This study aims to describe the clinical features, hospital management, and outcomes of patients with interstitial lung disease (ILD) and acute respiratory failure (ARF) admitted to the respiratory intermediate care units (RICUs).
Material and methodsAn observational study was conducted on ILD patients admitted to the RICU between June 1, 2021, and May 30, 2024. Main variables analysed included demographics, non-invasive respiratory support (NIRS) types, ILD-related variables, lung transplant outcomes, and survival rates.
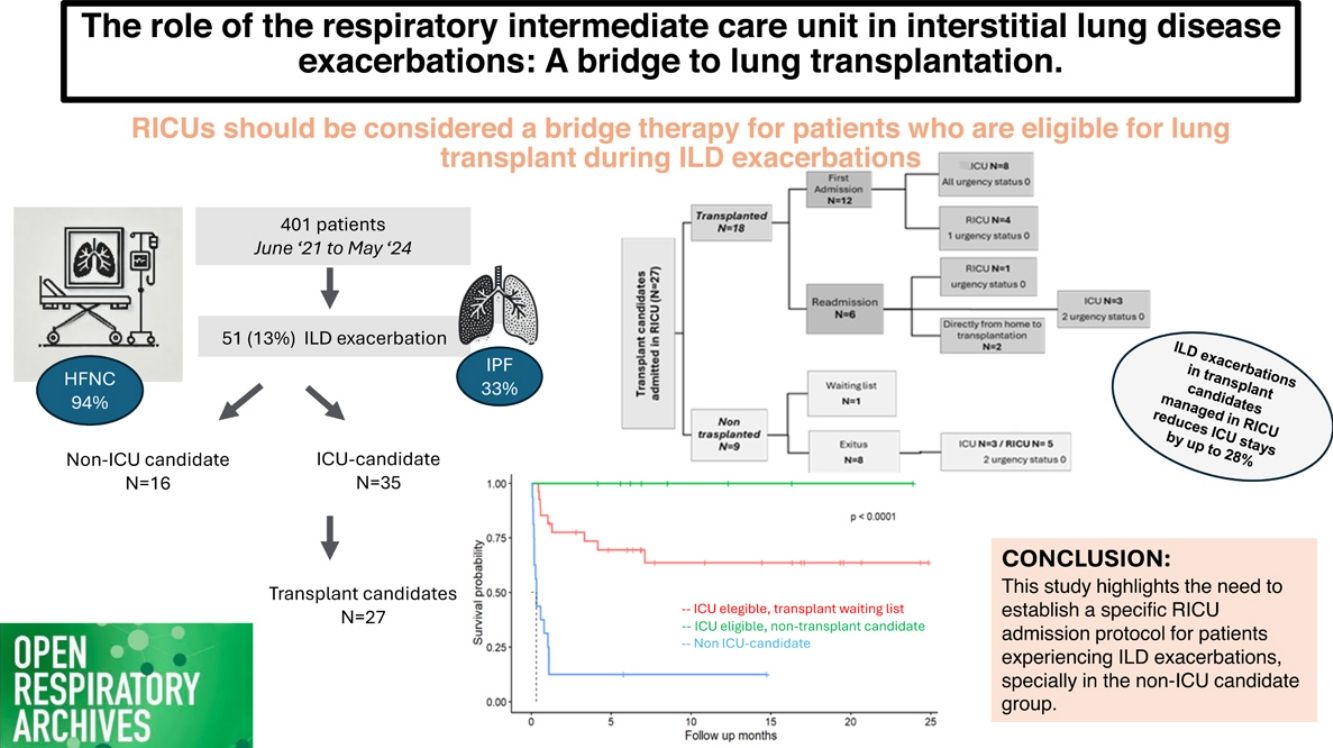
ResultsOf the 401 patients admitted, 51 (13%) had ILD, of whom 34 (67%) were male. Idiopathic pulmonary fibrosis (IPF) was the most common condition (33%), and high-flow oxygen therapy (HFOT) was the primary treatment (94%). Infection (29%) and disease progression (36%) were the main hospitalization causes. Of the 27 patients (53%) evaluated for transplantation, 18 (36%) underwent a lung transplant, with 5 (28%) directly transplanted from the RICU and 55% (N=15) were added to the emergency-driven list. Overall survival rates for ICU-eligible patients were 89% at one month, 77% at six months, and 72% at one year, while non-ICU eligible had lower survival probabilities of 31%, 13%, and 13%, respectively.
ConclusionsThe RICU should be considered a bridge therapy for patients who are ICU candidates and eligible for lung transplant during ILD exacerbations, potentially reducing ICU admissions. However, patients who are not ICU candidates face high short- and medium-term mortality risks. The significance of this study highlights the need to establish a specific RICU admission protocol for patients experiencing ILD exacerbations.
Describir las características clínicas, el manejo hospitalario y el desenlace de los pacientes con enfermedad pulmonar intersticial difusa ingresados por insuficiencia respiratoria aguda en la unidad de cuidados intermedios respiratorios (UCIR).
Material y métodosSe llevó a cabo un estudio observacional de una cohorte de pacientes con EPID ingresados en la UCIR entre el 1 de junio de 2021 y el 30 de mayo de 2024. Las principales variables analizadas fueron demográficas, tipo de soporte respiratorio no invasivo, relacionadas con la EPID, el trasplante y la supervivencia.
ResultadosDe los 401 pacientes admitidos en UCIR al año, 51 (13%) tenían diagnóstico de EPID, siendo un 67% varones. La patología más frecuente fue la FPI (33%), y el SRNI más empleado fue la oxigenoterapia de alto flujo (OAF) en un 94%. Las causas más frecuentes de ingreso fueron infección (29%) y progresión de enfermedad (36%). De los 27 pacientes evaluados para trasplante, finalmente se trasplantaron 18 (36%), 5 (28%) directamente desde UCIR, y el 55% (n=15) requirieron priorización a nivel nacional. La supervivencia global estimada de los pacientes candidatos a UCI al mes fue del 89%, a los 6 meses del 77% y al año del 72%, mientras que los pacientes no candidatos a UCI tenían menores probabilidad de supervivencia, siendo del 31%, del 13% y del 13%, respectivamente.
ConclusionesLas UCIR deben ser consideradas como puente al trasplante en pacientes candidatos a trasplante durante las exacerbaciones de EPID, para reducir ingresos en la UCI. Sin embargo, los pacientes con techo terapéutico UCIR enfrentan una mortalidad a medio y a corto plazo elevada. El presente estudio sirve como base para enfatizar la necesidad de crear protocolos específicos de ingreso en la UCIR en pacientes con exacerbación de EPID.
Interstitial lung diseases (ILDs) constitute a heterogeneous group of disorders affecting the interstitial space, leading to inflammation and/or fibrosis and a progressive decline in lung function. ILDs are characterized by high morbidity and mortality rates.1 Although treatment for ILDs has evolved significantly in recent decades, lung transplantation remains the only therapy at advanced stages with a meaningful impact on survival.2 Nevertheless, the optimal management of ILD patients remains challenging, especially during acute exacerbations. In-hospital mortality from an acute ILD exacerbation can reach 50%, and up to 90% if invasive ventilatory support is required.3,4 For this reason, mechanical ventilation and ECMO (extracorporeal membrane oxygenation) are typically reserved for patients who are candidates for lung transplantation.
Respiratory intermediate care units (RICUs) are defined as areas dedicated to the monitoring and care of patients with acute respiratory failure who require non-invasive respiratory support (NIRS), such as non-invasive mechanical ventilation (NIMV) and/or high-flow oxygen therapy (HFOT) as part of their treatment.5 These units admit patients who do not require or do not benefit from admission to an intensive care unit (ICU), but due to their clinical complexity, would not receive adequate care in a standard hospital ward.6 RICUs enable continuous monitoring and specialized treatments, easing ICU burdens, optimizing resources, and improving clinical outcomes by lowering mortality and costs.6,7
The benefits of these units were particularly evident during the COVID-19 pandemic.8,9 Additionally, these units have proven valuable for managing all pathologies causing severe acute respiratory failure, including ILDs. RICUs offer hope for ILD patients, especially with the use of HFOT. Studies suggest that NIRS may effectively manage acute ILD exacerbations, significantly improving oxygenation and reducing the need for mechanical intubation.10,11
The median survival for patients with acute exacerbations of idiopathic pulmonary fibrosis (IPF) is only 3–4 months, with 46% of deaths preceded by an exacerbation.4 Consequently, many of these patients are often considered unsuitable for invasive measures unless they are eligible for lung transplantation.
Given that our centre has both RICU and lung transplant unit, we aim to describe and analyse the impact of RICU admission on patients with ILD, including both those eligible for lung transplantation and those who are not. Through this research, we seek to provide insight into the benefits and challenges associated with this therapeutic approach, contributing to the development of specific protocols to improve medical care for this patient.
MethodsStudy designAn observational study was conducted on a retrospective cohort of patients with interstitial lung disease (ILD) admitted to the respiratory intermediate care unit (RICU) of tertiary care university hospital between June 1, 2021, and May 30, 2024.
Characteristics of the unit and study populationThe study included all patients diagnosed with ILD admitted to RICU with acute respiratory failure. The RICU has 6 beds during the winter, reduced to 5 in summer. Our hospital has been a transplant centre since 1991, performing over 1000 transplants.
Admission criteria for the respiratory intermediate care unit (RICU)ICU admission eligibility includes candidates for lung transplantation or intubation and patients with a RICU therapeutic ceiling. An admission protocol identifies patients likely to benefit from intermediate care, based on:
- •
Age and frailty: For patients over 70, the Frailty Index between 1 and 6 is favourable with a Charlson Index below 4 and Barthel Score below 65.
- •
Disease prognosis and reversibility: The recovery potential and prognosis of the underlying condition. An APACHE II score below 20 is preferred, as multi-organ failure complicates outcomes.
- •
Respiratory support and oxygenation status: Patients should ideally tolerate prior non-invasive respiratory support (NIRS); poor tolerance suggests a challenging prognosis. PaO2/FiO2 ratio over 100 or SpO2/FiO2 ratio above 148 correlates with better prognosis.
Admission decisions are individualized, with these criteria as indicators rather than strict requirements.
Data collectionPatient data was obtained through electronic medical records, ensuring confidentiality in accordance with the Ethics Committee. The Research Ethics Committee of the Hospital approved its realization as it complies with all the ethical requirements.
VariablesMain variables were demographics, comorbidities (hypertension, dyslipidaemia, smoking), and those related to ILD. The primary types of ILD were divided in three main groups:
- •
Idiopathic pulmonary fibrosis (IPF)
- •
Non-IPF pulmonary fibrosis: interstitial pneumonia with autoimmune features (IPAF), ILD secondary to connective tissue disease, fibrotic nonspecific interstitial pneumonia (NSIP), unclassifiable fibrosing ILD, fibrotic hypersensitivity pneumonitis (HP), post-adult respiratory distress (including post-COVID fibrosis), and interstitial involvement with metastatic calcification.
- •
Others: Cryptogenic organizing pneumonia, Langerhans cell histiocytosis, drug-induced pneumonitis, asbestosis, and acute interstitial pneumonia.
Types of ILD exacerbations included idiopathic acute exacerbation, disease progression, infection, and heart failure. To assess patient condition severity and prioritize waiting list placement based on survival probability, the lung allocation score (LAS) was used. Upon UCRI admission, variables related to dates of admission/discharge, origin unit, and 90-day readmission were recorded. Respiratory support types were documented: conventional oxygen, high-flow nasal cannula (HFNC), non-invasive ventilation (NIV), or CPAP. The use of combined therapy, NIV+HFNC or CPAP+HFNC, was indicated for patients with hypercapnic respiratory failure, refractory hypoxemic respiratory failure unresponsive to HFNC, or patients diagnosed with OSA or radiological atelectasis, respectively. Additional variables included blood gas parameters at admission (pH, pO2, pCO2, HCO3) and UCRI-related prognostic scales, such as:
- •
Rox index: SpO2/FiO2/RR at admission.
- •
Charlson Index: Predicts 10-year life expectancy based on age and comorbidities.
- •
APACHE-II scale: Records worst physiological parameters in the first 24h of UCRI admission.
Finally, transplantation variables included candidates on the list, those accepted but not yet listed, and those removed from the list at admission. The date of transplantation, need for “Urgency 0” status, days on ECMO before transplant, intubation prior to transplant, and survival rates at one, six, and twelve months were recorded.
Statistical analysisDescriptive analyses of categorical variables were conducted using absolute and relative frequencies, while numerical variables were analysed using mean, standard deviation, median, and 25th and 75th percentiles. Croup comparisons employed the Wilcoxon test for numerical variables, and Chi-square or Fisher's exact test for categorical variables. Kaplan–Meier survival analysis was employed to estimate the survival function and compare survival curves among different groups using the log-rank test. Competing risk analysis determines the cumulative incidence of transplantation versus death. Cox regression modelled the relationship of variables with survival, with a significance level set at 0.05. R version 4.3.3 was the statistical software used.
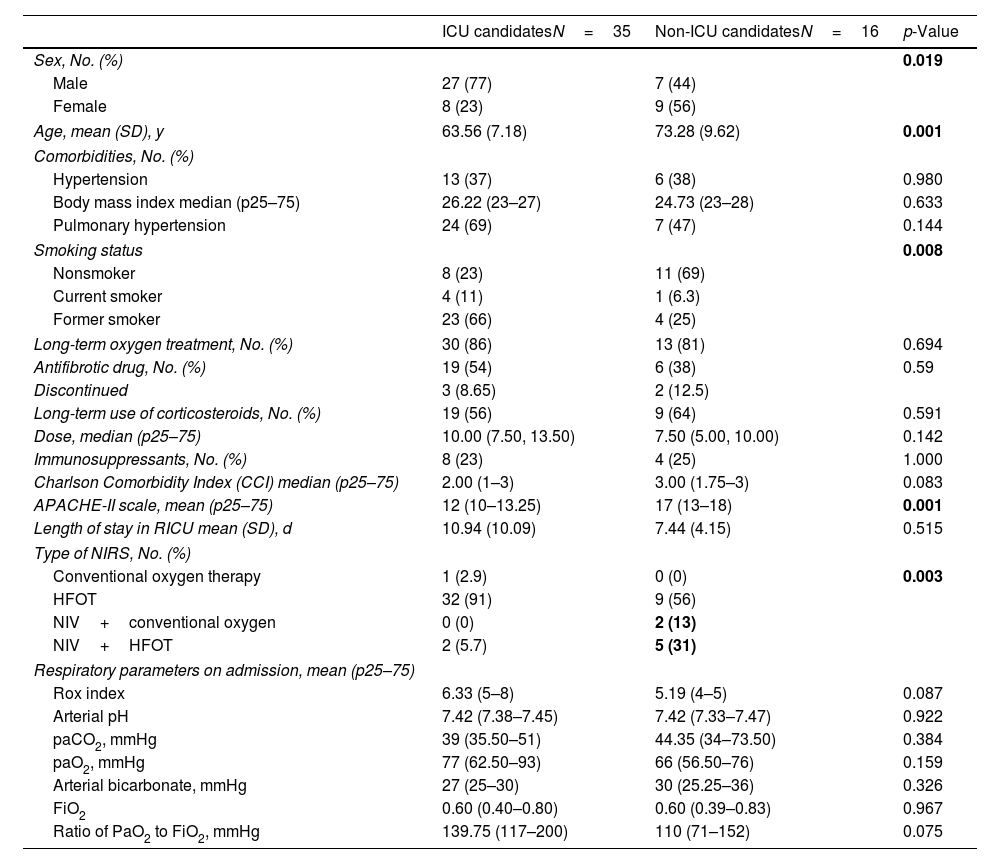
ResultsGeneralA total of 51 patients diagnosed with ILD were analysed out of the 401 patients treated in the RICU during the study period. The characteristics of the cohort are presented in Table 1, categorized by ICU eligibility at admission.
Baseline characteristics, comorbidities, chronic treatments, prognostic scales, types of NIRS, and respiratory parameters upon admission.
| ICU candidatesN=35 | Non-ICU candidatesN=16 | p-Value | |
|---|---|---|---|
| Sex, No. (%) | 0.019 | ||
| Male | 27 (77) | 7 (44) | |
| Female | 8 (23) | 9 (56) | |
| Age, mean (SD), y | 63.56 (7.18) | 73.28 (9.62) | 0.001 |
| Comorbidities, No. (%) | |||
| Hypertension | 13 (37) | 6 (38) | 0.980 |
| Body mass index median (p25–75) | 26.22 (23–27) | 24.73 (23–28) | 0.633 |
| Pulmonary hypertension | 24 (69) | 7 (47) | 0.144 |
| Smoking status | 0.008 | ||
| Nonsmoker | 8 (23) | 11 (69) | |
| Current smoker | 4 (11) | 1 (6.3) | |
| Former smoker | 23 (66) | 4 (25) | |
| Long-term oxygen treatment, No. (%) | 30 (86) | 13 (81) | 0.694 |
| Antifibrotic drug, No. (%) | 19 (54) | 6 (38) | 0.59 |
| Discontinued | 3 (8.65) | 2 (12.5) | |
| Long-term use of corticosteroids, No. (%) | 19 (56) | 9 (64) | 0.591 |
| Dose, median (p25–75) | 10.00 (7.50, 13.50) | 7.50 (5.00, 10.00) | 0.142 |
| Immunosuppressants, No. (%) | 8 (23) | 4 (25) | 1.000 |
| Charlson Comorbidity Index (CCI) median (p25–75) | 2.00 (1–3) | 3.00 (1.75–3) | 0.083 |
| APACHE-II scale, mean (p25–75) | 12 (10–13.25) | 17 (13–18) | 0.001 |
| Length of stay in RICU mean (SD), d | 10.94 (10.09) | 7.44 (4.15) | 0.515 |
| Type of NIRS, No. (%) | |||
| Conventional oxygen therapy | 1 (2.9) | 0 (0) | 0.003 |
| HFOT | 32 (91) | 9 (56) | |
| NIV+conventional oxygen | 0 (0) | 2 (13) | |
| NIV+HFOT | 2 (5.7) | 5 (31) | |
| Respiratory parameters on admission, mean (p25–75) | |||
| Rox index | 6.33 (5–8) | 5.19 (4–5) | 0.087 |
| Arterial pH | 7.42 (7.38–7.45) | 7.42 (7.33–7.47) | 0.922 |
| paCO2, mmHg | 39 (35.50–51) | 44.35 (34–73.50) | 0.384 |
| paO2, mmHg | 77 (62.50–93) | 66 (56.50–76) | 0.159 |
| Arterial bicarbonate, mmHg | 27 (25–30) | 30 (25.25–36) | 0.326 |
| FiO2 | 0.60 (0.40–0.80) | 0.60 (0.39–0.83) | 0.967 |
| Ratio of PaO2 to FiO2, mmHg | 139.75 (117–200) | 110 (71–152) | 0.075 |
No.: number of patients; SD: standard deviation; %: percentage; y: years; p25–75: p25–75th percentile; TNF: tumour necrosis factor; DMARD: disease-modifying antirheumatic drug; RICU: respiratory intermediate care unit; NIRS: non-invasive support; HFOT: high flow oxygen therapy; NIV: non-invasive ventilation; FiO2: fraction of inspired oxygen; mmHg: millimetres of mercury.
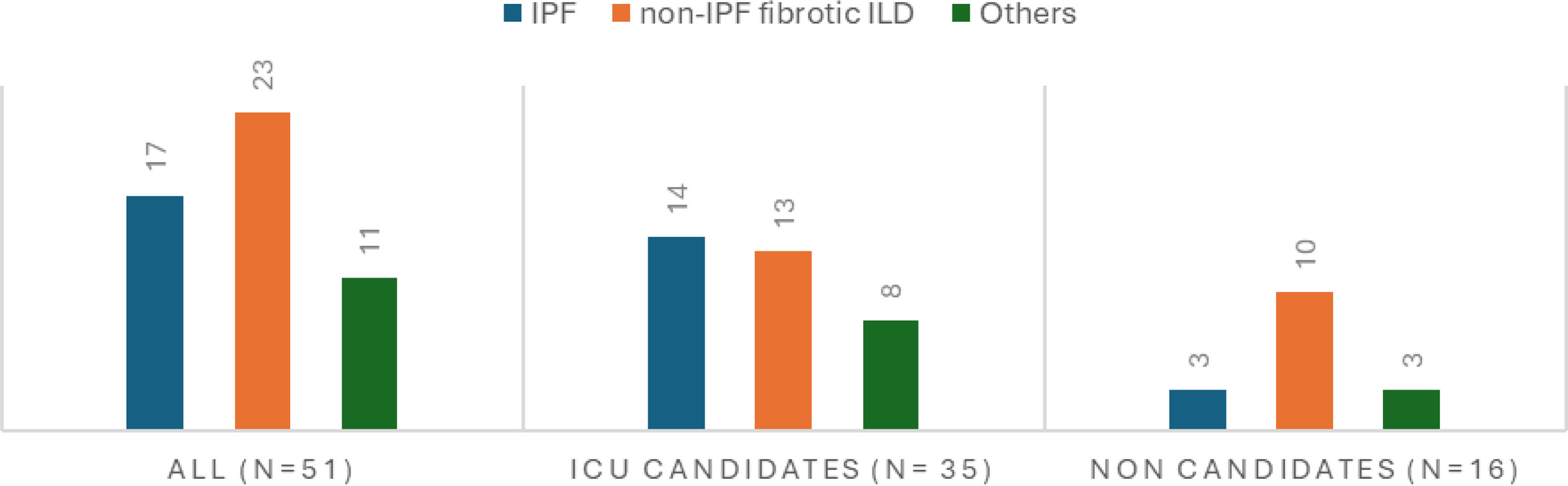
The ILD diagnosis upon admission was highly variable, so they were grouped into three categories (see “Statistical analysis” section). Since non-IPF fibrotic ILDs consist of several types of ILDs, idiopathic pulmonary fibrosis (IPF) is the most common disease in both groups, accounting for 33% of cases. It was more prevalent among ICU-eligible patients (40%, 14 vs. 3) (see Fig. 1). The main causes of admission included respiratory infection (N=20, 39%), followed by disease progression (N=15, 29%), of whom 14 were ICU candidates. Idiopathic exacerbation occurred in 11 patients (6 ICU eligible vs. 5 non-eligible), and heart failure in 2 (4%). Additionally, three patients were admitted for monitoring purposes due to complications from diagnostic bronchoscopy, a case of recovered cardiac arrest in a non-ICU-eligible patient who passed away three days after admission, and a patient on the transplant waiting list admitted for the initiation of pulmonary vasodilators. Furthermore, 41% of patients experienced with right heart failure related to pulmonary hypertension secondary to their advanced respiratory disease.
Regarding NIRS in the RICU, 48 (94%) patients received HFNC, while 7 (14%) patients required a combination with NIV due to ventilatory failure with respiratory acidosis (see Table 1).
The readmission rate during the first month post-RICU discharge was 9.8%. Primary causes were progression (31%) and infection (31%), followed by heart failure (14%). Two patients were readmitted and transplanted immediately. Only one non-ICU-eligible patient was readmitted to the unit.
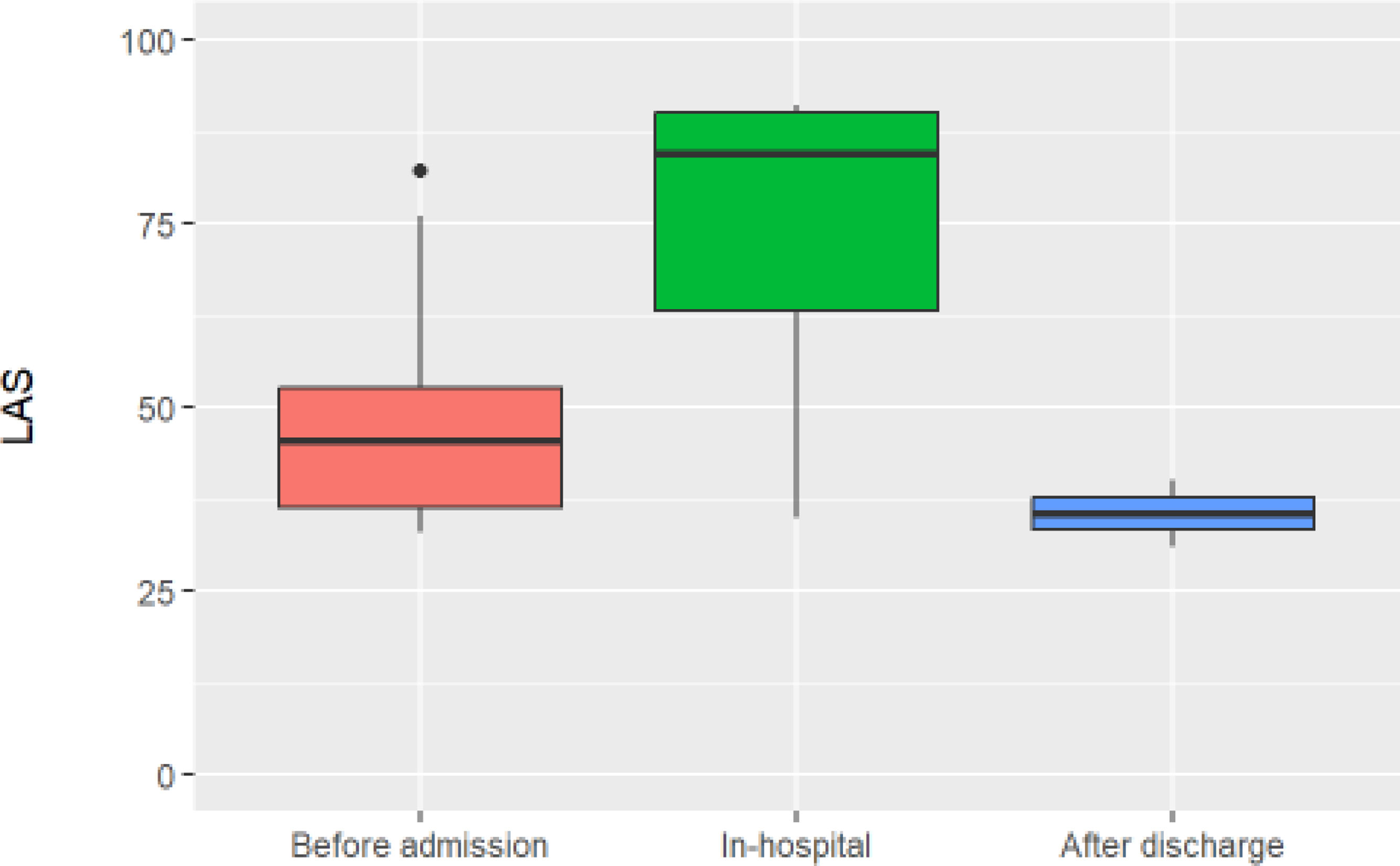
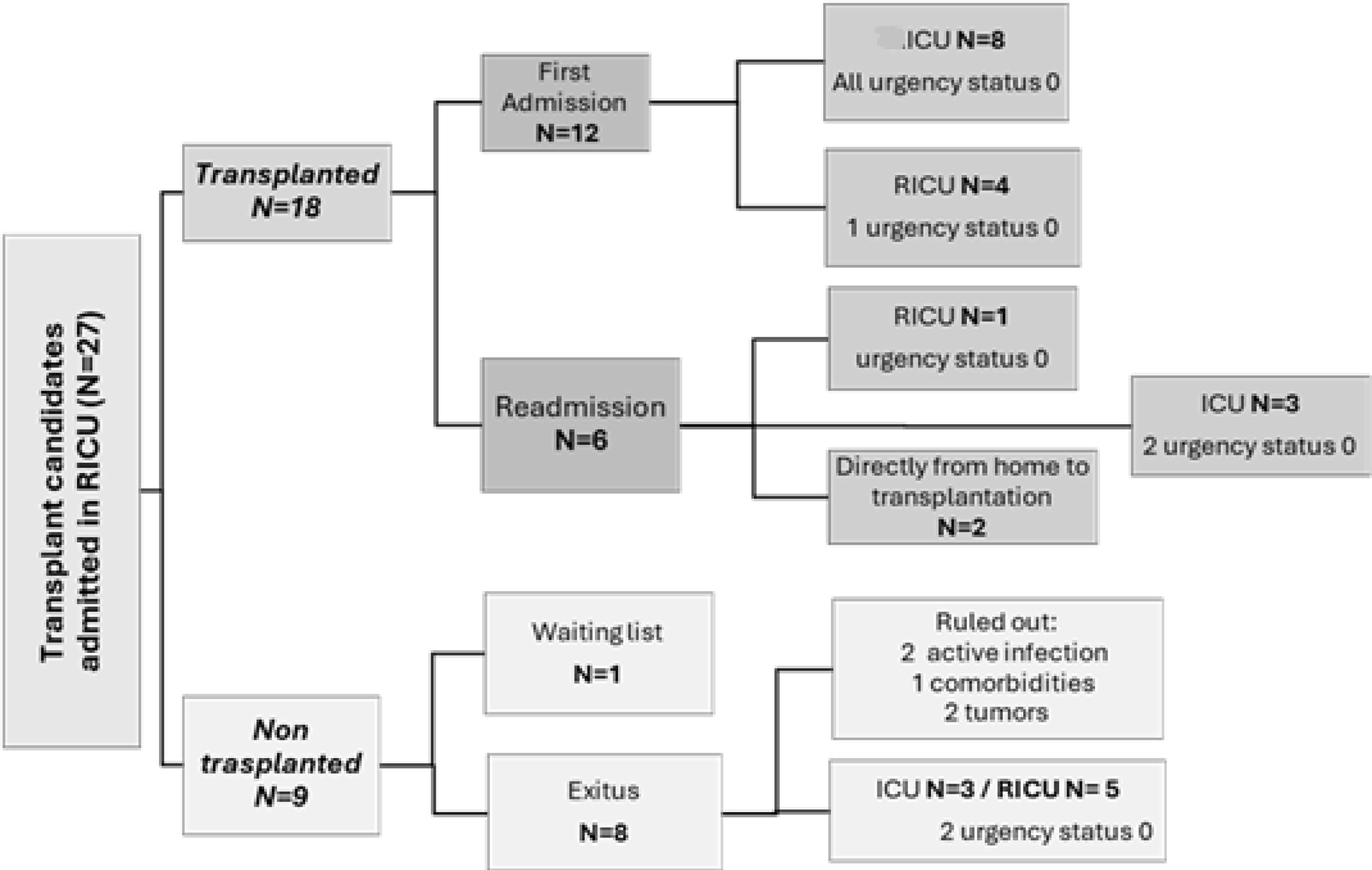
Subgroup of patients under lung transplant evaluationA total of 27 patients (52.94%) were being evaluated for lung transplantation. Of these, 14 (52%) were already on the transplant waiting list upon RICU admission, with a median lung allocation score (LAS) of 46 (37–53). Ten patients were evaluated and added to the list during their RICU stay, with a median LAS of 84 (63–90). Two patients were not listed until after discharge, with a median LAS of 35 (33–37) (see Fig. 2). One patient passed away after being accepted by the transplant committee but was not officially added to the list. Of the 27 transplant candidates, 18 patients (66%) were eventually transplanted; representing 36% of all RICU admissions for ILD at our centre (see Fig. 3). All patients transferred to ICU during their first admission, were transplant candidates (N=10, 19.6%), all as “Urgency 0” status. The average ICU stay before transplantation was 3.88 days (±1.54), while the average RICU stay was 13 days (±14). The median time to transplantation was 0.77 months (0.26–2.12). A total of five transplants were performed directly from RICU (28%).
Fifteen patients (55%) were prioritized under “Urgency 0” status (N=27), three of them transplanted directly from the RICU and two from the ICU without requiring ECMO neither invasive ventilation, only HFNC. All the rest (N=10), required ECMO, five of whom also needed invasive ventilation (two of these patients died before transplantation due to right ventricular failure). The other five underwent “awake ECMO” combined with HFNC; two of them for 2 and 3 days prior transplantation, and the remaining three just hours before being transferred to the operating room, for safety reasons (see Fig. 3).
Survival analysisAmong the 14 patients (27%) who died during their RICU stay, the median time to death was 10 days (5.75–18). Main causes of death were disease progression (64%); heart failure (21%), infection (7%) and advanced tumours (7%).
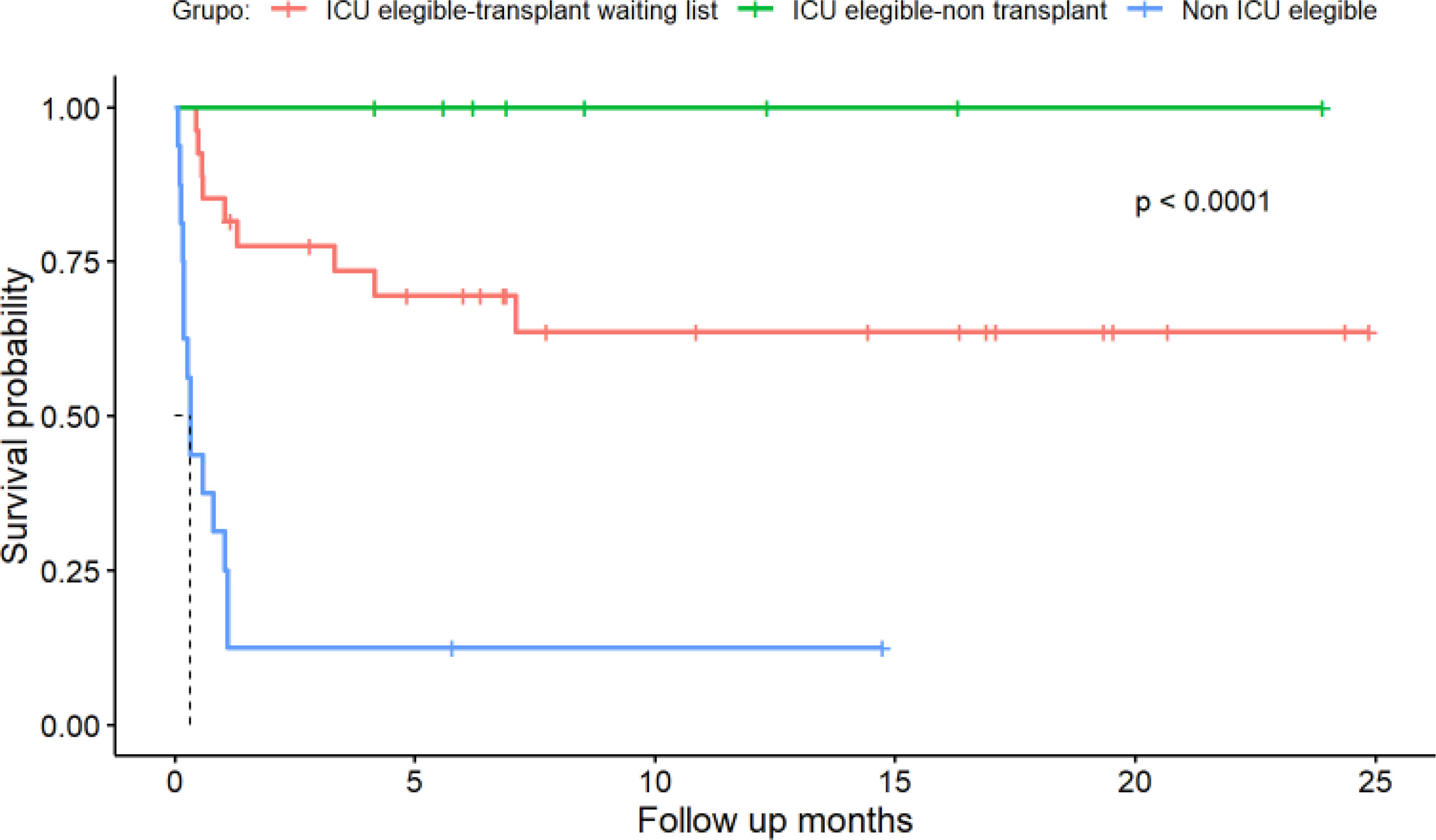
By the end of the follow-up, 23 patients (45%) had died. In this cohort, Kaplan–Meier survival estimates demonstrated significant differences between ICU-eligible and non-eligible patients (Fig. 4A, p=<0.0001). ICU-eligible patients exhibited survival rates of 89% at one month, 77% at six months, and 72% at one year. Non-ICU-eligible patients had survival rates of 31%, 13%, and 13%, respectively.
Non-ICU-eligible patients who were not on the transplant waiting list either required transfer to the ICU or died during their hospital stay or the follow-up period (see Fig. 4, p<0.0001). In the subgroup of patients undergoing transplant evaluation, four patients (14%) died during hospitalization; two of them died in the ICU under national urgency status, and two died in the RICU (one was removed from the transplant list due to an active infection, and the other due to disease progression).
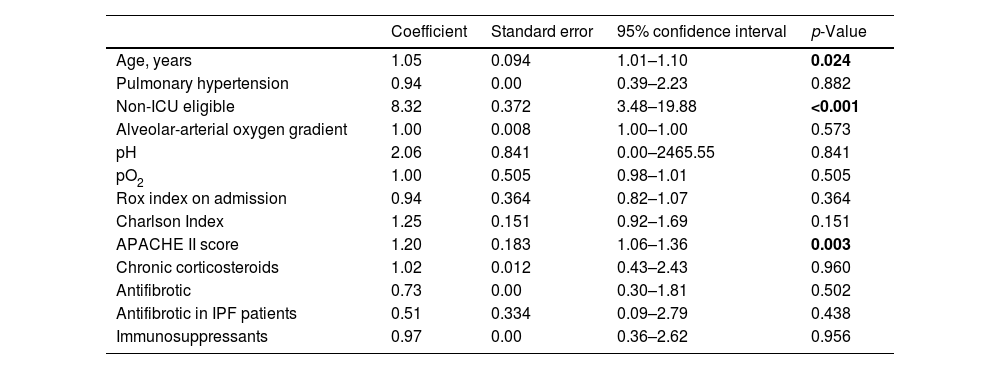
The univariable analysis revealed that age, APACHE score, and the therapeutic ceiling status were associated with survival (see Table 2). Multivariable analysis confirmed that being ICU eligible was the sole independent variable significantly impacting survival (HR 6.12; CI 2.08–18.01; p<0.001).
Univariate survival analysis.
| Coefficient | Standard error | 95% confidence interval | p-Value | |
|---|---|---|---|---|
| Age, years | 1.05 | 0.094 | 1.01–1.10 | 0.024 |
| Pulmonary hypertension | 0.94 | 0.00 | 0.39–2.23 | 0.882 |
| Non-ICU eligible | 8.32 | 0.372 | 3.48–19.88 | <0.001 |
| Alveolar-arterial oxygen gradient | 1.00 | 0.008 | 1.00–1.00 | 0.573 |
| pH | 2.06 | 0.841 | 0.00–2465.55 | 0.841 |
| pO2 | 1.00 | 0.505 | 0.98–1.01 | 0.505 |
| Rox index on admission | 0.94 | 0.364 | 0.82–1.07 | 0.364 |
| Charlson Index | 1.25 | 0.151 | 0.92–1.69 | 0.151 |
| APACHE II score | 1.20 | 0.183 | 1.06–1.36 | 0.003 |
| Chronic corticosteroids | 1.02 | 0.012 | 0.43–2.43 | 0.960 |
| Antifibrotic | 0.73 | 0.00 | 0.30–1.81 | 0.502 |
| Antifibrotic in IPF patients | 0.51 | 0.334 | 0.09–2.79 | 0.438 |
| Immunosuppressants | 0.97 | 0.00 | 0.36–2.62 | 0.956 |
Our cohort analysis was conducted by dividing patients into two groups: ICU candidates or non-candidates. Epidemiological differences were present at the time of admission, with ICU candidates being younger (63±7 vs. 73±9 years) and predominantly male (77% vs. 44%). These differences related to the fact that 76% of ICU candidates being evaluated for lung transplantation, who must meet strict selection criteria, such as age. Individuals over 70 have a higher probability of post-transplant failure.12 Although age is not an exclusion criterion, it explains differences between groups. The predominance of males (40% vs. 19%) can also be attributed to a higher prevalence of IPF in this subgroup, as male sex is a risk factor,13 as well as tobacco exposure (77% vs. 32%). Our data align with the National Transplant Organization (ONT) for the year 2023 (65% males and 35% females).14 IPF is the most prevalent, worst-prognosis pulmonary disease and the most frequently transplanted in Spain, and in our centre.15,16
In our cohort, 58.8% (N=30) of patients were receiving or had previously received antifibrotic therapy, including 82% (N=14) of those diagnosed with IPF. Among non-IPF patients, only 11 (47%) had initiated antifibrotic treatment, primarily due to the late approval of nintedanib for progressive pulmonary fibrosis in September 2022. Additionally, some patients did not meet the treatment criteria, and in those who were not ICU candidates, its use was restricted due to advanced age and associated comorbidities.
ICU candidates have a slightly lower Charlson Comorbidity Index (CCI) score (1–2 vs. 2–3), though this difference is not statistically significant. This finding indicates that the differences between groups cannot be attributed to CCI-based comorbidities. However, significant differences were observed in APACHE II scores with non-ICU-eligible patients more severely ill at the RICU admission (13 vs. 17). No significant differences were observed in respiratory parameters at admission, although non-ICU candidates had a higher mean pCO2, lower P/F ratio, and lower I. Rox at admission, consistent with the APACHE scale. Only three patients exceeded 20 points on the APACHE scale and had a CCI greater than 4, which are two criteria considered for RICU admission at our hospital. Therefore, selecting RICU candidates with pulmonary disease aligns with our protocol.
ICU candidates also had a longer hospital stay (11 vs. 7 days), though this was not statistically significant. Twelve patients were placed on the transplant list during their RICU stay or after discharge. Six underwent transplantation during the same hospitalization, requiring multiple assessments (cardiac catheterization, CT scans, pulmonary and cardiac ultrasound …) and evaluations by specialists (rehabilitation, thoracic surgery, cardiology, etc.). Inclusion on the transplant list requires clinical stability, the daily rehabilitation ability, and adequate nutrition, all contributing to longer stay in the unit.
These patients face high mortality due to respiratory illness, reflected in a higher LAS score than outpatients (84±63–90 vs. 46±37–53). Before the pandemic, our centre lacked an RICU, and all such patients required ICU admission, with associated costs and loss of specialization. We have not found studies specifically addressing RICY benefits for this subgroup of patients. In our cohort, prioritized transplant candidates were transferred to the ICU, except one patient, when ECMO was necessary. This is reflected in their short ICU stay (3.88 days in ICU vs. 13 days in RICU). Avoiding ICU transfer is preferred as mechanical ventilation increases mortality by up to 90%.3 Furthermore, there is strong evidence that HFNC prevent lung function deterioration and intubation due to its clinical and physiological benefits, such as alveolar recruitment, humidification, heating, enhanced secretion clearance, and reduced dead space.17,18 It also reduces sedative use, length of stay,11 intubation rates,19 and in some studies, short-term mortality reduction has also been observed.20 In our cohort, 94% of patients received HFOT despite a mean P/F ratio lower than 150 at admission, but with a Rox index greater than 3. Data were not collected to analyse the evolution of Rox index during the first 24h of admission. Nine patients required NIV, always used in combination with another therapy (high-flow oxygen or conventional oxygen), and the indication was respiratory acidosis, more frequent among non-ICU candidates, related to greater admission severity.
Twenty-eight percent of patients were transplanted directly from RICU, highlighting RICU's efficiency as a bridge to transplantation. This approach benefits patients who experience exacerbation without being on the transplant list, or even without having initiated the process, which can take months on an outpatient, risking high mortality. Although we do not have a specific cost-effectiveness study of our unit, Heili-Frades et al.6 demonstrated that high-complexity units reduce costs and complications associated with prolonged ICU stays.
In terms of mortality, usually over half of the patients admitted for exacerbation die in-hospital,10 while our cohort's RICU mortality was 27%, rising to 45% long-term. Mortality was lower in transplant candidates, representing 14%. Conversely, non-ICU-eligible patients experience high mortality within the first 10 days. Peters et al.21 proposed HFOT as an effective respiratory support for patients with a do-not-intubate order and hypoxemic respiratory failure, including patients with pulmonary fibrosis. However, we observed that despite respiratory support, over 70% of non-ICU candidates die, prompting us to reconsider initial admission selection for these patients while maintaining HFOT as palliative care to alleviate dyspnoea in selected cases.
The average time from admission to death was significantly longer for transplant candidates (47.44 days) compared to non-candidates (6.36 days). Log-rank tests confirmed that survival curve differences were statistically significant. Multivariable analysis revealed that the therapeutic ceiling criterion for RICU is associated with high mortality, which serves to modify our admission criteria, and encourage new protocols, recognizing that pulmonary disease exacerbations inherently have high mortality.
The limitations of this study include a small sample size, lack of a control group, and limited follow-up to one year, which limits statistical power and prevents thorough evaluation of long-term outcomes, survival rates, and quality of life post-discharge.
Additionally, the mortality rate for ILD exacerbation in our cohort was lower, as 35% of patients admitted underwent lung transplantation, contributing to improved outcomes.
ConclusionsPatients with acute exacerbations of ILD and a therapeutic ceiling in the RICU exhibit a high mortality rate (85%), even when meeting the established admission criteria (Charlson Comorbidity Index <4, APACHE <20). For this subgroup, stricter admission criteria may be necessary, while HFNC should be maintained as a palliative care option outside the unit. The APACHE scale and the concept of a therapeutic ceiling are valuable tools in predicting survival for ILD patients admitted to the RICU. Additionally, RICUs could serve as a bridge to lung transplantation for ILD patients experiencing acute exacerbations, as these units help stabilize patients within the transplant eligibility window, facilitating the transplant evaluation without requiring intubation or mechanical ventilation. In out cohort RICU admission has the potential to reduce or even avoid pre-transplant ICU stays by up to 28%, thereby decreasing ICU-related complications and associated healthcare costs. This study aims to provide a foundation for developing a specific admission protocol for the RICU tailored to patients with acute ILD exacerbations.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsAll authors were involved in the conception and design of the work; acquisition, analysis, interpretation of data, drafting the work, revising it critically for important intellectual content.
Conflicts of interestThe authors declare not having any conflicts of interest that may be considered to influence directly or indirectly the content of the manuscript.