenfeld Junior, Bernardo Uribe, Bibiana Saravia, Caio Vinicius De Meira Grava Simioni, Carlo Piero Lastarria, Carlos Alberto, Carlos Perdomo Rivera, Carlos Tschiedel Farias, Carlos Vargas Blas, Cecilia Odette Cardenas Bedecarratz, Célia Aparecida De Paula Roesler, Claudia Baptista Tavares, Cristian Sedano, Cristina Pérez, Doctorovich Eduardo Daniel, Eduardo De Almeida Guimarães Nogueira, Eduardo Odir Ribeiro Leal, Elder Machado Sarmento, Eliana Meire Melhado, Eliseo Barral, Elza Magalhaes Silva, Ernesto Bancalari, Federico Buonanotte, Felipe Reinaldo Deus Ramos Santos, Fidel Ernesto Sobrino Mejía, Gabriel Taricani Kubota, Gustavo Fischbein, Héctor Elías Cruz Segura, Héctor Noe Bazaldua Avila, Ildefonso Rodríguez Leyva, Joao Jose Carvalh, Joe Muñoz, Jorge Alberto Giglio, Jorge Erick Sinche Flores, Jorge Tacconi, Jose Alex Cabrejo Bravo, Jose David Martínez, José Jesús De Paz, José Miguel Láinez, Julio Pascual Gómez, Karol Vargas Hurtado, Leandro Cortoni Calia, Leonardo De Sousa Bernardes, Leonardo Sebastián Ayala, Lucas Bonamico, Lucio Óscar Gonzales Cuchallo, Luis Roberto Partida Medina, Luis Sanin, Luiz Paulo Queiroz, Manuel Gudiño Castelazo, Marcelo Mariano Da Silva, Marcio Nattan Portes Souza, Marco Antonio Martínez Gurrola, Marco Antonio Morais Pinto Ribeiro Serodio, Marco Lisicki, María Elena Novoa Mosquera, María Eugenia Tejada, María Kari A Velez Jiménez, María Loreto Cid Jeffs, María Teresa Reyes Álvarez, Mariana Lujan Rosas, Mario Fernando Prieto Peres, Marta Liliana Ramos Romero, Mauro Eduardo Jurno, Michel Volcy Gomez, Miguel Osorno Guerra, Murilo Rubens Schaefer, Nelson Barrientos, Norma Regina, Pereira Fleming, Olivier, Marina Alejandra, Óscar Pradilla, Osvaldo Carlos Bruera, Oswaldo Couto Junior, Pablo Irimia, Pablo Schubaroff, Patricia Lorena Pardo DÍaz, Paula Cavanzo Henao, Pedro André Kowacs, Raúl Federico Pelli Noble, Raul Juliet Pérez, Renata Lysia Soares Lima Farneze, Renato Arruda, Reydmar Lopez González, Samuel Díaz Insa, Sebastian Gabriel Villate, Sergio Francisco Ramírez Garcia, Silvina Mariel Miranda, Stephania Bohórquez Valderrama, Viviana Rocchi, and Yessika Milena Rojas Villegas.