An 82-year-old woman with a history of polycythemia vera on treatment with hydroxycarbamide 500mg/day presented with asthenia, odynophagia and dry cough for two weeks. Physical examination showed generalized hypophonesis with bibasal rales. Chest X-ray showed a diffuse bilateral interstitial pattern and scant bilateral pleural effusion. Blood tests showed hemoglobin 5.5g/dL, leukocytosis (17,100/μL), neutropenia (700/Al), monocytosis (12,500/μL), elevated LDH (660IU/L) and NT-proBNP (2740pg/ml). Microbiological tests (PCR in nasopharyngeal exudate for SARS-CoV-2/VSR/Influenza A and B, sputum culture, blood cultures and antigenuria) did not detect microorganisms. Peripheral blood smear showed 72% blasts with lax chromatin, with one to four nucleoli and poor nucleus-to-cytoplasm ratio, which was mainly agranular and without splinters. The patient was admitted and treatment was started with meropenem 1g IV every 8h, linezolid 600mg IV every 12h, hydroxycarbamide dose was increased to 500mg every 12h, furosemide 20mg IV every 12h, in addition to administration of blood products until hemoglobin figures of 9.2g/dL were reached.
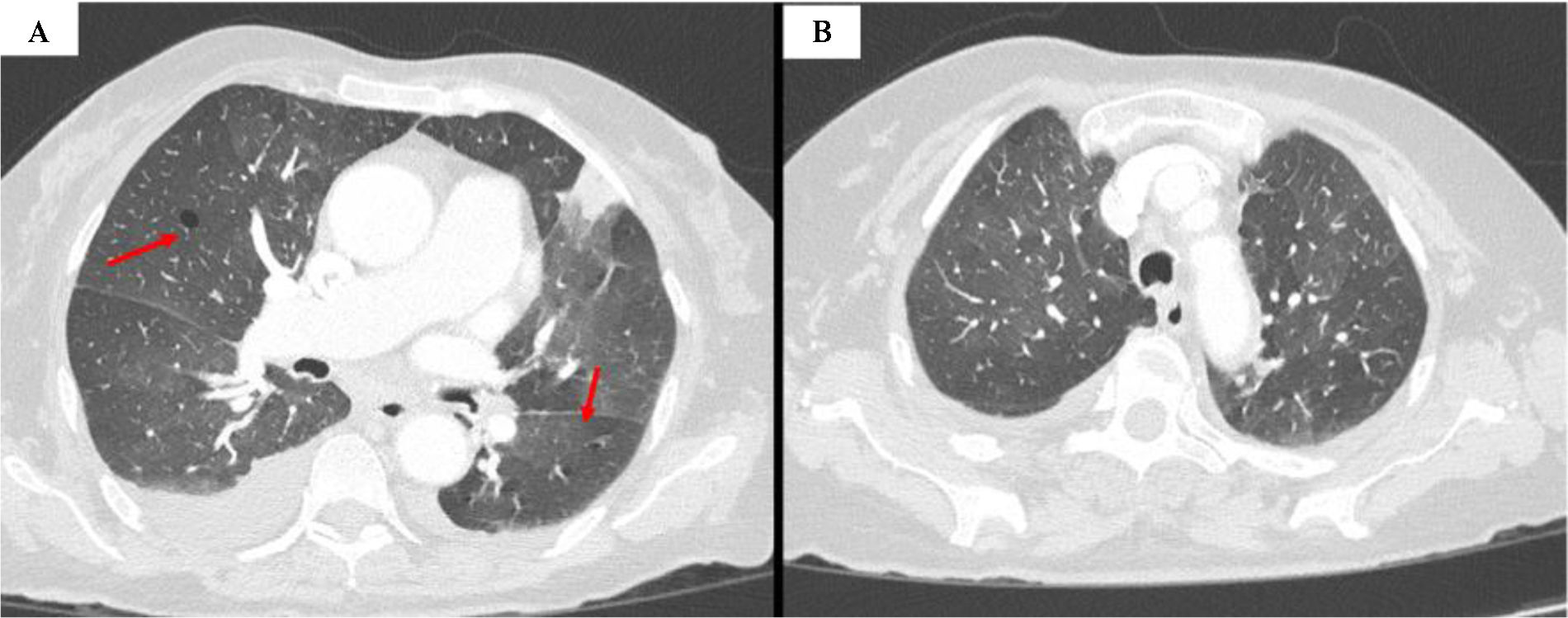
After a bone marrow biopsy, the diagnosis of acute myeloid leukemia (AML) with complex karyotype, inversion of chromosome 16 (inv16) was confirmed, but it was decided to postpone the initiation of hypomethylation until the resolution of the respiratory symptoms. After five days of antibiotic treatment the patient presented persistent cough, increased asthenia, fever, exertional dyspnea and required oxygen therapy. The control X-ray showed a worsening of the interstitial pattern, with a decrease in pleural effusion. Blood tests showed the appearance of eosinophilia (3300/μL) and a decrease in NT-proBNP levels, while the rest of the parameters showed no significant changes with respect to the admission analysis. Thoracic computed tomography (CT) showed ground-glass opacities and cystic lesions (Fig. 1). Bronchoscopy and bronchoalveolar lavage (BAL) were performed, and PCR for Pneumocystis jirovecii was positive (10,000 genome copies/ml). Treatment with trimethoprim/sulfamethoxazole 160/800mg IV every 12h and methylprednisolone 40mg IV every 24h was started. After four days with this treatment she presented unfavorable evolution, appearance of hypoxemic respiratory failure and subsequent death.
Cases of PCP in acute myeloid leukemia and myelodysplastic syndromes are rare, the incidence of developing PCP in AML is 6.8% and the risk may increase by 2% for each cycle of chemotherapy.1 One of the reasons for this is that P. jirovecci interacts with lymphocytes and in AML the main abnormality is found in the myeloid precursors 2. On the other hand, alveolar macrophages (AM) do not seem to be severely damaged, even when the patient receives bone marrow suppression therapy for AML, as these cells have a high regenerative capacity.3 It does not seem that the use of monoclonal antibodies in AML particularly affects AM, as they have a very low expression of the surface marker CD33 (myeloid cell marker).2,3
So far, this would be the second described case of de novo leukemia presenting with acute PJP. An increased risk of complications associated with inv16 AML has been suggested, such as: eosinophilia, organizing pneumonia or bronchiolitis obliterans, which in up to 54% of cases may be expressed as acute respiratory distress syndrome.4,5 With this in mind, it appears that our patient's eosinophilia was another complication associated with her AML. De novo AML presenting initially as a severe P. jirovecii infection is extremely rare, so the risk of opportunistic infections in this form of leukemia needs to be studied.
Ethical approval and informed consentWe have the approval of the ethics committee and the informed consent of the patients included in this research.
FundingThis research has not received any specific grants from agencies in the public, commercial or for-profit sectors.
Confidentiality of dataThe authors declare that they have followed their institution's protocols on the publication of patient data.
Declaration of generative AI and AI-assisted technologies in the writing processThe authors declare that they have not used any type of generative artificial intelligence in the writing of this manuscript or for the creation of figures, graphs, tables or their corresponding captions or legends.
Conflicts of interestThe authors state that they have no conflict of interests.