Diabetic kidney disease features certain clinical and laboratorial characteristics that differ from chronic kidney disease of other etiologies. We performed a transversal study comparing some of these characteristics and assessed potential associations among blood pressure, plasma phosphate concentration and estimated glomerular filtration rate between patients with diabetic and non-diabetic chronic kidney disease.
We found a positive correlation between both systolic and diastolic blood pressure and the plasma phosphate concentration in the diabetic kidney disease group, but not in the non-diabetic group. Also, diastolic blood pressure was negatively correlated with the estimated glomerular filtration rate in the diabetic group, yet not in the non-diabetic group.
In conclusion, these data support the hypothesis of a close link between systolic and diastolic blood pressure and hyperphosphatemia, as well as between diastolic blood pressure and estimated glomerular filtration rate, in patients with diabetic kidney disease. Therapeutic approaches directed at these factors might prove to be important to delay the decline of renal function in the subgroup of patients with diabetic kidney disease.
Chronic kidney disease (CKD) represents a major public health problem due to the elevated prevalence of associated cardiovascular disease.1
Diabetic nephropathy is one of the major complications of diabetes mellitus (DM) and is the leading cause of chronic kidney disease and end-stage renal disease worldwide.2–4 Clinical features and treatments of diabetic and non-diabetic CKD are quite different, thus it is important to distinguish between both etiologies in an early stage of the natural history of the disease.5
Recent studies suggest that an inherited predisposition to high blood pressure (BP) may confer susceptibility to diabetic nephropathy, supporting the claim that hypertension (HT) is an independent risk factor for diabetic nephropathy.5,6 The mechanisms behind this link remain complex but include the stimulation of the sympathetic nervous system and activation of the systemic renin-angiotensin system (RAS), therefore leading to retention of water and sodium.5 Furthermore, activated intrarenal RAS has shown to play an important role in the pathogenesis of HT and CKD, specially in patients with diabetic nephropathy.7,8 Intrarenal generation of angiotensin II is increased in these patients, contributing to the progression of diabetic nephropathy via several hemodynamic, tubular and growth-promoting actions.9 This view is supported by studies showing that treatments for high blood pressure and that target the RAS slow the decline of glomerular filtration rate (GFR), postponing end-stage CKD.2,5 Also, a recent study showed that systolic blood pressure (SBP) and diastolic blood pressure (DBP) had both lower values in patients with non-diabetic CKD.5
HT and hyperphosphatemia have been associated with vascular calcification, an important predictor of progression of the CKD, frequently since early stages of the disease.10,11 Type 2 diabetic patients tend to have particularly severe vascular calcification phenomena.10,11 Beside vascular calcification, other harmful effects of phosphate overload include direct induction of chronic systemic inflammation, malnutrition and increased activation of the intrarenal RAS.12–14 These are secondary to impaired clearance of the dietary phosphate as kidney function declines.12,13 Thus, it is plausible that antiphosphate treatment may slow GFR deterioration, specially in patients with diabetic CKD.
The current study attempted to explore potential relations between some of the factors that have been associated to the progression of CKD. Among the available data, we selected BP and plasma phosphate concentration to conduct a more detailed analysis.15
MethodsThis cross-sectional study was carried out at the Nephrology Department of the Health Service of Madeira Autonomous Region (SESARAM, E.P.E).
Study design and patientsThe Low Clearance Consultation (LCC) is a service provided by the Department of Nephrology and comprises a regular clinic and laboratorial follow-up of CKD patients who reach eGFR equal or below 40ml/min/1.73m2. The study was designed to include all patients registered in the LCC in December 2015. Exclusion criteria were initiation of renal replacement therapy and occurrence of death in the specified period.
A total of 53 patients were enrolled in the study and classed in two groups, according to the etiology of the CKD: diabetic CKD versus non-diabetic CKD.
Clinical and laboratory assessmentsOffice BP was assessed in a sitting position, after at least 5minutes of rest, using a calibrated sphygmomanometer with appropriately sized cuffs in the upper dominant arm. We registered the mean of three measurements, performed with a two minute interval.
The mean blood samples were collected early in the morning, after a night of fasting. Estimated glomerular filtration rate (eGFR) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula.
EndpointsThe primary objective of the study was to compare clinical and analytical characteristics between the two groups.
The secondary objectives were to evaluate the correlation between 1) eGFR and BP in diabetic versus non-diabetic CKD; 2) plasma phosphate concentration and BP in diabetic versus non-diabetic CKD.
Statistical analysisCategorical variables were expressed as percentages and continuous variables as medians with the interquartile range (IQR: 25th–75th percentiles).
Baseline characteristics are shown according to the etiological groups of chronic kidney disease: diabetic versus non-diabetic. Relationships between etiological groups and baseline characteristics were investigated by Pearson chi-square or Fisher test analyses for percentages and by univariate linear regression analyses for etiological groups versus continuous distribution variables. Adjustment for confounding variables was applicable when p-value<0.1. Potential correlations of eGFR and plasma phosphate concentration with SBP and DBP were explored using figures showing regression lines with interquartile ranges and Pearson's correlation test.
A probability value of <0.05 was considered to confer statistical significance. Analyses and graphs were performed with Stata software® version 12 (Stata Corp, Texas, USA).
ResultsClinical and analytical characteristics were available for all the 53 patients included in the study: 30 patients with diabetic CKD and 23 patients with non-diabetic CKD.
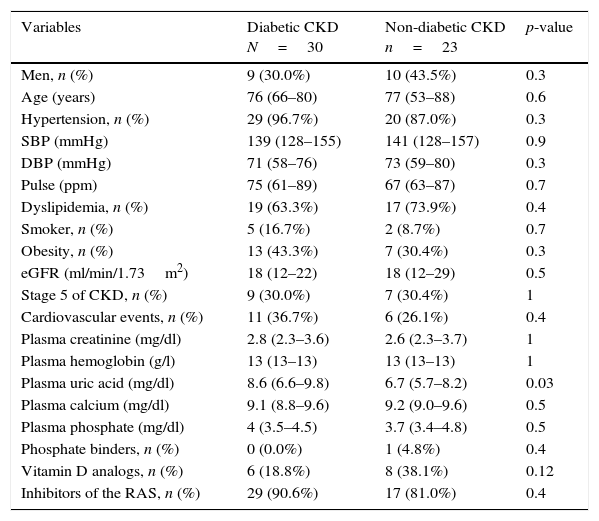
Comparison of patients with diabetic CKD and non-diabetic CKDCompared with patients of the non-diabetic CKD group, those of the diabetic CKD group had higher plasma uric acid (8.6mg/dl [IQR 6.6–9.8] vs 6.7mmHg [IQR 5.7–8.2], p=0.03). The two groups did not differ in other baseline characteristics (Table 1).
Baseline characteristics of patients with diabetic and non-diabetic CKD.
| Variables | Diabetic CKD N=30 | Non-diabetic CKD n=23 | p-value |
|---|---|---|---|
| Men, n (%) | 9 (30.0%) | 10 (43.5%) | 0.3 |
| Age (years) | 76 (66–80) | 77 (53–88) | 0.6 |
| Hypertension, n (%) | 29 (96.7%) | 20 (87.0%) | 0.3 |
| SBP (mmHg) | 139 (128–155) | 141 (128–157) | 0.9 |
| DBP (mmHg) | 71 (58–76) | 73 (59–80) | 0.3 |
| Pulse (ppm) | 75 (61–89) | 67 (63–87) | 0.7 |
| Dyslipidemia, n (%) | 19 (63.3%) | 17 (73.9%) | 0.4 |
| Smoker, n (%) | 5 (16.7%) | 2 (8.7%) | 0.7 |
| Obesity, n (%) | 13 (43.3%) | 7 (30.4%) | 0.3 |
| eGFR (ml/min/1.73m2) | 18 (12–22) | 18 (12–29) | 0.5 |
| Stage 5 of CKD, n (%) | 9 (30.0%) | 7 (30.4%) | 1 |
| Cardiovascular events, n (%) | 11 (36.7%) | 6 (26.1%) | 0.4 |
| Plasma creatinine (mg/dl) | 2.8 (2.3–3.6) | 2.6 (2.3–3.7) | 1 |
| Plasma hemoglobin (g/l) | 13 (13–13) | 13 (13–13) | 1 |
| Plasma uric acid (mg/dl) | 8.6 (6.6–9.8) | 6.7 (5.7–8.2) | 0.03 |
| Plasma calcium (mg/dl) | 9.1 (8.8–9.6) | 9.2 (9.0–9.6) | 0.5 |
| Plasma phosphate (mg/dl) | 4 (3.5–4.5) | 3.7 (3.4–4.8) | 0.5 |
| Phosphate binders, n (%) | 0 (0.0%) | 1 (4.8%) | 0.4 |
| Vitamin D analogs, n (%) | 6 (18.8%) | 8 (38.1%) | 0.12 |
| Inhibitors of the RAS, n (%) | 29 (90.6%) | 17 (81.0%) | 0.4 |
Data are expressed as medians (interquartile range) unless otherwise indicated; DBP: diastolic blood pressure; SBP: systolic blood pressure; eGFR: estimated glomerular filtration rate (calculated using the Chronic Kidney Disease Epidemiology Collaboration formula); RAS: renin-angiotensin system.
In our sample, SBP was similar between genders (141mmHg [IQR 129–157] in men vs 140mmHg [IQR 123–155] in women, p=0.9) and the same was verified in diabetic and non-diabetic CKD groups (p=0.3 vs p=0.3).
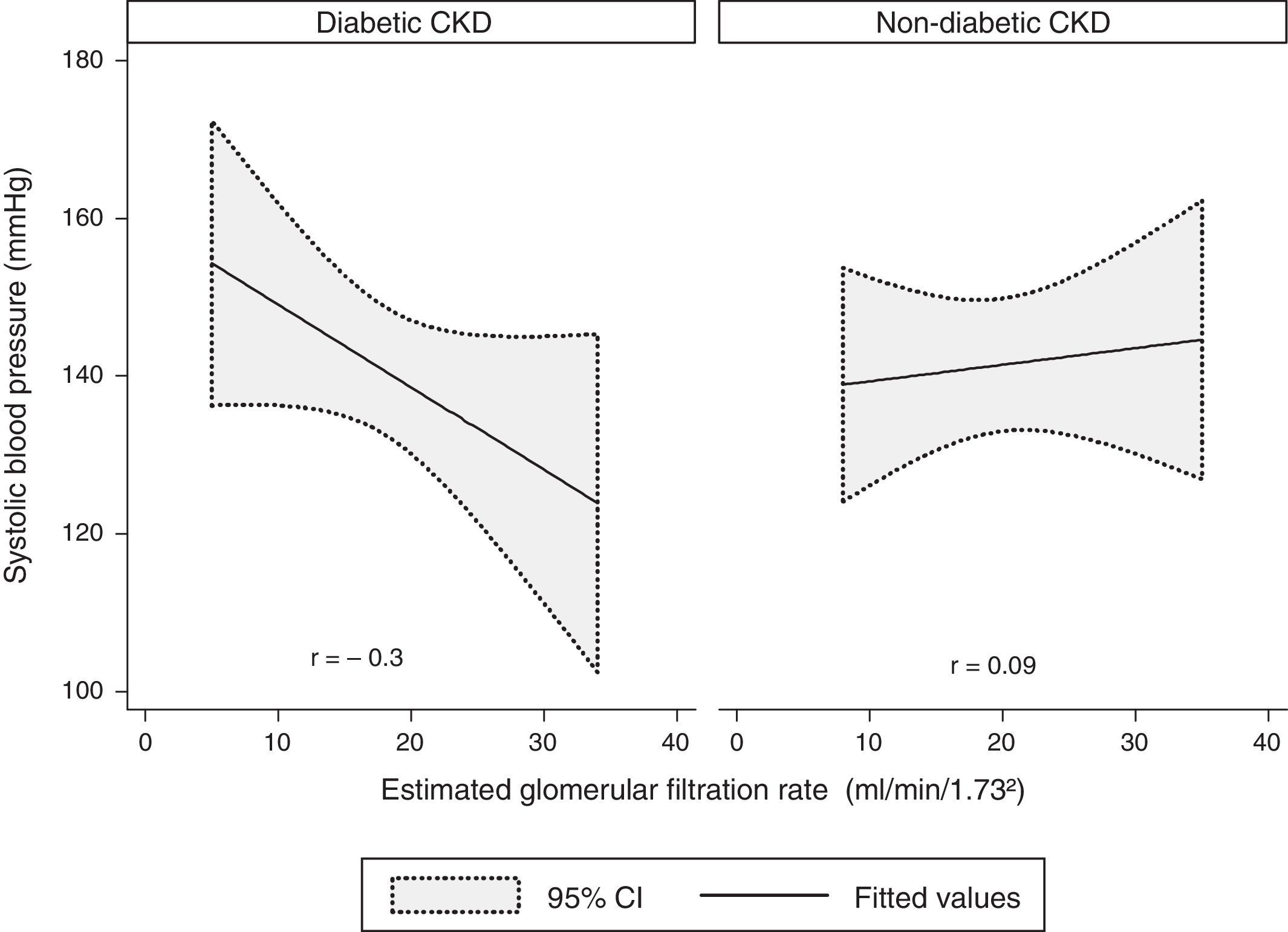
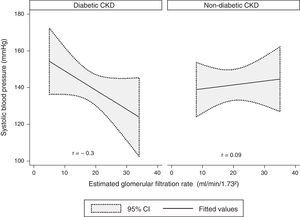
A modest negative correlation was found between the SBP and the eGFR in the whole sample, but it showed no statistical significance (r=−0.1, p=0.4). Concerning the groups, there was a moderate negative correlation between the SBP and the eGFR in the diabetic CKD group, although without statistical significance (r=−0.3, p=0.09). No correlation was found in the non-diabetic CKD group (r=0.09, p=0.7) (Fig. 1).
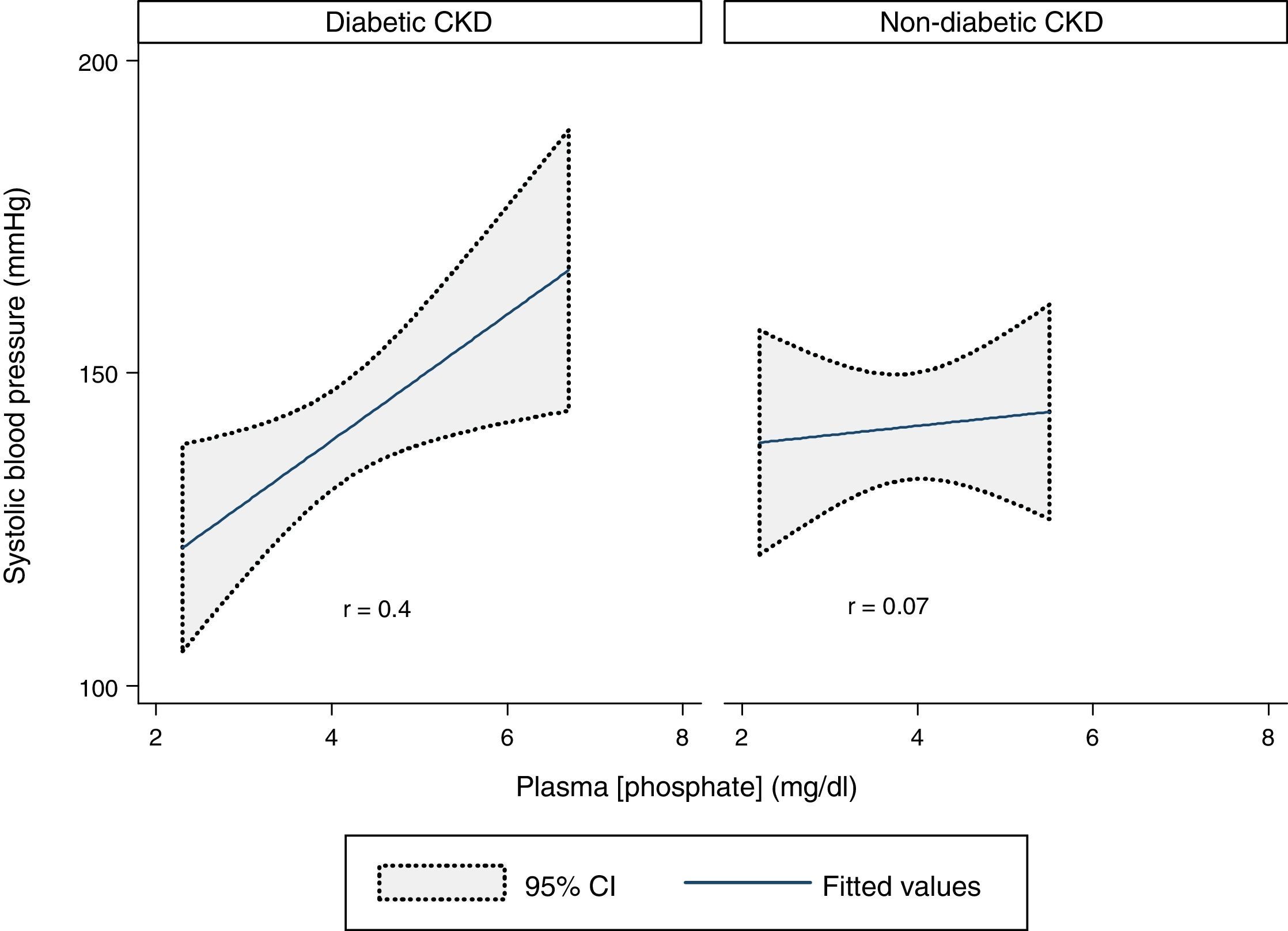
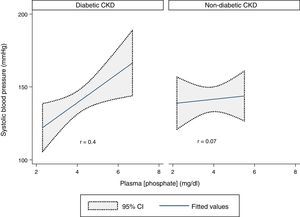
A statistically significant moderate positive correlation was verified between SBP and plasma phosphate concentration in the whole sample (r=0.3, p=0.03). Analyzing the two groups, there was a statistically significant moderate positive correlation in the diabetic CKD group (r=0.4, p=0.02) but no correlation was found in the non-diabetic CKD group (r=0.07, p=0.7) (Fig. 2).
Diastolic blood pressureSimilarly to SBP, DBP did not differ between men and women in our sample (72mmHg [IQR 59–76] in men vs 71mmHg [IQR 58–78] in women, p=0.9) or in the diabetic and non-diabetic CKD groups (p=0.4 vs p=0.1).
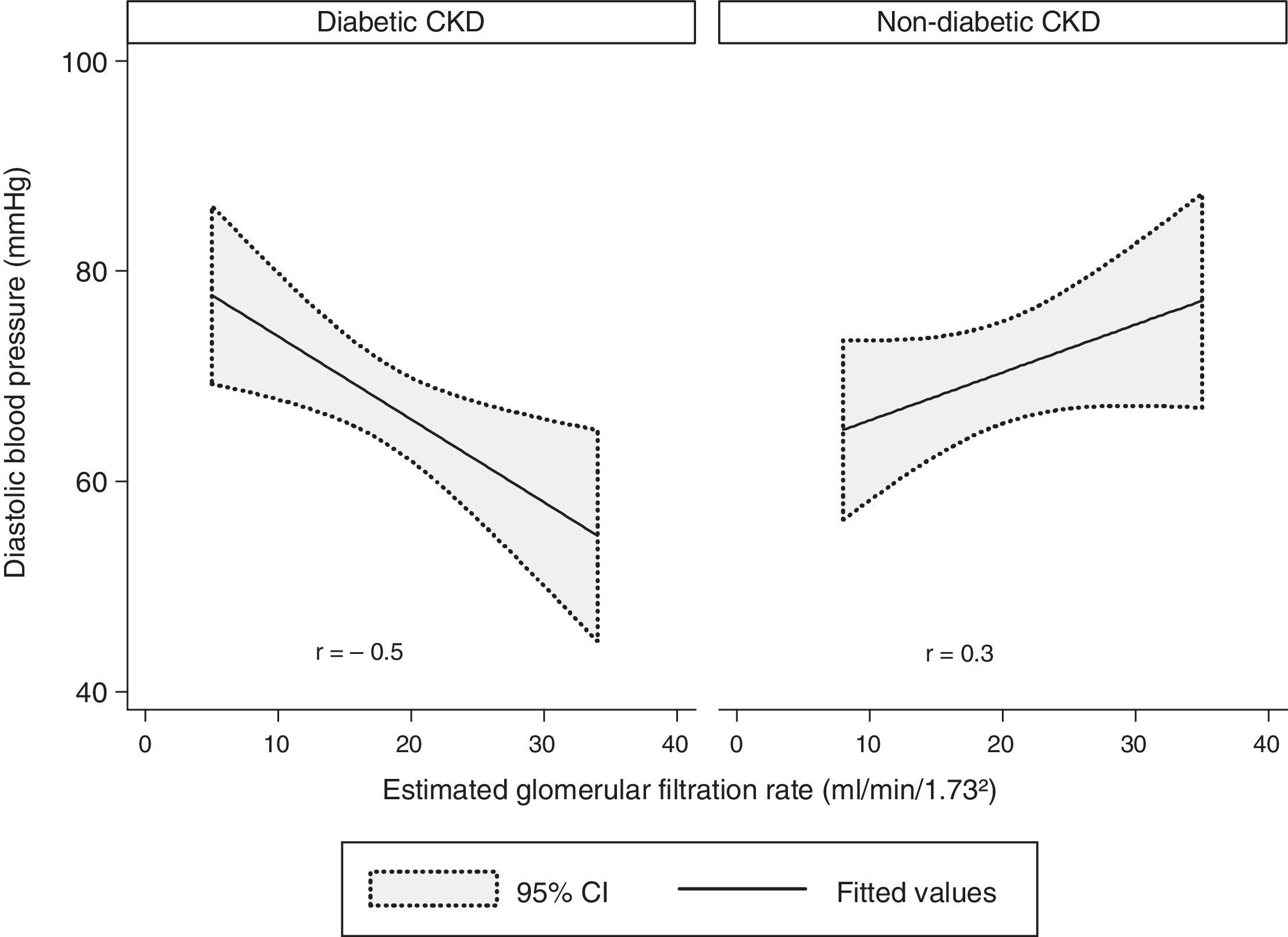
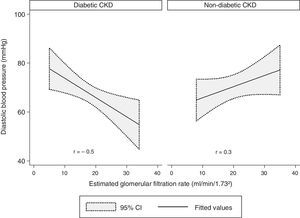
There was a modest negative correlation between the DBP and the eGFR in our sample, although without statistical significance (r=−0.1, p=0.7). Concerning the groups, there was a statistically significant and strong negative correlation between the DBP and the eGFR in the diabetic CKD group (r=−0.5, p=0.009). In the non-diabetic CKD group, no statistically significant correlation was found between DBP and eGFR (r=−0.3, p=0.1) (Fig. 3).
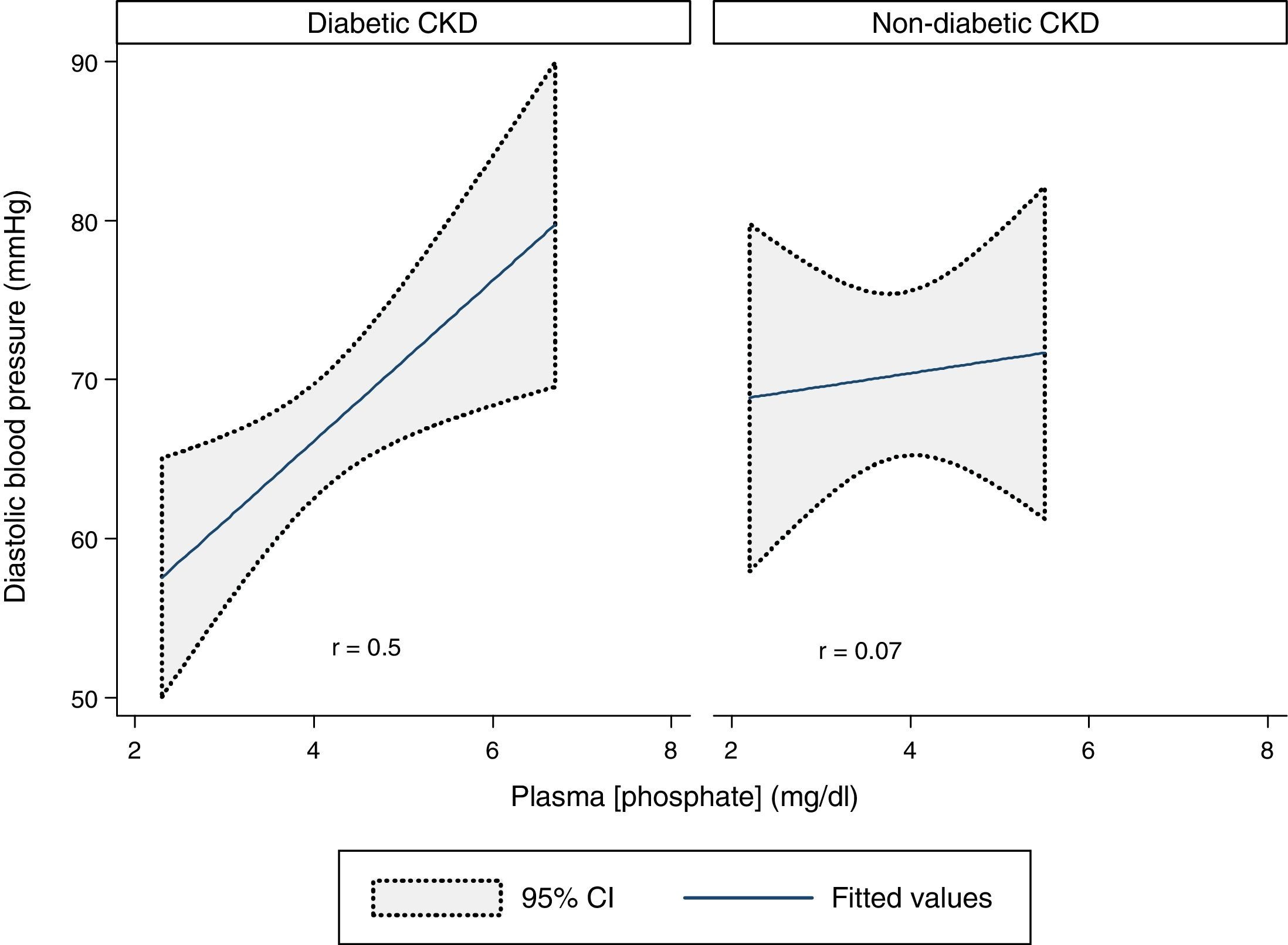
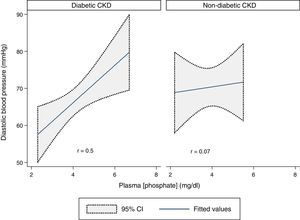
DBP and plasma phosphate concentration showed a statistically significant moderate positive correlation in the whole sample (r=0.3, p=0.03). That correlation was stronger in the diabetic CKD group (r=0.5, p=0.009) but was absent in the non-diabetic CKD group (r=0.07, p=0.8) (Fig. 4).
DiscussionThe current study intended to explore differences and establish associations between relevant variables associated to progression of CKD, according to the diabetic and non-diabetic etiology of the disease.
No statistically significant difference was obtained among the totality of baseline characteristics compared between the diabetic and the non-diabetic CKD groups.
Our study showed a positive correlation between SBP and plasma phosphate concentration, which was due to a moderate positive correlation in the diabetic CKD group. The same results were obtained between DBP and plasma phosphate concentration. This demonstrates that BP, either systolic or diastolic, is closely associated to an increase of plasma phosphate retention in diabetic CKD, but not in non-diabetic CKD patients.
No statistically significant correlation was observed between SBP and the eGFR in our study. Analysis between DBP and the eGFR showed a strong negative correlation in the diabetic CKD group. This suggests that association between BP and renal function deterioration in CKD is limited to the diastolic component of BP and to diabetic CKD.
The main strength of our study was the use of rigorous procedures to assess clinical and biological parameters. As potential limitations, we mention the small sample size and the impossibility to estimate the sodium intake through the urinary sodium excretion dosing. Nevertheless, we were able to demonstrate statistically significant differences in correlations between selected characteristics in diabetic and non-diabetic CKD groups. Further studies, specifically designed for the purpose, are necessary to support the obtained results and to explore potential causality hypotheses that may arise among the described associations.
Sources of fundingNone.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank Dr. Carina Graça for the support provided for the realization of the current scientific work.